Eriodictyol and Homoeriodictyol Improve Memory Impairment in Aβ25–35-Induced Mice by Inhibiting the NLRP3 Inflammasome
Abstract
:1. Introduction
2. Results
2.1. Eri and Hom Cross the Blood–Brain Barrier
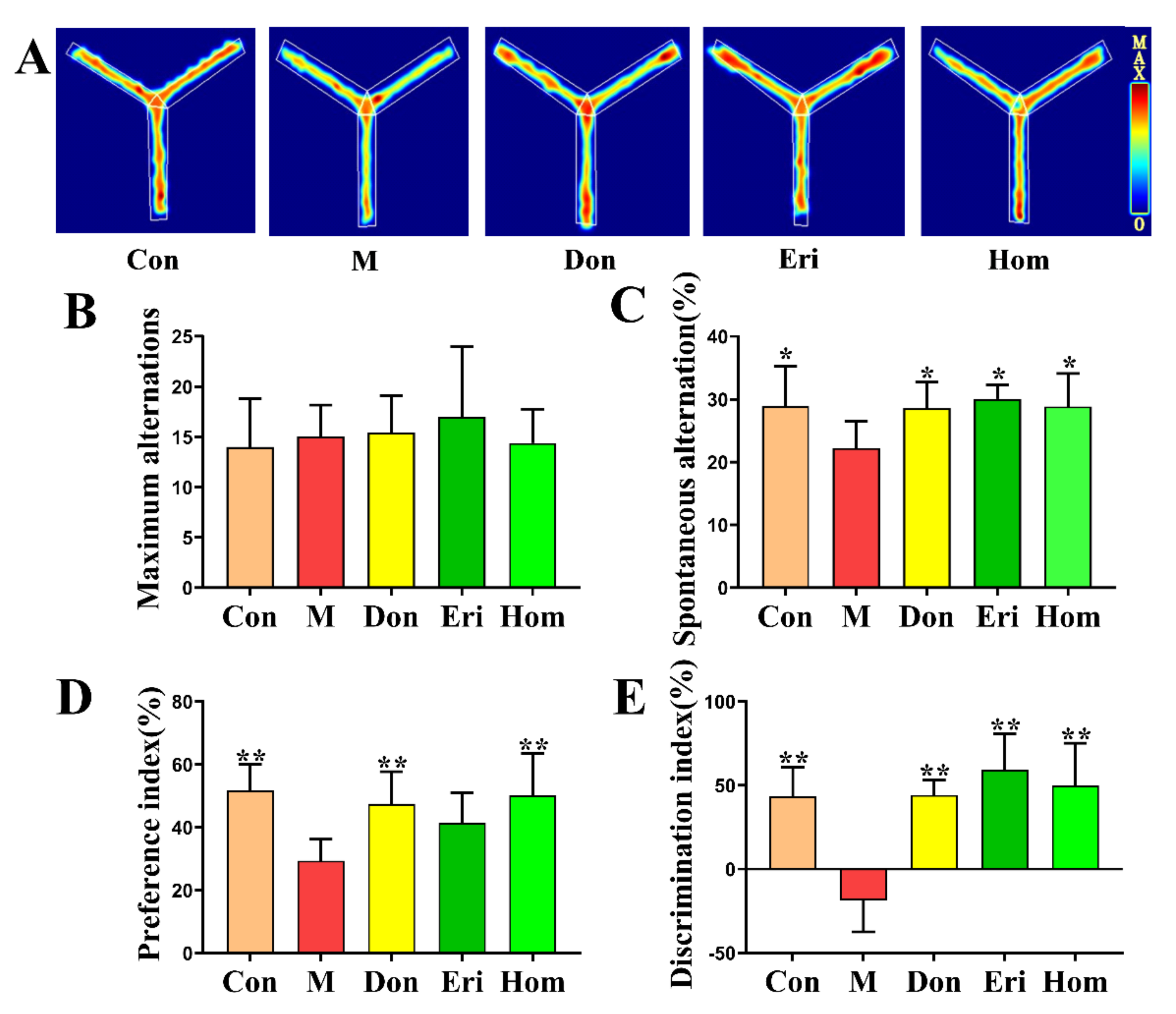
2.2. Eri and Hom Improve Aβ25–35-Induced Memory Impairment and Cognitive Dysfunction in Mice
2.3. Eri and Hom Alleviate Hippocampal Neuronal Damage Induced by Aβ25–35
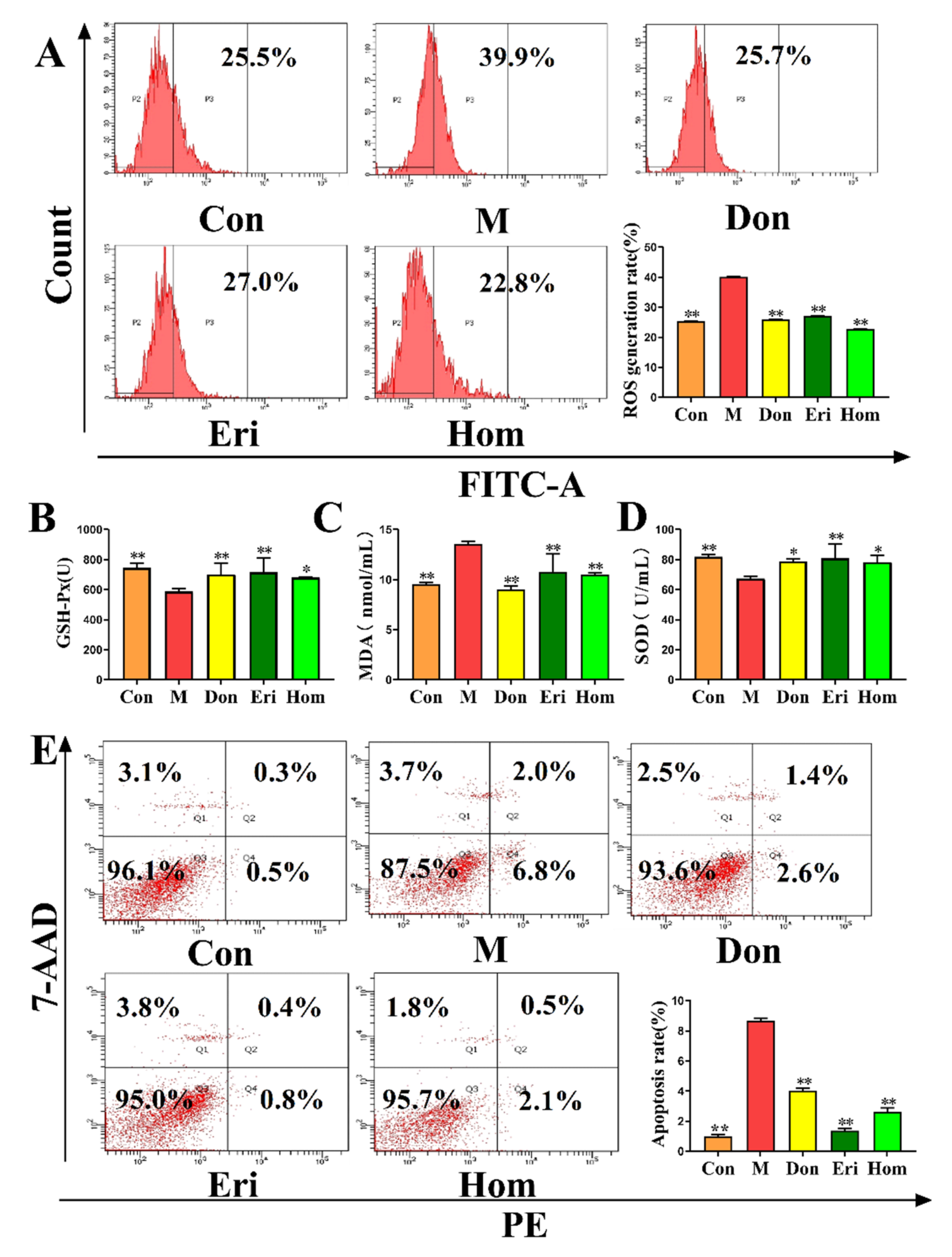
2.4. Eri and Hom Reduce Oxidative Stress in the Brain of Aβ25–35-Induced Mice
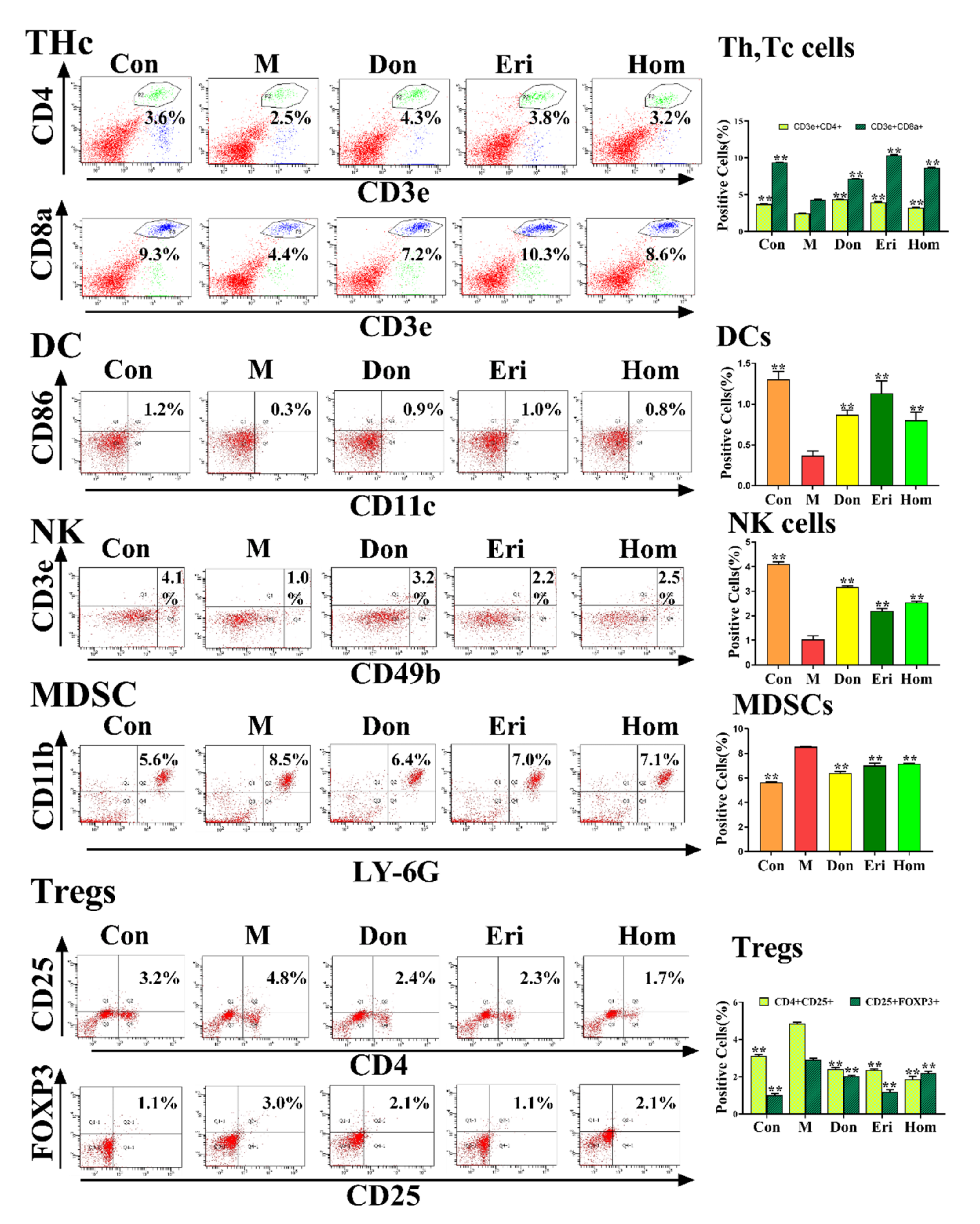
2.5. Eri and Hom Regulate the Level of Immune Cells in Aβ25–35-Induced Mice
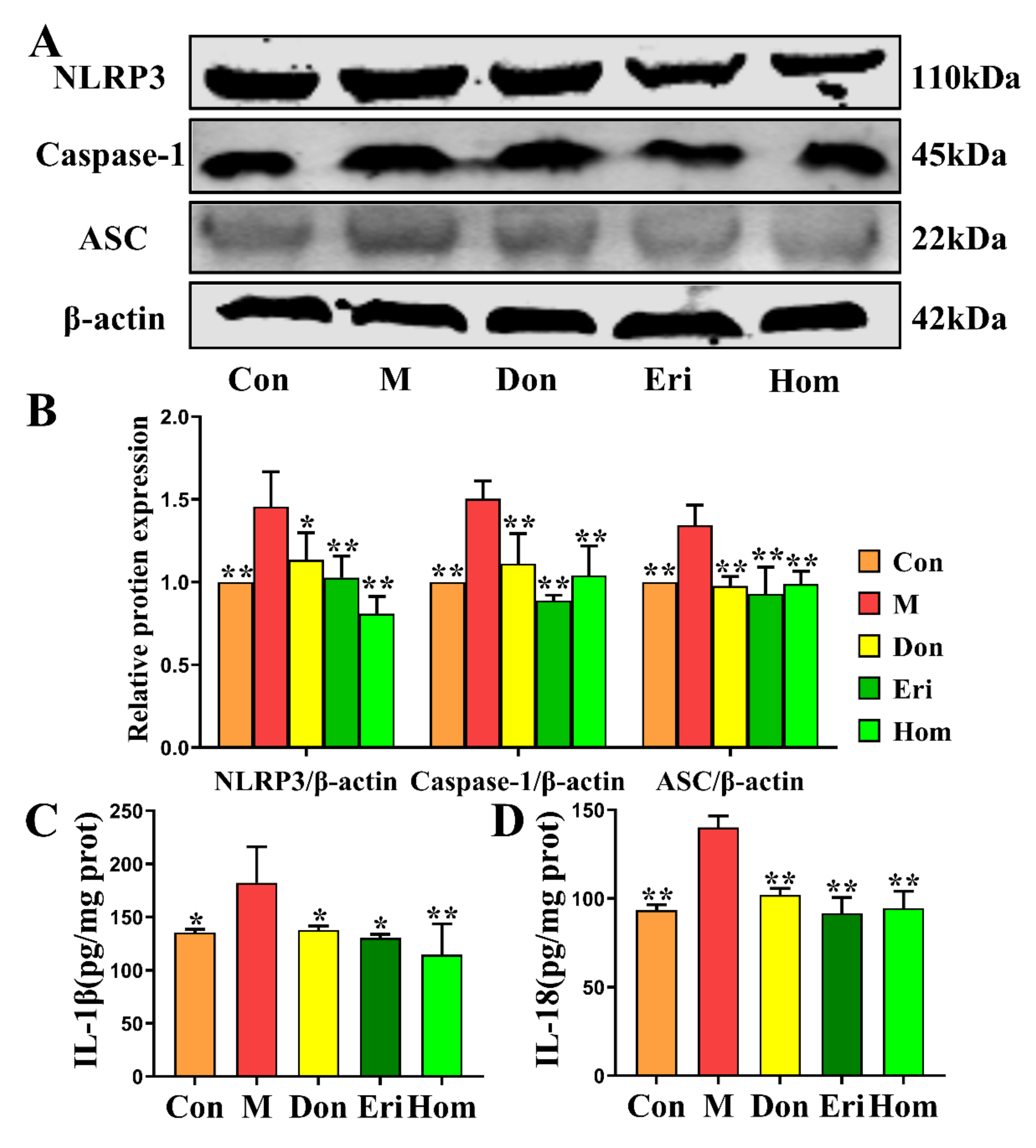
2.6. Eri and Hom Inhibit NLRP3 Inflammasome Activation
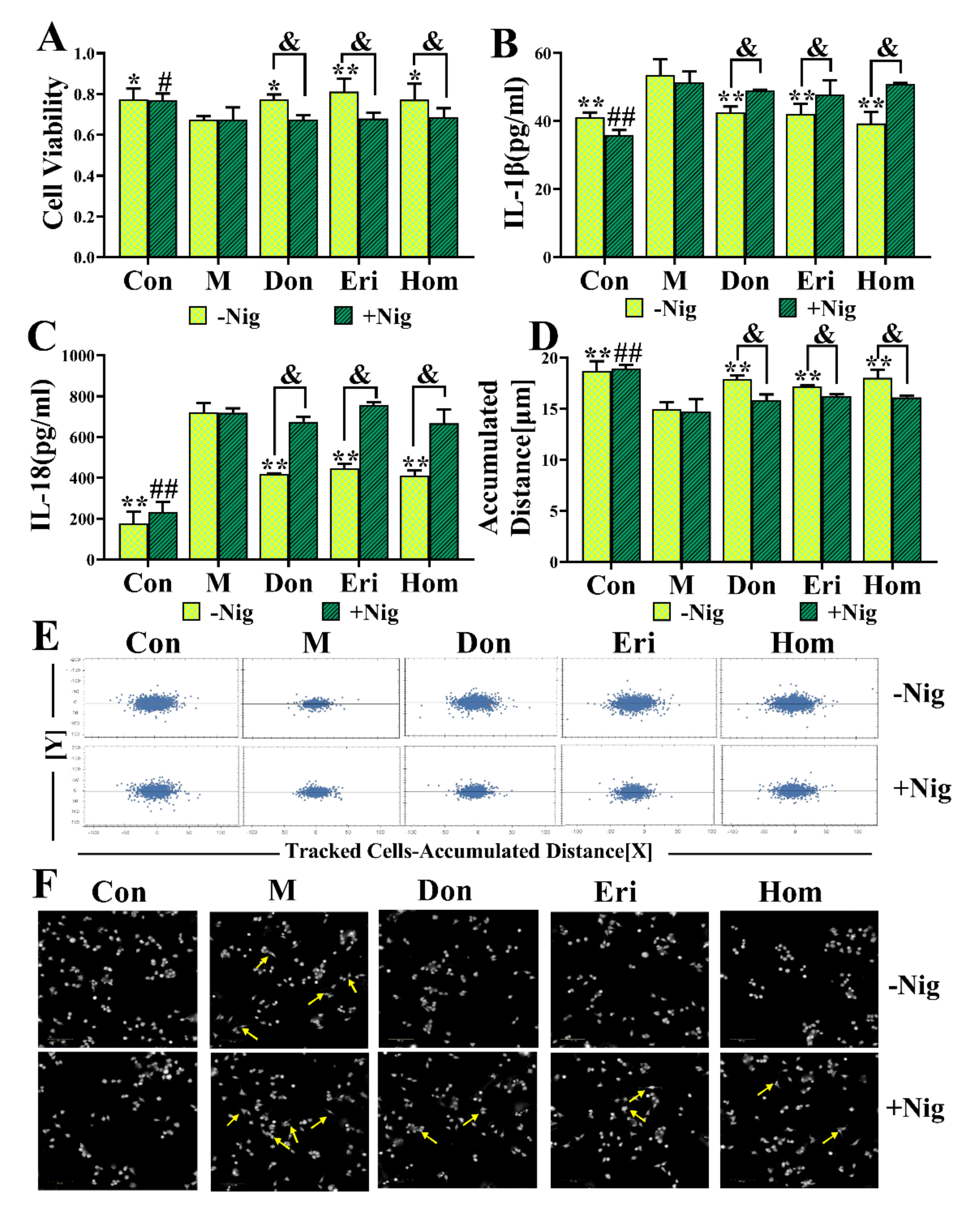
2.7. Eri and Hom Inhibit N9 Microglia via the NLRP3 Inflammasome
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Treatment Schedule
4.3. Behavioral Analysis
4.4. Pharmacokinetic Study of Eriodictyol and Homoeriodictyol in Mouse Brain Tissue
4.5. Histopathology of Brain Tissues
4.6. Biochemical Indexes Assay
4.7. Flow Cytometry Analysis of Immune Cells
4.8. Flow Cytometry Analysis of Reactive Oxygen Species (ROS) and Apoptosis Markers in Primary Brain Cells
4.9. Western Blot Analysis
4.10. Near-Infrared In Vivo Imaging
4.11. Cell Culture and Treatment
4.12. Cell Viability Assay
4.13. Analysis of Cytokines in the Cell Supernatant by ELISA
4.14. Cell Migration Assay
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisbet, R.M.; Van der Jeugd, A.; Leinenga, G.; Evans, H.T.; Janowicz, P.W.; Gotz, J. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain 2017, 140, 1220–1230. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, S.; Yuan, P.; Wu, Y.; Schrag, M.; Paradise, S.; Grutzendler, J.; De Camilli, P.; Ferguson, S.M. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc. Natl. Acad. Sci. USA 2015, 112, E3699–E3708. [Google Scholar] [CrossRef] [Green Version]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein aggregation in the brain: The molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhang, J.; Yang, G. Mechanisms of NLRP3 Inflammasome Activation: Its Role in the Treatment of Alzheimer’s Disease. Neurochem. Res. 2020, 45, 2560–2572. [Google Scholar] [CrossRef]
- Cameron, B.; Landreth, G.E. Inflammation, microglia, and Alzheimer’s disease. Neurobiol. Dis. 2010, 37, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef]
- Feng, Y.S.; Tan, Z.X.; Wu, L.Y.; Dong, F.; Zhang, F. The involvement of NLRP3 inflammasome in the treatment of Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 101192. [Google Scholar] [CrossRef]
- Villarejo-Galende, A.; González-Sánchez, M.; Blanco-Palmero, V.A.; Llamas-Velasco, S.; Benito-León, J. Non-steroidal Anti-inflammatory Drugs as Candidates for the Prevention or Treatment of Alzheimer’s Disease: Do they Still Have a Role? Curr. Alzheimer Res. 2020, 17, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid. Med. Cell Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Shi, H.; Zhu, X.; Wei, X.; Ren, M.; Han, M.; Ren, D.; Lou, H. Eriodictyol Attenuates beta-Amyloid 25-35 Peptide-Induced Oxidative Cell Death in Primary Cultured Neurons by Activation of Nrf2. Neurochem. Res. 2015, 40, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-kappaB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Hadacek, F.; Bachmann, G.; Weckwerth, W.; Kubicova, L. Pro- and Antioxidant Activity of Three Selected Flavan Type Flavonoids: Catechin, Eriodictyol and Taxifolin. Int. J. Mol. Sci. 2016, 17, 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.F.; Guo, H.J.; Huang, Y.; Wu, C.T.; Zhang, X.F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp. Ther. Med. 2015, 10, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Alqasoumi, S.I.; Basudan, O.A.; Alam, P.; Abdel-Kader, M.S. Antioxidant study of flavonoid derivatives from the aerial parts of Rhus natalensis growing in Saudi Arabia. Pak. J. Pharm. Sci. 2016, 29, 97–103. [Google Scholar]
- Madeswaran, A.; Umamaheswari, M.; Asokkumar, K.; Sivashanmugam, T.; Subhadradevi, V.; Jagannath, P. Discovery of potential cyclooxygenase inhibitors using in silico docking studies. Bangladesh J. Pharmacol. 2012, 7, 21–27. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ho, D.K.; Cassady, J.M.; Cook, V.M.; Baird, W.M. Isolation of potential cancer chemopreventive agents from Eriodictyon californicum. J. Nat. Prod. 1992, 55, 357–363. [Google Scholar] [CrossRef]
- Yi, Y.S. Regulatory Roles of Flavonoids on Inflammasome Activation during Inflammatory Responses. Mol. Nutr. Food Res. 2018, 62, e1800147. [Google Scholar] [CrossRef]
- Hanapi, N.A.; Arshad, A.S.M.; Abdullah, J.M.; Muhammad, T.S.T.; Yusof, S.R. Blood-Brain Barrier Permeability of Asiaticoside, Madecassoside and Asiatic Acid in Porcine Brain Endothelial Cell Model. J. Pharm. Sci. 2021, 110, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurother. J. Am. Soc. Exp. Neurother. 2015, 12, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, M.; Dawson, H.N.; Binder, L.I.; Vitek, M.P.; Ferreira, A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 6364–6369. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Burgaletto, C.; Munafo, A.; Di Benedetto, G.; De Francisci, C.; Caraci, F.; Di Mauro, R.; Bucolo, C.; Bernardini, R.; Cantarella, G. The immune system on the TRAIL of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 298. [Google Scholar] [CrossRef]
- Van Zeller, M.; Dias, D.; Sebastiao, A.M.; Valente, C.A. NLRP3 Inflammasome: A Starring Role in Amyloid-beta- and Tau-Driven Pathological Events in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal. Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Pooler, A.M.; Polydoro, M.; Maury, E.A.; Nicholls, S.B.; Reddy, S.M.; Wegmann, S.; William, C.; Saqran, L.; Cagsal-Getkin, O.; Pitstick, R.; et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 2015, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Zeng, M.; Zhang, Q.; Zhang, B.; Cao, Y.; Wu, Y.; Feng, W.; Zheng, X. Amentoflavone Ameliorates Memory Deficits and Abnormal Autophagy in Aβ25–35-Induced Mice by mTOR Signaling. Neurochem. Res. 2021, 46, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, CD001190. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, B.; Burns, A. Donepezil for Alzheimer’s disease. Expert Rev. Neurother. 2007, 7, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Pedros, I.; Petrov, D.; Allgaier, M.; Sureda, F.; Barroso, E.; Beas-Zarate, C.; Auladell, C.; Pallas, M.; Vazquez-Carrera, M.; Casadesus, G.; et al. Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1556–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaroudi, W.; Garami, J.; Garrido, S.; Hornberger, M.; Keri, S.; Moustafa, A.A. Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev. Neurosci. 2017, 28, 705–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Xu, Y.; Wang, X.; Guo, C.; Wang, T.; Wang, Z.Y. Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and Mitigates Alzheimer’s-Like Pathology via Nrf2-TXNIP-TrX Axis. Antioxid. Redox Signal. 2019, 30, 1411–1431. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Dong, D.; Jiao, Q.; Pan, H.; Ma, L.; Wang, R. Sarsasapogenin-AA13 ameliorates Abeta-induced cognitive deficits via improving neuroglial capacity on Abeta clearance and antiinflammation. CNS Neurosci. Ther. 2017, 23, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wise, L.; Fukuchi, K.I. TLR4 Cross-Talk with NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Severini, C.; Barbato, C.; Di Certo, M.G.; Gabanella, F.; Petrella, C.; Di Stadio, A.; de Vincentiis, M.; Polimeni, A.; Ralli, M.; Greco, A. Alzheimer’s Disease: New Concepts on the Role of Autoimmunity and NLRP3 Inflammasome in the Pathogenesis of the Disease. Curr. Neuropharmacol. 2021, 19, 498–512. [Google Scholar] [CrossRef]
- Hirshman, N.A.; Hughes, F.M., Jr.; Jin, H.; Harrison, W.T.; White, S.W.; Doan, I.; Harper, S.N.; Leidig, P.D.; Purves, J.T. Cyclophosphamide-induced cystitis results in NLRP3-mediated inflammation in the hippocampus and symptoms of depression in rats. Am. J. Physiol. Ren. Physiol. 2020, 318, F354–F362. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Srivastava, S.Y.; Brickey, W.J.; Iocca, H.; Toews, A.; Morrison, J.P.; Chen, V.S.; Gris, D.; Matsushima, G.K.; Ting, J.P. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 2010, 30, 15811–15820. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, A.; Takeuchi, H.; Takahashi, K.; Tanaka, F. Microglia in Alzheimer’s Disease: Risk Factors and Inflammation. Front. Neurol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt-Johnson, S.K.; Brutkiewicz, R.R. The Complexity of Microglial Interactions with Innate and Adaptive Immune Cells in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 592359. [Google Scholar] [CrossRef] [PubMed]
- Bossu, P.; Spalletta, G.; Caltagirone, C.; Ciaramella, A. Myeloid Dendritic Cells are Potential Players in Human Neurodegenerative Diseases. Front. Immunol. 2015, 6, 632. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. The potential importance of myeloid-derived suppressor cells (MDSCs) in the pathogenesis of Alzheimer’s disease. Cell Mol. Life Sci. 2018, 75, 3099–3120. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Bausinger, H.; de la Salle, H. Autoimmunity mediated by innate immune effector cells. Trends Immunol. 2001, 22, 300–301. [Google Scholar] [CrossRef]
- Thome, A.D.; Faridar, A.; Beers, D.R.; Thonhoff, J.R.; Zhao, W.; Wen, S.; Pascual, B.; Masdeu, J.C.; Appel, S.H. Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, K.; Hu, Y.; Wang, X. Expression of Immune Related Genes and Possible Regulatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2021, 12, 768966. [Google Scholar] [CrossRef]
- Laudisi, F.; Spreafico, R.; Evrard, M.; Hughes, T.R.; Mandriani, B.; Kandasamy, M.; Morgan, B.P.; Sivasankar, B.; Mortellaro, A. Cutting edge: The NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J. Immunol. 2013, 191, 1006–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celias, D.P.; Corvo, I.; Silvane, L.; Tort, J.F.; Chiapello, L.S.; Fresno, M.; Arranz, A.; Motran, C.C.; Cervi, L. Cathepsin L3 from Fasciola hepatica Induces NLRP3 Inflammasome Alternative Activation in Murine Dendritic Cells. Front. Immunol. 2019, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, H.; Srinivasan, L.; Shah, S.; Mayer-Barber, K.D.; Sher, A.; Sutterwala, F.S.; Briken, V. Mycobacterium tuberculosis infection of dendritic cells leads to partially caspase-1/11-independent IL-1beta and IL-18 secretion but not to pyroptosis. PLoS ONE 2012, 7, e40722. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Xu, G.; Wang, Y.; Zhan, X.; Gao, Y.; Wang, Z.; Fu, S.; Shi, W.; Hou, X.; Wang, C.; et al. Bavachin enhances NLRP3 inflammasome activation induced by ATP or nigericin and causes idiosyncratic hepatotoxicity. Front. Med. 2021, 15, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, G.; Ma, L.; Shi, W.; Wang, Z.; Zhan, X.; Qin, N.; He, T.; Guo, Y.; Niu, M.; et al. Icariside I specifically facilitates ATP or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic hepatotoxicity. Cell Commun. Signal. 2021, 19, 13. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, S.H. Ameliorating Activity of Ishige okamurae on the Amyloid Beta-Induced Cognitive Deficits and Neurotoxicity through Regulating ERK, p38 MAPK, and JNK Signaling in Alzheimer’s Disease-Like Mice Model. Mol. Nutr. Food Res. 2020, 64, e1901220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xiao, D.; Zhao, Y.; Tan, B.; Long, Z.; Yu, L.; He, G. Enhanced Autolysosomal Function Ameliorates the Inflammatory Response Mediated by the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 629891. [Google Scholar] [CrossRef]
- Lee, J.; Cho, E.; Kwon, H.; Jeon, J.; Jung, C.J.; Moon, M.; Jun, M.; Lee, Y.C.; Kim, D.H.; Jung, J.W. The fruit of Crataegus pinnatifida ameliorates memory deficits in β-amyloid protein-induced Alzheimer’s disease mouse model. J. Ethnopharmacol. 2019, 243, 112107. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, e55718. [Google Scholar] [CrossRef]
- Zeng, M.; Li, M.; Zhang, B.; Li, B.; Kan, Y.; Zheng, X.; Feng, W. Camellia oil inhibits oxidative stress and inflammatory response to ameliorate LPS-induced acute kidney injury via downregulation of TLR4-mediated activation of the NF-κB/AP-1/IRF3 and NLRP3 pathways. J. Funct. Foods 2020, 68, 103908. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, M.; Li, B.; Kan, Y.; Zhang, B.; Zheng, X.; Feng, W. Raw and salt-processed Achyranthes bidentata attenuate LPS-induced acute kidney injury by inhibiting ROS and apoptosis via an estrogen-like pathway. Biomed. Pharmacother. 2020, 129, 110403. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zeng, M.; Wu, Y.; Wang, S.; Zhang, B.; Zhang, J.; Kan, Y.; Li, B.; Cao, B.; Zheng, X.; et al. Acetone Extract of Cornus officinalis Leaves Exerts Anti-Melanoma Effects via Inhibiting STAT3 Signaling. Onco. Targets Ther. 2021, 14, 3487–3501. [Google Scholar] [CrossRef] [PubMed]


| Compound | Calibration Curve | Correlation Coefficient (R2) | Content (ng/g) |
|---|---|---|---|
| Eriodictyol | Y = 0.109 × X + 0.195 | 0.992 | 7.5 |
| Homoeriodictyol | Y = 0.026 × X + 0.057 | 0.993 | 205.3 |
| Compound | Ionic Type | RT/min | Parent Ion m/z | Product Ion m/z | DP/v | CE/v |
|---|---|---|---|---|---|---|
| Eriodictyol | M-H | 5.91 | 286.9 | 150.9 | 36 | 18 |
| Homoeriodictyol | M-H | 6.96 | 300.9 | 150.9 | 42 | 28 |
| Quercetin | M-H | 6.06 | 300.9 | 150.9 | 42 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Zeng, M.; Wang, S.; Cao, B.; Liu, M.; Zhang, Y.; Jia, J.; Zhang, Q.; Zhang, B.; Wang, R.; et al. Eriodictyol and Homoeriodictyol Improve Memory Impairment in Aβ25–35-Induced Mice by Inhibiting the NLRP3 Inflammasome. Molecules 2022, 27, 2488. https://doi.org/10.3390/molecules27082488
Guo P, Zeng M, Wang S, Cao B, Liu M, Zhang Y, Jia J, Zhang Q, Zhang B, Wang R, et al. Eriodictyol and Homoeriodictyol Improve Memory Impairment in Aβ25–35-Induced Mice by Inhibiting the NLRP3 Inflammasome. Molecules. 2022; 27(8):2488. https://doi.org/10.3390/molecules27082488
Chicago/Turabian StyleGuo, Pengli, Mengnan Zeng, Shengchao Wang, Bing Cao, Meng Liu, Yuhan Zhang, Jufang Jia, Qinqin Zhang, Beibei Zhang, Ru Wang, and et al. 2022. "Eriodictyol and Homoeriodictyol Improve Memory Impairment in Aβ25–35-Induced Mice by Inhibiting the NLRP3 Inflammasome" Molecules 27, no. 8: 2488. https://doi.org/10.3390/molecules27082488






