Electrochemical Synthesis of Plasmonic Nanostructures
Abstract
:1. Introduction
2. Optical Properties of Metal Nanostructures
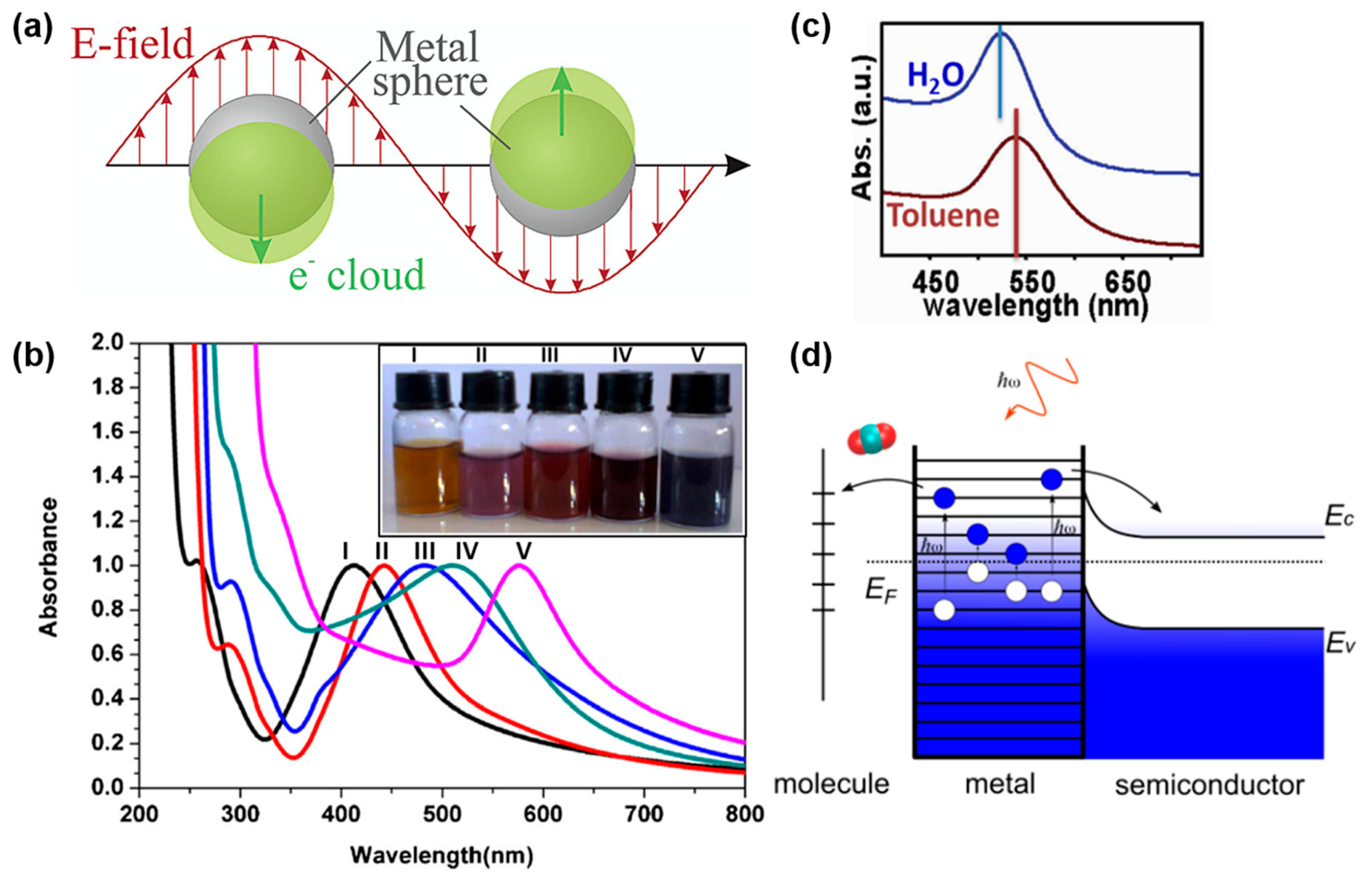
2.1. Spherical Nanoparticles
2.2. Anisotropic Nanoparticles
2.3. Controlling Distances within Metal Nanostructures in One, Two, and Three Dimensions
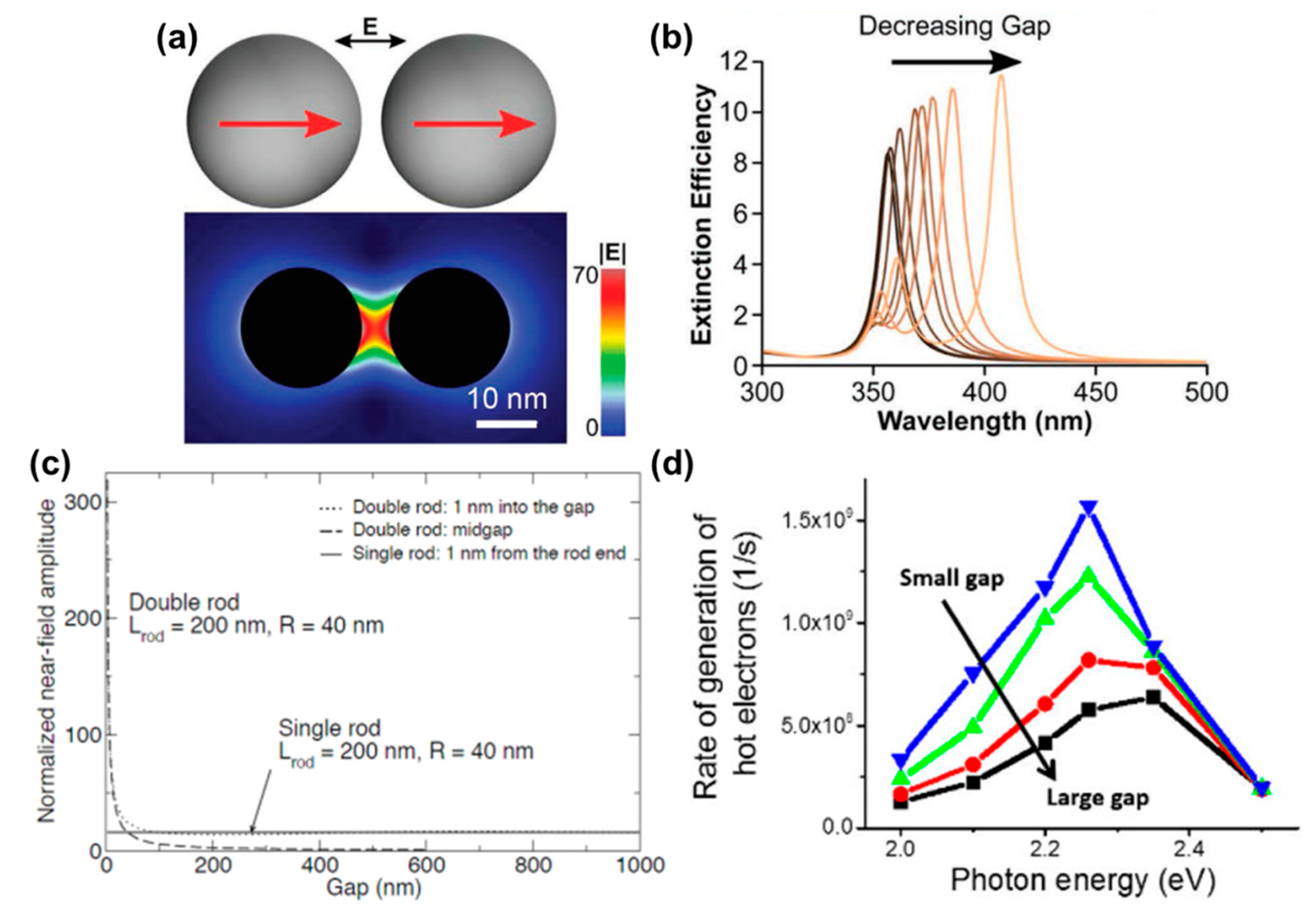
2.3.1. Nanoscale Gaps (1D)
2.3.2. One-, Two-, and Three-Dimensional Plasmonic Arrays (1D, 2D, and 3D)
3. Template-Free Electrochemical Synthesis of Metal Nanostructures
3.1. Electrochemical Deposition
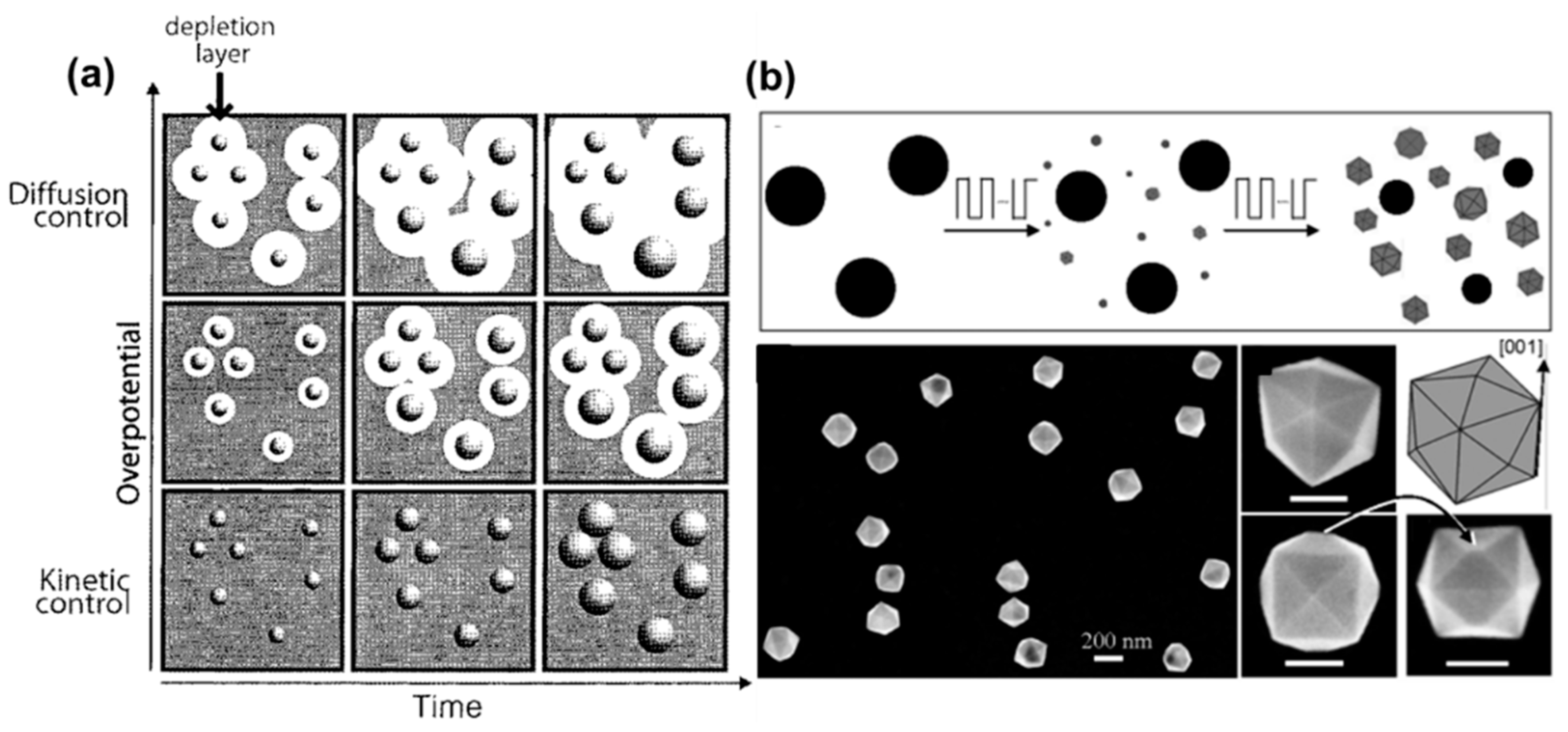
3.1.1. Synthesis of Monodisperse NPs
3.1.2. Synthesis of Anisotropic NPs
3.2. Ostwald Electrochemical Ripening
3.3. Electrochemical Dealloying
4. Templated Electrochemical Syntheses
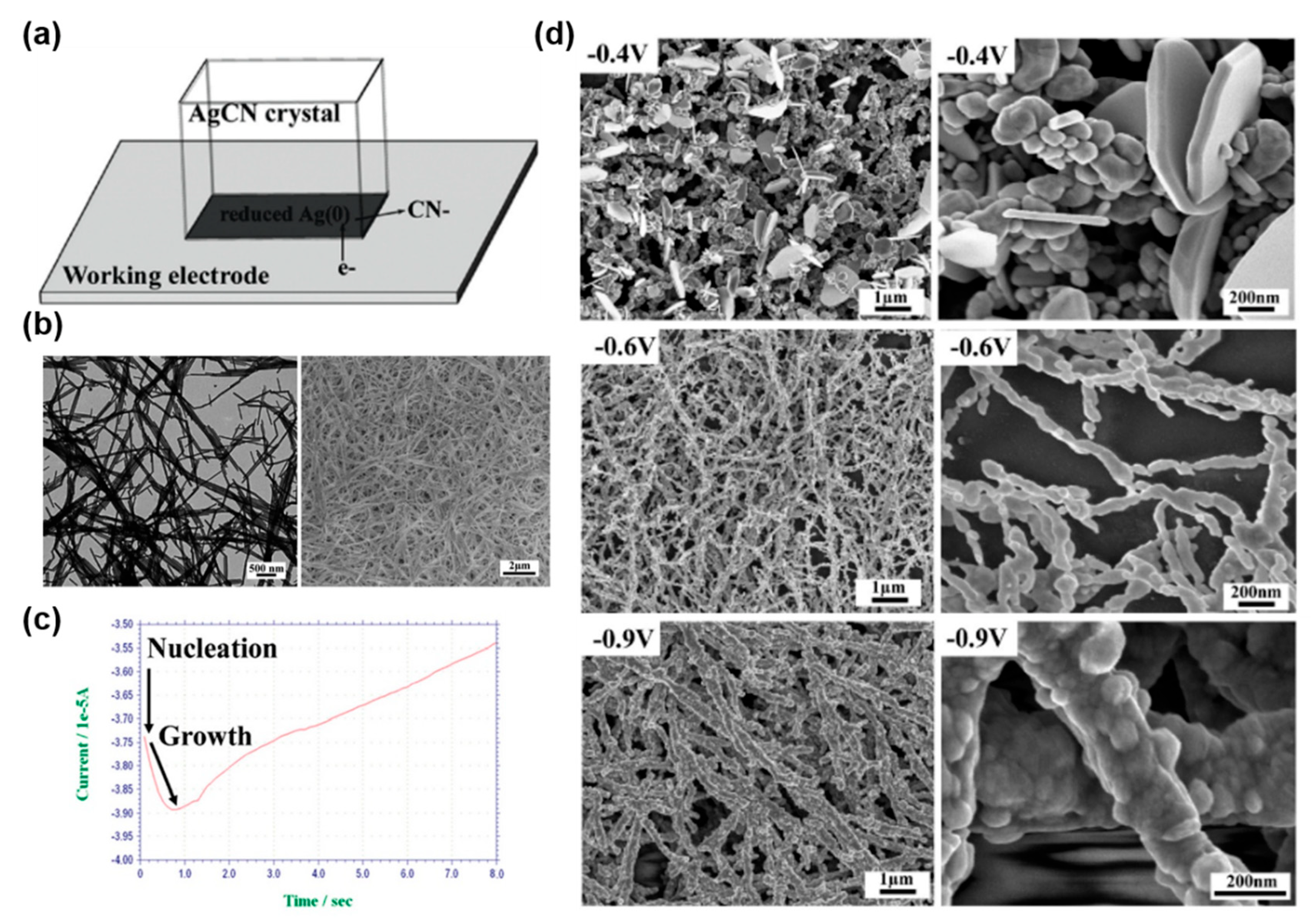
4.1. Sacrificial Templates: Electrochemical Reduction of Non-Zero-Valent Metal Nanostructures
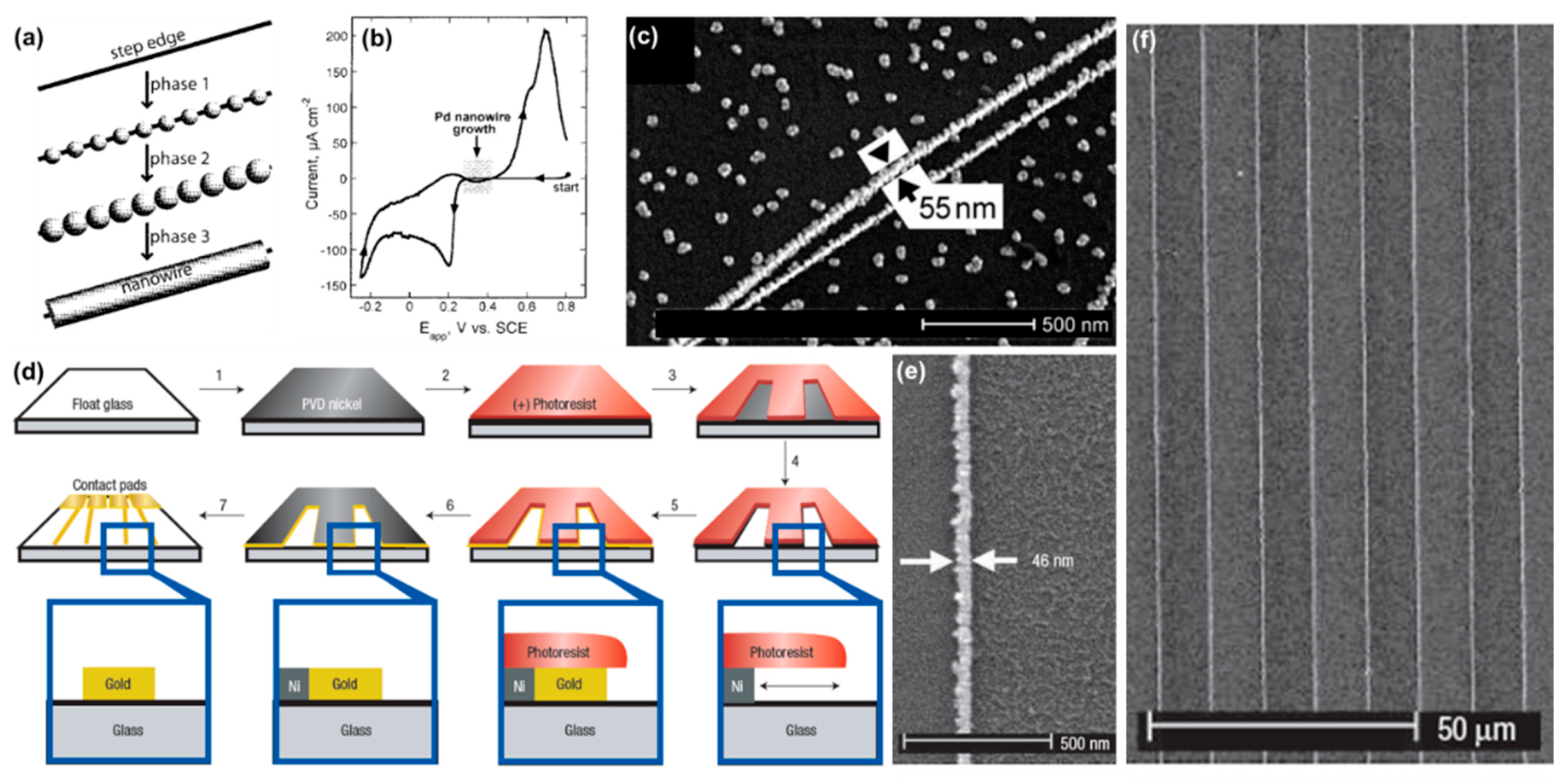
4.2. Patterned Planar Electrodes
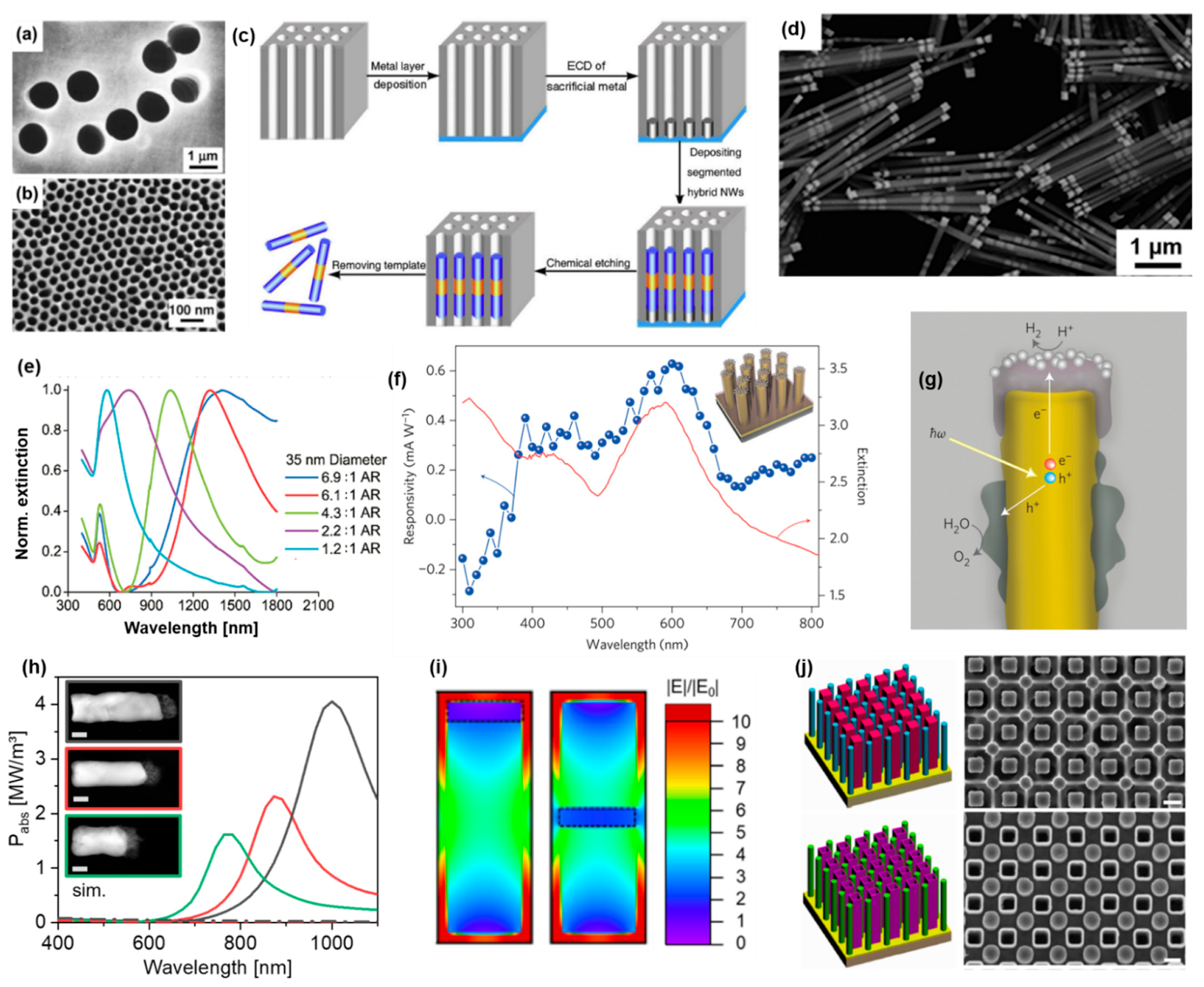
4.3. Porous Membranes
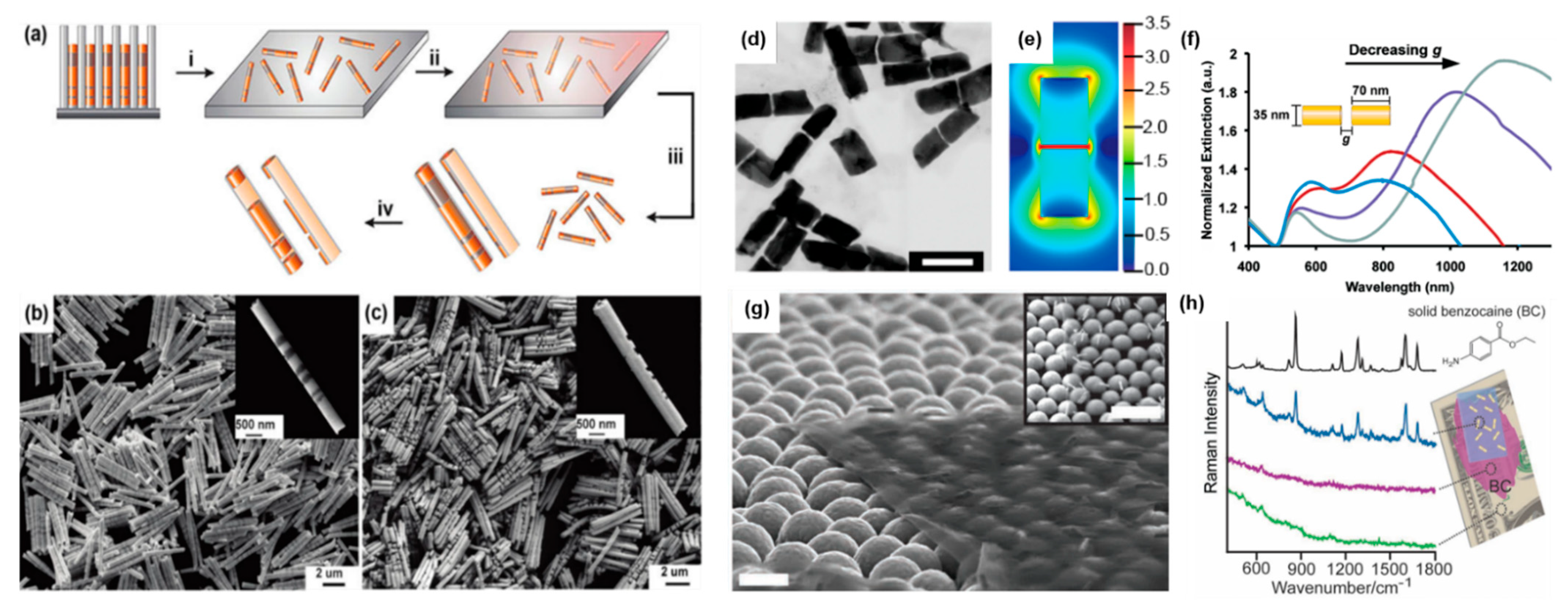
4.3.1. Nanorod Synthesis
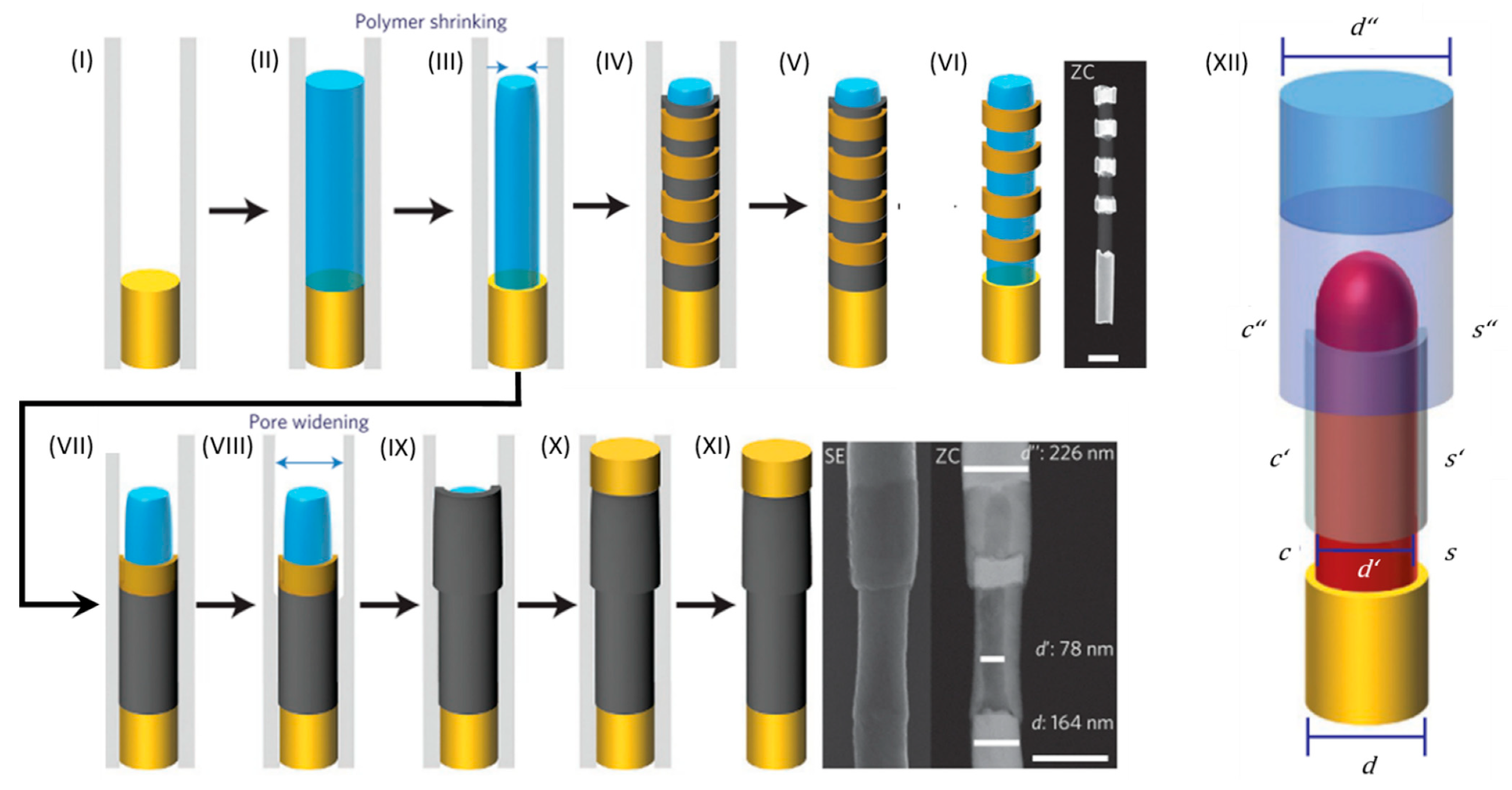
4.3.2. On-Wire Lithography
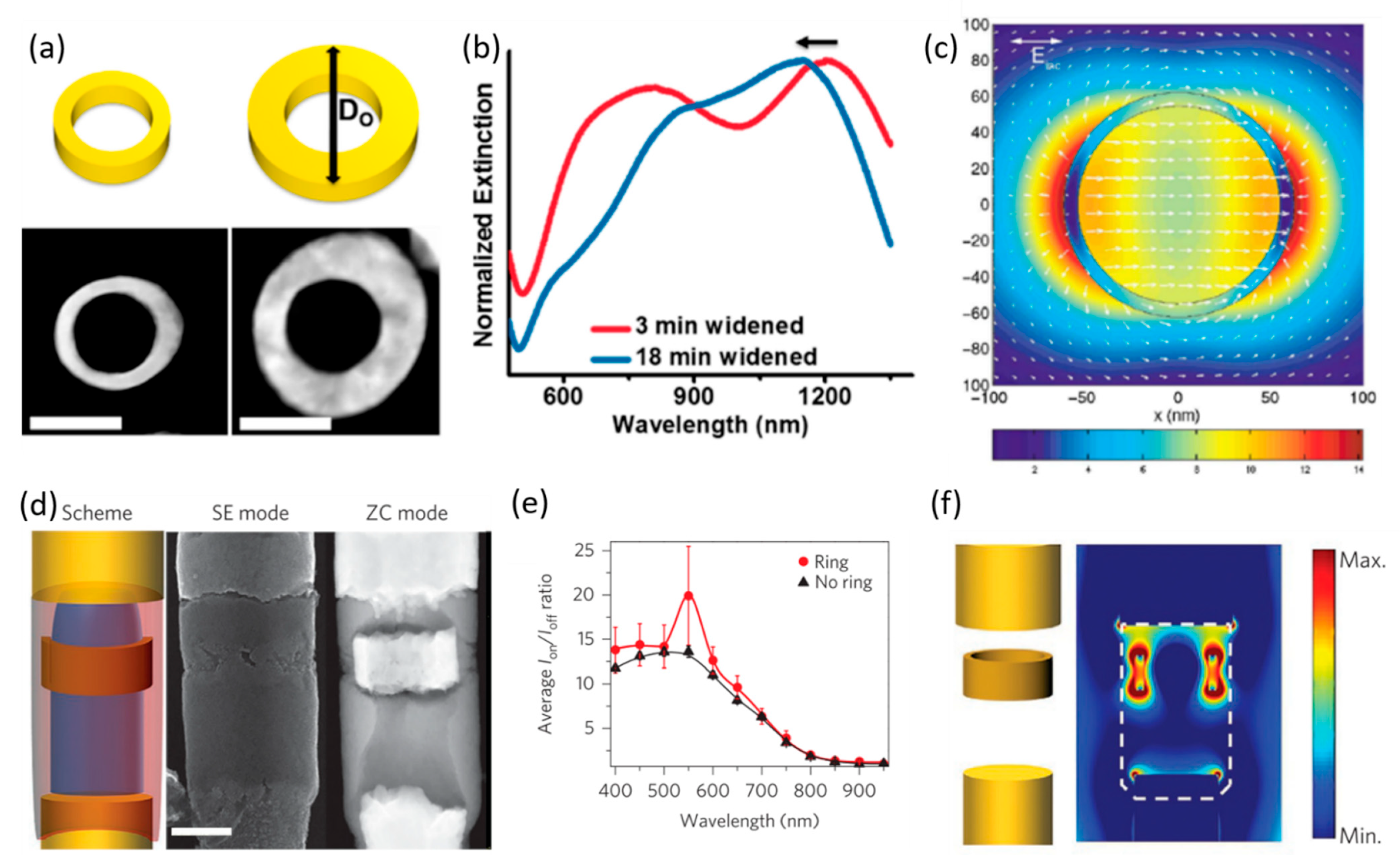
4.3.3. Coaxial Lithography
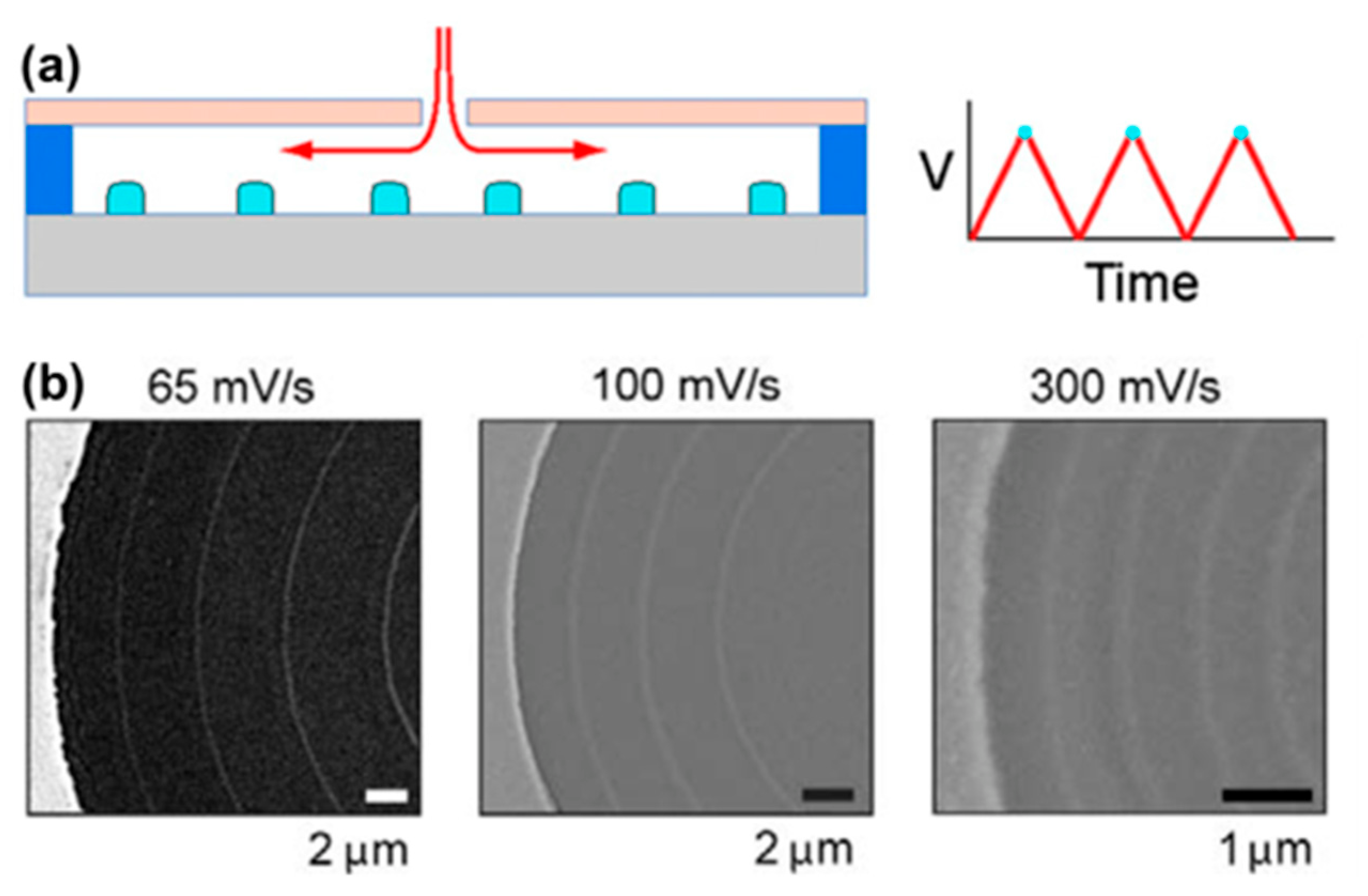
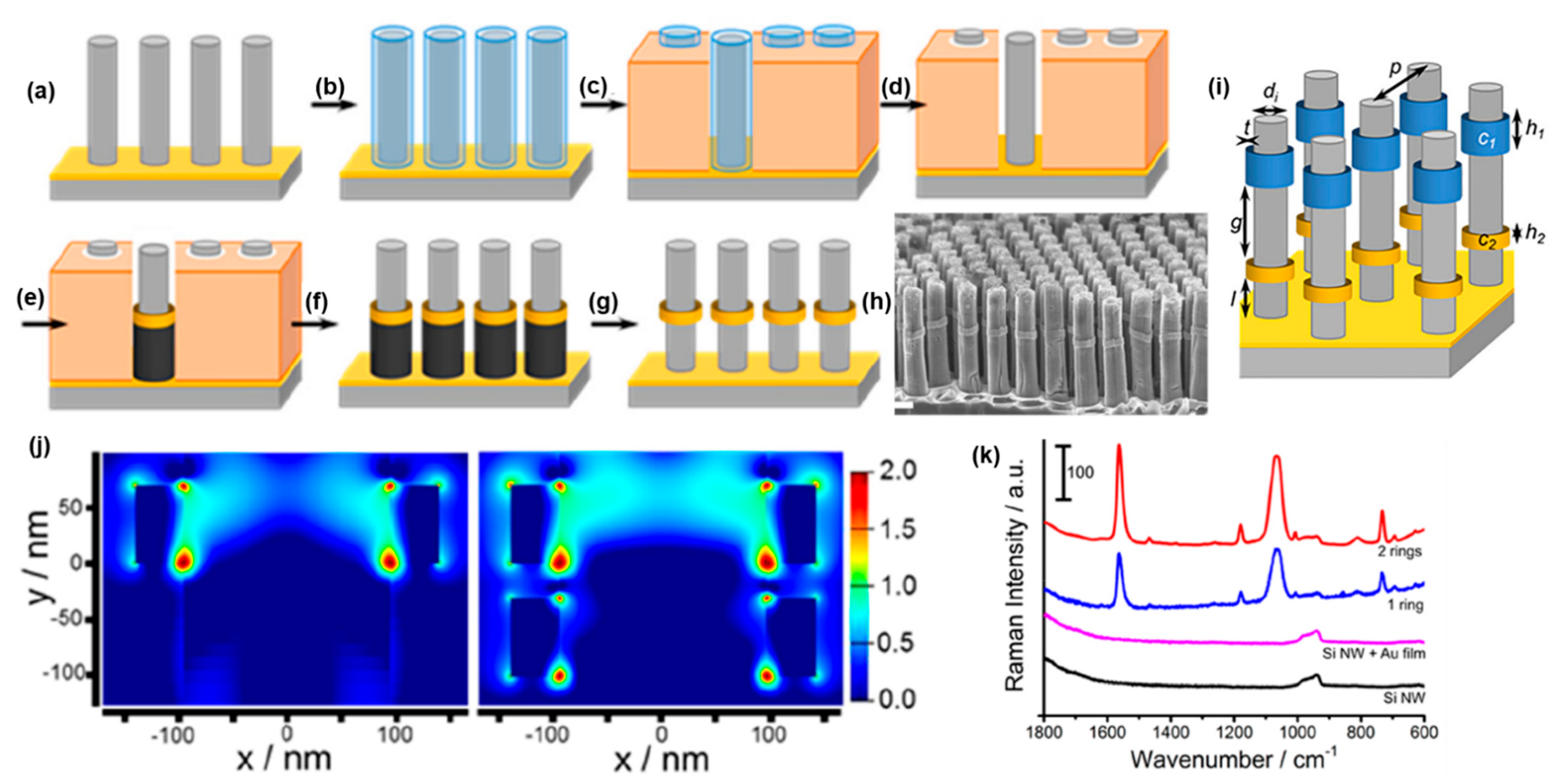
4.4. Silicon Nanowire Arrays (3DEAL)
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kreibig, U.V.M. Optical Properties of Metal Clusters, 1st ed.; Springer: Berlin, Germany, 1995. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications, 1st ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Pelton, M.; Aizpurua, J.; Bryant, G. Metal-nanoparticle plasmonics. Laser Photonics Rev. 2008, 2, 136–159. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R.; Ray, K.; Chowdhury, M.; Szmacinski, H.; Fu, Y.; Zhang, J.; Nowaczyk, K. Plasmon-controlled fluorescence: A new paradigm in fluorescence spectroscopy. Analyst 2008, 133, 1308–1346. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfrinato, V.R.; Zhang, L.; Su, D.; Duan, H.; Hobbs, R.G.; Stach, E.A.; Berggren, K.K. Resolution limits of electron-beam lithography toward the atomic scale. Nano Lett. 2013, 13, 1555–1558. [Google Scholar] [CrossRef]
- Balčytis, A.; Nishijima, Y.; Krishnamoorthy, S.; Kuchmizhak, A.; Stoddart, P.R.; Petruškevičius, R.; Juodkazis, S. From Fundamental toward Applied SERS: Shared Principles and Divergent Approaches. Adv. Opt. Mater. 2018, 6, 1800292. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Ferry, V.E.; Munday, J.N.; Atwater, H.A. Design considerations for plasmonic photovoltaics. Adv. Mater. 2010, 22, 4794–4808. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced Raman scattering from individual au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, J.; Prodan, E.; Nordlander, P. Quantum description of the plasmon resonances of a nanoparticle dimer. Nano Lett. 2009, 9, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.B.; Mirkin, C.A.; Schatz, G.C. Optical Properties of One-, Two-, and Three-Dimensional Arrays of Plasmonic Nanostructures. J. Phys. Chem. C 2016, 120, 816–830. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- McMahon, J.M.; Li, S.; Ausman, L.K.; Schatz, G.C. Modeling the Effect of Small Gaps in Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2011, 116, 1627–1637. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. Engl. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-Metal Nanocrystals with Controlled Shapes for Catalytic and Electrocatalytic Applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- O’Brien, M.N.; Jones, M.R.; Brown, K.A.; Mirkin, C.A. Universal noble metal nanoparticle seeds realized through iterative reductive growth and oxidative dissolution reactions. J. Am. Chem. Soc. 2014, 136, 7603–7606. [Google Scholar] [CrossRef]
- Personick, M.L.; Mirkin, C.A. Making sense of the mayhem behind shape control in the synthesis of gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 18238–18247. [Google Scholar] [CrossRef]
- Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Chu, Z.; Pavan, G.M.; Klajn, R. Electrostatic co-assembly of nanoparticles with oppositely charged small molecules into static and dynamic superstructures. Nat. Chem. 2021, 13, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Osberg, K.D.; Macfarlane, R.J.; Langille, M.R.; Mirkin, C.A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kim, T.W.; Kubota, S.R.; Cardiel, A.C.; Cha, H.G.; Choi, K.S. Electrochemical Synthesis of Photoelectrodes and Catalysts for Use in Solar Water Splitting. Chem. Rev. 2015, 115, 12839–12887. [Google Scholar] [CrossRef] [PubMed]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Li, G.R.; Xu, H.; Lu, X.F.; Feng, J.X.; Tong, Y.X.; Su, C.Y. Electrochemical synthesis of nanostructured materials for electrochemical energy conversion and storage. Nanoscale 2013, 5, 4056–4069. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Qin, L.; Park, S.; Huang, L.; Mirkin, C.A. On-wire lithography. Science 2005, 309, 113–115. [Google Scholar] [CrossRef] [Green Version]
- Ozel, T.; Bourret, G.R.; Mirkin, C.A. Coaxial lithography. Nat. Nanotechnol. 2015, 10, 319–324. [Google Scholar] [CrossRef]
- Wendisch, F.J.; Saller, M.S.; Eadie, A.; Reyer, A.; Musso, M.; Rey, M.; Vogel, N.; Diwald, O.; Bourret, G.R. Three-Dimensional Electrochemical Axial Lithography on Si Micro- and Nanowire Arrays. Nano Lett. 2018, 18, 7343–7349. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Blaber, M.G.; Arnold, M.D.; Ford, M.J. A review of the optical properties of alloys and intermetallics for plasmonics. J. Phys. Condens. Matter 2010, 22, 143201. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Govorov, A.O. Amplified Generation of Hot Electrons and Quantum Surface Effects in Nanoparticle Dimers with Plasmonic Hot Spots. J. Phys. Chem. C 2016, 120, 19329–19339. [Google Scholar] [CrossRef]
- Hartland, G.V.; Besteiro, L.V.; Johns, P.; Govorov, A.O. What’s so Hot about Electrons in Metal Nanoparticles? ACS Energy Lett. 2017, 2, 1641–1653. [Google Scholar] [CrossRef] [Green Version]
- Malathi, S.; Ezhilarasu, T.; Abiraman, T.; Balasubramanian, S. One pot green synthesis of Ag, Au and Au-Ag alloy nanoparticles using isonicotinic acid hydrazide and starch. Carbohydr. Polym. 2014, 111, 734–743. [Google Scholar] [CrossRef]
- Goulet, P.J.; Bourret, G.R.; Lennox, R.B. Facile phase transfer of large, water-soluble metal nanoparticles to nonpolar solvents. Langmuir 2012, 28, 2909–2913. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Bourret, G.R.; Ozel, T.; Blaber, M.; Shade, C.M.; Schatz, G.C.; Mirkin, C.A. Long-range plasmophore rulers. Nano Lett. 2013, 13, 2270–2275. [Google Scholar] [CrossRef]
- Piaskowski, J.; Ibragimov, A.; Wendisch, F.J.; Bourret, G.R. Selective Enhancement of Surface and Bulk E-Field within Porous AuRh and AuRu Nanorods. J. Phys. Chem. C 2021, 125, 27661–27670. [Google Scholar] [CrossRef] [PubMed]
- Rycenga, M.; Langille, M.R.; Personick, M.L.; Ozel, T.; Mirkin, C.A. Chemically isolating hot spots on concave nanocubes. Nano Lett. 2012, 12, 6218–6222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aizpurua, J.; Hanarp, P.; Sutherland, D.S.; Kall, M.; Bryant, G.W.; Garcia de Abajo, F.J. Optical properties of gold nanorings. Phys. Rev. Lett. 2003, 90, 057401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, E.M.; Alegret, J.; Kall, M.; Sutherland, D.S. Sensing characteristics of NIR localized surface plasmon resonances in gold nanorings for application as ultrasensitive biosensors. Nano Lett. 2007, 7, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Osberg, K.D.; Rycenga, M.; Bourret, G.R.; Brown, K.A.; Mirkin, C.A. Dispersible surface-enhanced Raman scattering nanosheets. Adv. Mater. 2012, 24, 6065–6070. [Google Scholar] [CrossRef]
- Zou, S.; Schatz, G.C. Narrow plasmonic/photonic extinction and scattering line shapes for one and two dimensional silver nanoparticle arrays. Chem. Phys. 2004, 121, 12606–12612. [Google Scholar] [CrossRef]
- Zou, S.; Schatz, G.C. Theoretical studies of plasmon resonances in one-dimensional nanoparticle chains: Narrow lineshapes with tunable widths. Nanotechnology 2006, 17, 2813–2820. [Google Scholar] [CrossRef]
- Zou, S.; Schatz, G.C. Silver nanoparticle array structures that produce giant enhancements in electromagnetic fields. Chem. Phys. Lett. 2005, 403, 62–67. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Adelmann, C.; Zhao, L.; De Gendt, S. Advanced Interconnects: Materials, Processing, and Reliability. ECS J. Solid State Sci. Technol. 2014, 4, Y1–Y4. [Google Scholar] [CrossRef]
- Merchant, S.M.; Kang, S.H.; Sanganeria, M.; van Schravendijk, B.; Mountsier, T. Copper interconnects for semiconductor devices. JOM 2001, 53, 43–48. [Google Scholar] [CrossRef]
- Penner, R.M. Mesoscopic Metal Particles and Wires by Electrodeposition. J. Phys. Chem. B 2002, 106, 3339–3353. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Reiss, H. The Growth of Uniform Colloidal Dispersions. J. Chem. Phys. 1951, 19, 482–487. [Google Scholar] [CrossRef]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Xia, Y.; Gilroy, K.D.; Peng, H.C.; Xia, X. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chem. Int. Ed. Engl. 2017, 56, 60–95. [Google Scholar] [CrossRef]
- Langille, M.R.; Personick, M.L.; Zhang, J.; Mirkin, C.A. Defining rules for the shape evolution of gold nanoparticles. J. Am. Chem. Soc. 2012, 134, 14542–14554. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Chang, S.-S.; Lee, C.-L.; Wang, C.R.C. Gold Nanorods: Electrochemical Synthesis and Optical Properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Swetha, P.; Feng, S.-P. High-index facet defined shape-controlled electrochemical synthesis of nanocrystals: A mini review. Electrochem. Commun. 2018, 94, 64–69. [Google Scholar] [CrossRef]
- McDarby, S.P.; Personick, M.L. Potential-Controlled (R)Evolution: Electrochemical Synthesis of Nanoparticles with Well-Defined Shapes. ChemNanoMat 2021, 8, e202100472. [Google Scholar] [CrossRef]
- McDarby, S.P.; Wang, C.J.; King, M.E.; Personick, M.L. An Integrated Electrochemistry Approach to the Design and Synthesis of Polyhedral Noble Metal Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21322–21335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhang, D.; Su, N.Q.; Yang, W.; Everitt, H.O.; Liu, J. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 2017, 8, 14542. [Google Scholar] [CrossRef]
- Yu, N.F.; Tian, N.; Zhou, Z.Y.; Huang, L.; Xiao, J.; Wen, Y.H.; Sun, S.G. Electrochemical synthesis of tetrahexahedral rhodium nanocrystals with extraordinarily high surface energy and high electrocatalytic activity. Angew. Chem. Int. Ed. Engl. 2014, 53, 5097–5101. [Google Scholar] [CrossRef] [PubMed]
- Redmond, P.L.; Hallock, A.J.; Brus, L.E. Electrochemical ostwald ripening of colloidal ag particles on conductive substrates. Nano Lett. 2005, 5, 131–135. [Google Scholar] [CrossRef]
- Plieth, W.J. Electrochemical properties of small clusters of metal atoms and their role in the surface enhanced Raman scattering. J. Phys. Chem. 2002, 86, 3166–3170. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, O.S.; Zamborini, F.P. Size-dependent electrochemical oxidation of silver nanoparticles. J. Am. Chem. Soc. 2010, 132, 70–72. [Google Scholar] [CrossRef]
- Reyer, A.; Prinz, A.; Giancristofaro, S.; Schneider, J.; Bertoldo Menezes, D.; Zickler, G.; Bourret, G.R.; Musso, M.E. Investigation of Mass-Produced Substrates for Reproducible Surface-Enhanced Raman Scattering Measurements over Large Areas. ACS Appl. Mater. Interfaces 2017, 9, 25445–25454. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Xia, Q.; Williams, R.S. Fractal structure formation from Ag nanoparticle films on insulating substrates. Langmuir 2009, 25, 7222–7225. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Huang, Q.; Zhang, Y.; Tang, H.; Qian, Y.; Chen, B.; Wang, X. Ostwald-ripening-induced growth of parallel face-exposed Ag nanoplates on micro-hemispheres for high SERS activity. Chem. Eur. J. 2013, 19, 9211–9217. [Google Scholar] [CrossRef]
- Peng, H.I.; Krauss, T.D.; Miller, B.L. Aging induced Ag nanoparticle rearrangement under ambient atmosphere and consequences for nanoparticle-enhanced DNA biosensing. Anal. Chem. 2010, 82, 8664–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Rao, W.; Wang, D.; Kups, T.; Baradacs, E.; Parditka, B.; Erdelyi, Z.; Schaaf, P. Nanoporous Gold Nanoparticles and Au/Al2O3 Hybrid Nanoparticles with Large Tunability of Plasmonic Properties. ACS Appl. Mater. Interfaces 2017, 9, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tang, C.; Chen, G.; He, Z.; Wang, T.; He, X.; Yi, T.; Liu, Y.; Zhang, L.; Du, K. Toward the Limitation of Dealloying: Full Spectrum Responsive Ultralow Density Nanoporous Gold for Plasmonic Photocatalytic SERS. ACS Appl. Mater. Interfaces 2021, 13, 7735–7744. [Google Scholar] [CrossRef] [PubMed]
- Schubert, I.; Huck, C.; Kröber, P.; Neubrech, F.; Pucci, A.; Toimil-Molares, M.E.; Trautmann, C.; Vogt, J. Porous Gold Nanowires: Plasmonic Response and Surface-Enhanced Infrared Absorption. Adv. Opt. Mater. 2016, 4, 1838–1845. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Bourret, G.R.; Goulet, P.J.G.; Lennox, R.B. Synthesis of Porous Metallic Monoliths via Chemical Reduction of Au(I) and Ag(I) Nanostructured Sheets. Chem. Mater. 2011, 23, 4954–4959. [Google Scholar] [CrossRef]
- Bourret, G.R.; Lennox, R.B. Potential controlled electrochemical conversion of AgCN and Cu(OH)2 nanofibers into metal nanoparticles, nanoprisms, nanofibers, and porous networks. ACS Appl. Mater. Interfaces 2010, 2, 3745–3758. [Google Scholar] [CrossRef]
- Bourret, G.R.; Lennox, R.B. 1D Cu(OH)2 nanomaterial synthesis templated in water microdroplets. J. Am. Chem. Soc. 2010, 132, 6657–6659. [Google Scholar] [CrossRef]
- Lazzara, T.D.; Bourret, G.R.; Lennox, R.B.; van de Ven, T.G.M. Polymer Templated Synthesis of AgCN and Ag Nanowires. Chem. Mater. 2009, 21, 2020–2026. [Google Scholar] [CrossRef]
- Bourret, G.R.; Lennox, R.B. Electrochemical synthesis of Ag(0)/A2S heterojunctions templated on pre-formed Ag2S nanowires. Nanoscale 2011, 3, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Millstone, J.E.; Hurst, S.J.; Metraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal gold and silver triangular nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chao, L.; Wang, L.; Cheng, Y.; Xie, Q. Preparation of a Pt thin-film modified electrode for alkaline electrocatalytic oxidation of methanol by Cu(OH)2 electrodeposition and galvanic replacement reaction. Electrochim. Acta 2020, 330, 135234. [Google Scholar] [CrossRef]
- Himpsel, F.J.; Jung, T.; Ortega, J.E. Nanowires on Stepped Metal Surfaces. Surf. Rev. Lett. 2012, 4, 371–380. [Google Scholar] [CrossRef]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 2001, 293, 2227–2231. [Google Scholar] [CrossRef]
- Menke, E.J.; Thompson, M.A.; Xiang, C.; Yang, L.C.; Penner, R.M. Lithographically patterned nanowire electrodeposition. Nat. Mater. 2006, 5, 914–919. [Google Scholar] [CrossRef]
- Halpern, A.R.; Corn, R.M. Lithographically patterned electrodeposition of gold, silver, and nickel nanoring arrays with widely tunable near-infrared plasmonic resonances. ACS Nano 2013, 7, 1755–1762. [Google Scholar] [CrossRef]
- Kempa, T.J.; Bediako, D.K.; Kim, S.K.; Park, H.G.; Nocera, D.G. High-throughput patterning of photonic structures with tunable periodicity. Proc. Natl. Acad. Sci. USA 2015, 112, 5309–5313. [Google Scholar] [CrossRef] [Green Version]
- Goad, D.G.W.; Moskovits, M. Colloidal metal in aluminum-oxide. J. Appl. Phys. 1978, 49, 2929–2934. [Google Scholar] [CrossRef]
- Penner, R.M.; Martin, C.R. Preparation and electrochemical characterization of ultramicroelectrode ensembles. Anal. Chem. 1987, 59, 2625–2630. [Google Scholar] [CrossRef]
- Martin, C.R. Nanomaterials: A membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.J.; Payne, E.K.; Qin, L.; Mirkin, C.A. Multisegmented one-dimensional nanorods prepared by hard-template synthetic methods. Angew. Chem. Int. Ed. Engl. 2006, 45, 2672–2692. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, J.; Kurtz, J.; Mallouk, T.E.; Chan, M.H. Electrochemical Growth of Single-Crystal Metal Nanowires via a Two-Dimensional Nucleation and Growth Mechanism. Nano Lett. 2003, 3, 919–923. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Wang, H.; Shen, H.; Cheng, Q.; Yan, C.; Park, S. Electrodeposition of Rhodium Nanowires Arrays and Their Morphology-Dependent Hydrogen Evolution Activity. Nanomaterials 2017, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Routkevitch, D.; Bigioni, T.; Moskovits, M.; Xu, J.M. Electrochemical Fabrication of CdS Nanowire Arrays in Porous Anodic Aluminum Oxide Templates. J. Phys. Chem. 1996, 100, 14037–14047. [Google Scholar] [CrossRef]
- Wen, L.; Xu, R.; Mi, Y.; Lei, Y. Multiple nanostructures based on anodized aluminium oxide templates. Nat. Nanotechnol. 2017, 12, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, A.L.; Harris, N.; Banholzer, M.J.; Blaber, M.G.; Osberg, K.D.; Schatz, G.C.; Mirkin, C.A. Correlating nanorod structure with experimentally measured and theoretically predicted surface plasmon resonance. ACS Nano 2010, 4, 5453–5463. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shuford, K.L.; Bok, H.M.; Kim, S.K.; Park, S. Intraparticle surface plasmon coupling in quasi-one-dimensional nanostructures. Nano Lett. 2008, 8, 800–804. [Google Scholar] [CrossRef]
- Kim, C.; Hyeon, S.; Lee, J.; Kim, W.D.; Lee, D.C.; Kim, J.; Lee, H. Energy-efficient CO2 hydrogenation with fast response using photoexcitation of CO2 adsorbed on metal catalysts. Nat. Commun. 2018, 9, 3027. [Google Scholar] [CrossRef] [Green Version]
- Mubeen, S.; Lee, J.; Singh, N.; Kramer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef]
- Mubeen, S.; Lee, J.; Lee, W.R.; Singh, N.; Stucky, G.D.; Moskovits, M. On the plasmonic photovoltaic. ACS Nano 2014, 8, 6066–6073. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Meng, G.; Han, F. Porous AAO template-assisted rational synthesis of large-scale 1D hybrid and hierarchically branched nanoarchitectures. Prog. Mater. Sci. 2018, 95, 243–285. [Google Scholar] [CrossRef]
- Banholzer, M.J.; Qin, L.; Millstone, J.E.; Osberg, K.D.; Mirkin, C.A. On-wire lithography: Synthesis, encoding and biological applications. Nat. Protoc. 2009, 4, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Osberg, K.D.; Schmucker, A.L.; Senesi, A.J.; Mirkin, C.A. One-dimensional nanorod arrays: Independent control of composition, length, and interparticle spacing with nanometer precision. Nano Lett. 2011, 11, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Osberg, K.D.; Rycenga, M.; Harris, N.; Schmucker, A.L.; Langille, M.R.; Schatz, G.C.; Mirkin, C.A. Dispersible gold nanorod dimers with sub-5 nm gaps as local amplifiers for surface-enhanced Raman scattering. Nano Lett. 2012, 12, 3828–3832. [Google Scholar] [CrossRef]
- Chen, X.; Jeon, Y.M.; Jang, J.W.; Qin, L.; Huo, F.; Wei, W.; Mirkin, C.A. On-wire lithography-generated molecule-based transport junctions: A new testbed for molecular electronics. J. Am. Chem. Soc. 2008, 130, 8166–8168. [Google Scholar] [CrossRef]
- Qin, L.; Banholzer, M.J.; Millstone, J.E.; Mirkin, C.A. Nanodisk codes. Nano Lett. 2007, 7, 3849–3853. [Google Scholar] [CrossRef]
- Ozel, T.; Ashley, M.J.; Bourret, G.R.; Ross, M.B.; Schatz, G.C.; Mirkin, C.A. Solution-Dispersible Metal Nanorings with Deliberately Controllable Compositions and Architectural Parameters for Tunable Plasmonic Response. Nano Lett. 2015, 15, 5273–5278. [Google Scholar] [CrossRef]
- Lahav, M.; Weiss, E.A.; Xu, Q.; Whitesides, G.M. Core-shell and segmented polymer-metal composite nanostructures. Nano Lett. 2006, 6, 2166–2171. [Google Scholar] [CrossRef] [Green Version]
- Ozel, T.; Bourret, G.R.; Schmucker, A.L.; Brown, K.A.; Mirkin, C.A. Hybrid semiconductor core-shell nanowires with tunable plasmonic nanoantennas. Adv. Mater. 2013, 25, 4515–4520. [Google Scholar] [CrossRef]
- Bartschmid, T.; Wendisch, F.J.; Farhadi, A.; Bourret, G.R. Recent Advances in Structuring and Patterning Silicon Nanowire Arrays for Engineering Light Absorption in Three Dimensions. ACS Appl. Energy Mater. 2021. [Google Scholar] [CrossRef]
- Wendisch, F.J.; Abazari, M.; Mahdavi, H.; Rey, M.; Vogel, N.; Musso, M.; Diwald, O.; Bourret, G.R. Morphology-Graded Silicon Nanowire Arrays via Chemical Etching: Engineering Optical Properties at the Nanoscale and Macroscale. ACS Appl. Mater. Interfaces 2020, 12, 13140–13147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parameswaran, R.; Carvalho-de-Souza, J.L.; Jiang, Y.; Burke, M.J.; Zimmerman, J.F.; Koehler, K.; Phillips, A.W.; Yi, J.; Adams, E.J.; Bezanilla, F.; et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 2018, 13, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Vijselaar, W.; Westerik, P.; Veerbeek, J.; Tiggelaar, R.M.; Berenschot, E.; Tas, N.R.; Gardeniers, H.; Huskens, J. Spatial decoupling of light absorption and catalytic activity of Ni–Mo-loaded high-aspect-ratio silicon microwire photocathodes. Nat. Energy 2018, 3, 185–192. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, P.G.; Sandhel, A.; Wood, T.E.; Jelle, A.A.; Hoch, L.B.; Perovic, D.D.; Mims, C.A.; Ozin, G.A. Photomethanation of Gaseous CO2 over Ru/Silicon Nanowire Catalysts with Visible and Near-Infrared Photons. Adv. Sci. 2014, 1, 1400001. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Brittman, S.; Tang, J.; Fu, A.; Kornienko, N.; Kong, Q.; Yang, P. Single-nanowire photoelectrochemistry. Nat. Nanotechnol. 2016, 11, 609–612. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Chen, H.M.; Liu, B.; Yang, P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 2013, 13, 2989–2992. [Google Scholar] [CrossRef]
- Wu, Y.; Xiang, J.; Yang, C.; Lu, W.; Lieber, C.M. Single-crystal metallic nanowires and metal/semiconductor nanowire heterostructures. Nature 2004, 430, 61–65. [Google Scholar] [CrossRef]
- Glassner, S.; Zeiner, C.; Periwal, P.; Baron, T.; Bertagnolli, E.; Lugstein, A. Multimode silicon nanowire transistors. Nano Lett. 2014, 14, 6699–6703. [Google Scholar] [CrossRef]
- Liu, R.; Chen, R.; Elthakeb, A.T.; Lee, S.H.; Hinckley, S.; Khraiche, M.L.; Scott, J.; Pre, D.; Hwang, Y.; Tanaka, A.; et al. High Density Individually Addressable Nanowire Arrays Record Intracellular Activity from Primary Rodent and Human Stem Cell Derived Neurons. Nano Lett. 2017, 17, 2757–2764. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.H.; Aspetti, C.O.; Park, J.; Agarwal, R. Silicon coupled with plasmon nanocavity generates bright visible hot-luminescence. Nat. Photonics 2013, 7, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, D.; Aspetti, C.O.; Cargnello, M.; Ren, M.; Yoo, J.; Murray, C.B.; Agarwal, R. Engineering Localized Surface Plasmon Interactions in Gold by Silicon Nanowire for Enhanced Heating and Photocatalysis. Nano Lett. 2017, 17, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, K.Q.; Hu, Y.; Zhang, F.Q.; Hu, B.; Li, L.; Wang, M.; Meng, X.M.; Lee, S.T. Silicon/hematite core/shell nanowire array decorated with gold nanoparticles for unbiased solar water oxidation. Nano Lett. 2014, 14, 18–23. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Gao, Y.; Ma, J.; Long, R.; Wang, C.; Xiong, Y. Flexible Near-Infrared Photovoltaic Devices Based on Plasmonic Hot-Electron Injection into Silicon Nanowire Arrays. Angew. Chem. Int. Ed. Engl. 2016, 55, 4577–4581. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar] [CrossRef]
- Glassner, S.; Keshmiri, H.; Hill, D.J.; Cahoon, J.F.; Fernandez, B.; den Hertog, M.I.; Lugstein, A. Tuning Electroluminescence from a Plasmonic Cavity-Coupled Silicon Light Source. Nano Lett. 2018, 18, 7230–7237. [Google Scholar] [CrossRef]
- Milbrat, A.; Elbersen, R.; Kas, R.; Tiggelaar, R.M.; Gardeniers, H.; Mul, G.; Huskens, J. Spatioselective Electrochemical and Photoelectrochemical Functionalization of Silicon Microwires with Axial p/n Junctions. Adv. Mater. 2016, 28, 1400–1405. [Google Scholar] [CrossRef]
- Dasog, M.; Carim, A.I.; Yalamanchili, S.; Atwater, H.A.; Lewis, N.S. Profiling Photoinduced Carrier Generation in Semiconductor Microwire Arrays via Photoelectrochemical Metal Deposition. Nano Lett. 2016, 16, 5015–5021. [Google Scholar] [CrossRef] [Green Version]
- Wendisch, F.J.; Rey, M.; Vogel, N.; Bourret, G.R. Large-Scale Synthesis of Highly Uniform Silicon Nanowire Arrays Using Metal-Assisted Chemical Etching. Chem. Mater. 2020, 32, 9425–9434. [Google Scholar] [CrossRef]
- Wendisch, F.J.; Oberreiter, R.; Salihovic, M.; Elsaesser, M.S.; Bourret, G.R. Confined Etching within 2D and 3D Colloidal Crystals for Tunable Nanostructured Templates: Local Environment Matters. ACS Appl. Mater. Interfaces 2017, 9, 3931–3939. [Google Scholar] [CrossRef]
- Wendisch, F.J.; Abazari, M.; Werner, V.; Barb, H.; Rey, M.; Goerlitzer, E.S.A.; Vogel, N.; Mahdavi, H.; Bourret, G.R. Spatioselective Deposition of Passivating and Electrocatalytic Layers on Silicon Nanowire Arrays. ACS Appl. Mater. Interfaces 2020, 12, 52581–52587. [Google Scholar] [CrossRef] [PubMed]



| Technique | Advantages | Limitations | Spatial Resolution | Size Dispersity | References |
|---|---|---|---|---|---|
| ECD | Cost-effective and large scale Synthesis of NPs with complex morphologies Can improve our understanding of colloidal syntheses | Low yields compared to conventional wet-chemical colloidal syntheses | N.A. | <10–15% | [54,61,62,63,64] |
| Ostwald electrochemical ripening | Cost-effective Simple and large-scale Synthesis of a high density of nanoscale gaps/plasmonic hot spots | No precise control over interparticle distances and NP sizes | Nanometer-sized gaps | Rather large | [67,70,72] |
| Electrochemical dealloying | Cost-effective Simple and large-scale Can form porous metal thin films. | No precise control over distances between the nanoscale building blocks | Sub-50 nm features | Rather large | [74,76,78] |
| Electrochemical reduction of NZVN | Cost-effective Large-scale synthesis of Au, Ag, and Cu nanostructures with various geometries | Not suited to synthesize well-defined monodisperse nanostructures | N.A. | Rather large | [79,80,81,82,83] |
| ECD on patterned planar electrodes | Synthesis of metal/semiconductor nanowires with mm-scale lengths Synthesis of 2D nanoring arrays | Limited in throughput and accessibility by the patterning step or the surface topography of the surface used for ECD | Height: ca. 5 nm Width: ca. 40 nm | <10–15% | [54,87,88,89,90] |
| ECD within porous membranes | Large-scale Synthesis of multisegmented nanowires with controllable segment lengths. Can be used to prepare vertically oriented nanowire arrays Well-suited for plasmonics (pore diameters < 100 nm) | Requires a porous membrane The radial dimension is fixed by the membrane pore size | Sub-5 nm in axial dimension | <10–15% | [44,91,92,93,94,95,96,97,98,99,100,101,102,103,104] |
| OWL | Large-scale Precise synthesis of nm-sized gaps Synthesis of 1D gapped nanowire arrays | Requires a porous membrane The radial dimension is fixed by the membrane pore size | Sub-5 nm in axial dimension | <10–15% | [29,43,48,105,106,107,108,109] |
| COAL | Synthesis of metal nanorings with tunable dimensions Synthesis of core/multishell nanowires Synthesis of asymmetric nanowires | Requires a porous membrane Limited to materials that can be grown from solution (i.e., usually multicrystalline low-quality semiconductors) | Sub-10 nm in axial and radial dimensions | <10–15% | [30,110,111,112] |
| 3DEAL | Large-scale Compatible with single-crystal Si Can be used to pattern pre-synthesized Si microwire and nanowire arrays with well-defined metal nanorings and NPs at specific axial locations | To date, it requires a conducting metal film at the bottom of the wire array, which can be provided via metal-assisted chemical etching | Sub-10 nm in axial and radial dimensions | <10–15% | [31,113,114,131,132,133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piaskowski, J.; Bourret, G.R. Electrochemical Synthesis of Plasmonic Nanostructures. Molecules 2022, 27, 2485. https://doi.org/10.3390/molecules27082485
Piaskowski J, Bourret GR. Electrochemical Synthesis of Plasmonic Nanostructures. Molecules. 2022; 27(8):2485. https://doi.org/10.3390/molecules27082485
Chicago/Turabian StylePiaskowski, Joshua, and Gilles R. Bourret. 2022. "Electrochemical Synthesis of Plasmonic Nanostructures" Molecules 27, no. 8: 2485. https://doi.org/10.3390/molecules27082485





