Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations
Abstract
:1. Introduction
2. Results and Discussion
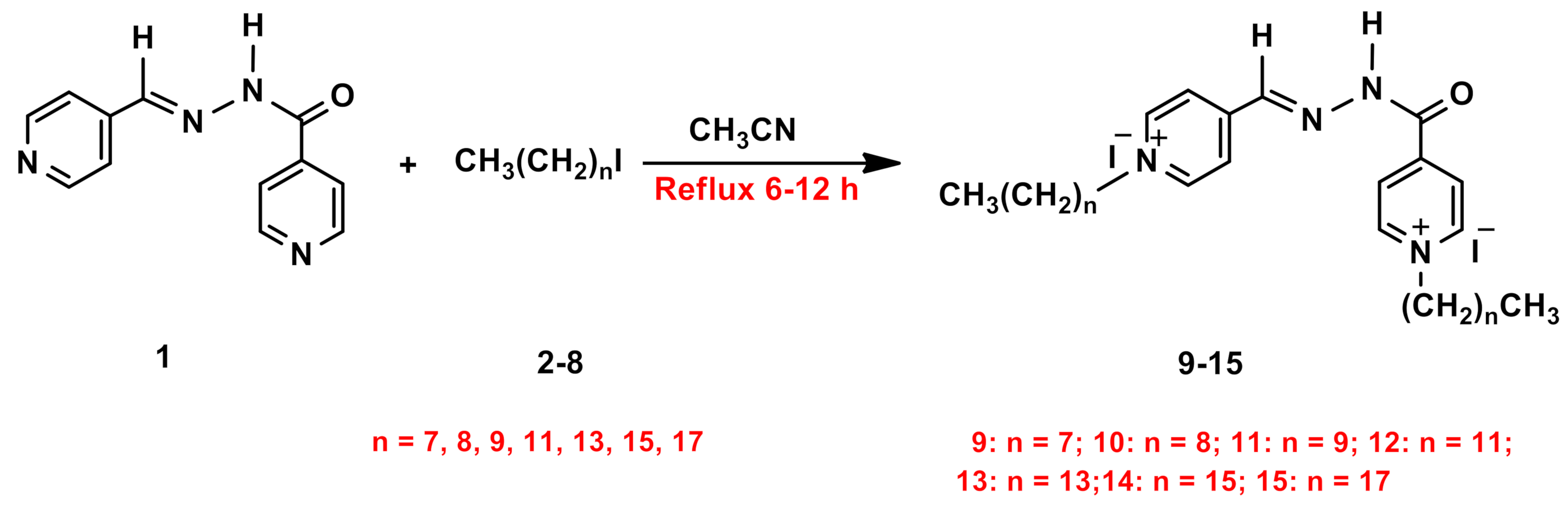
2.1. Synthesis and Characterization
2.2. Effects of Different Anions and Cations on the Thermal Stability
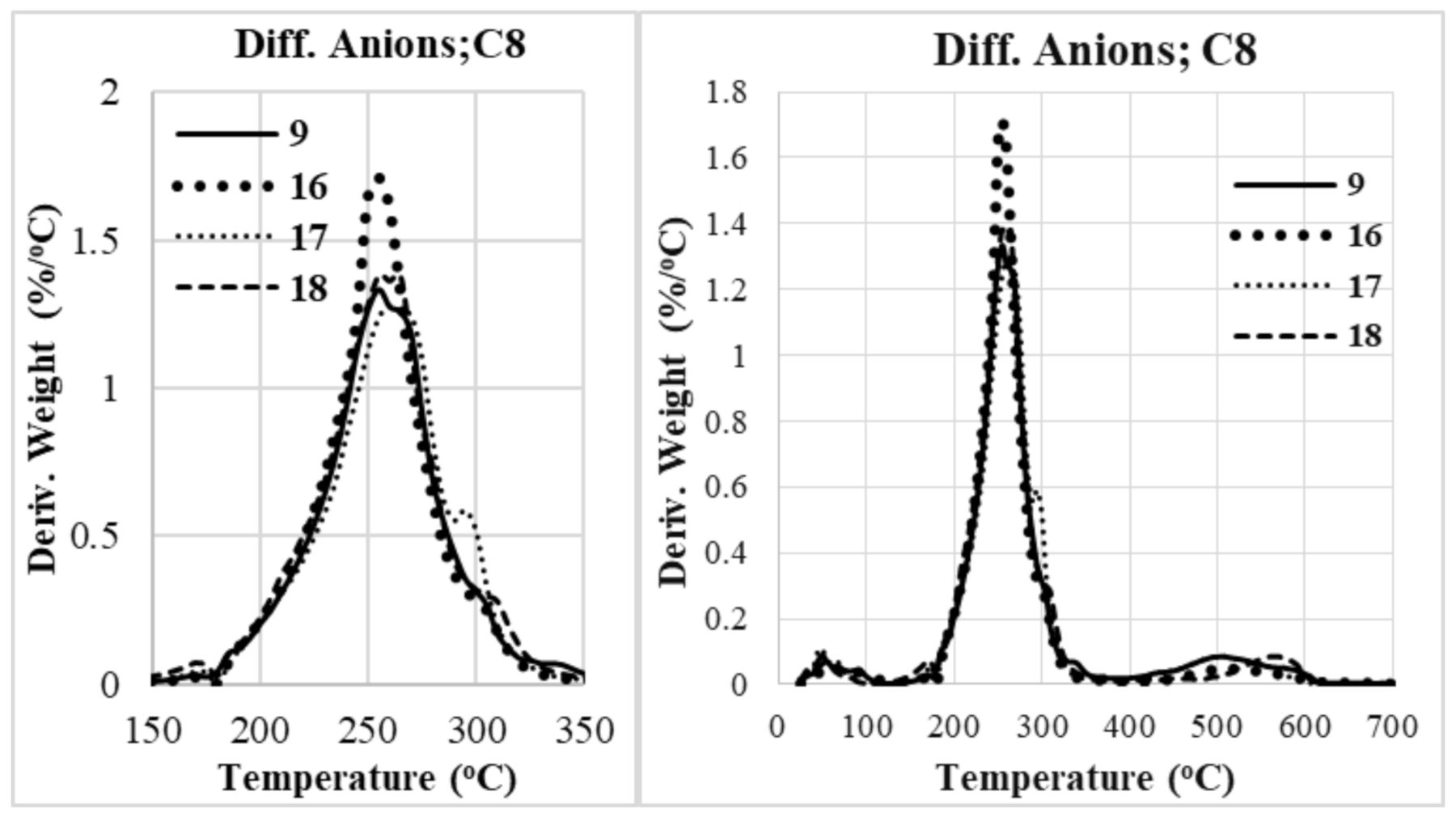
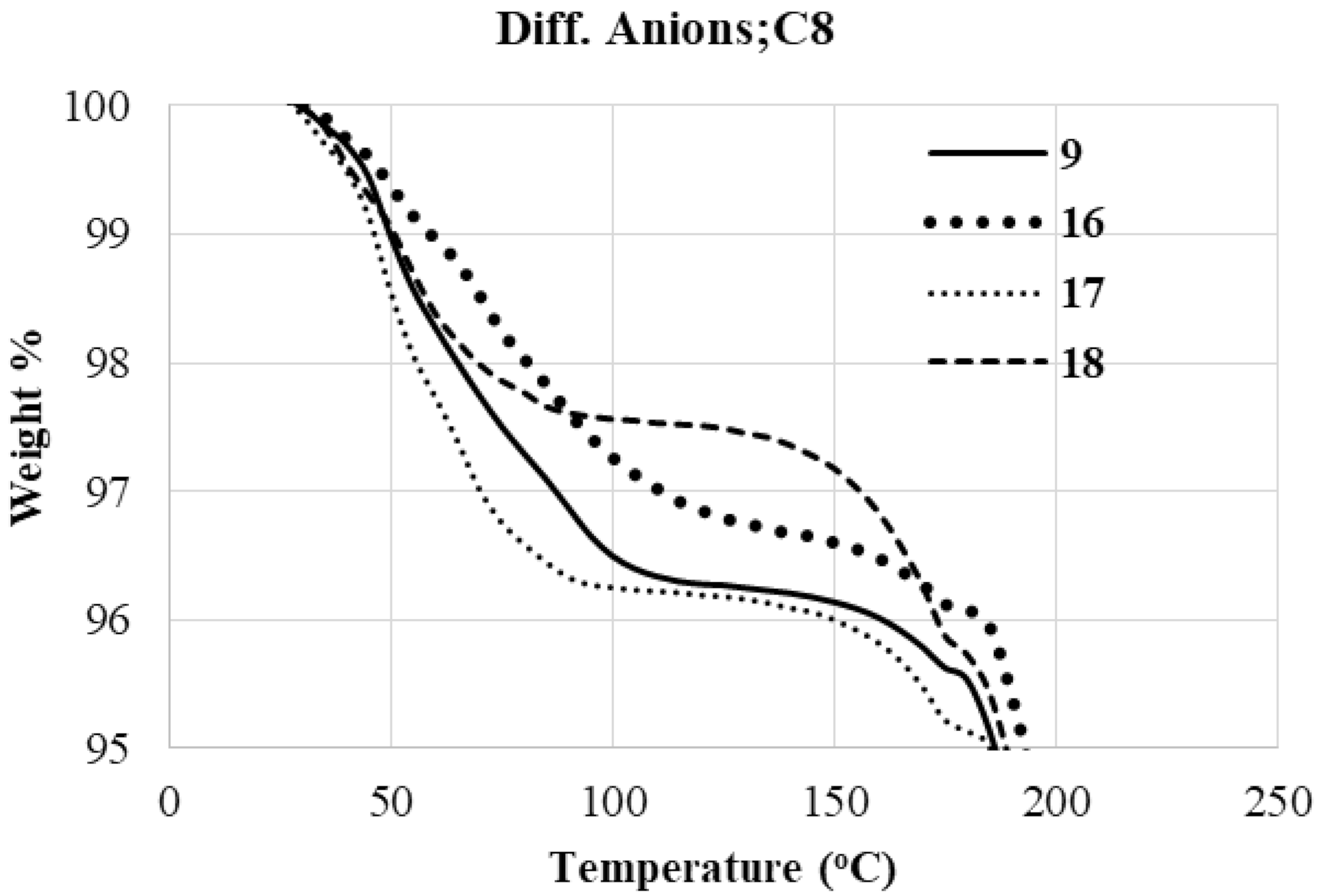
2.2.1. Thermogravimetric Analysis (TGA)
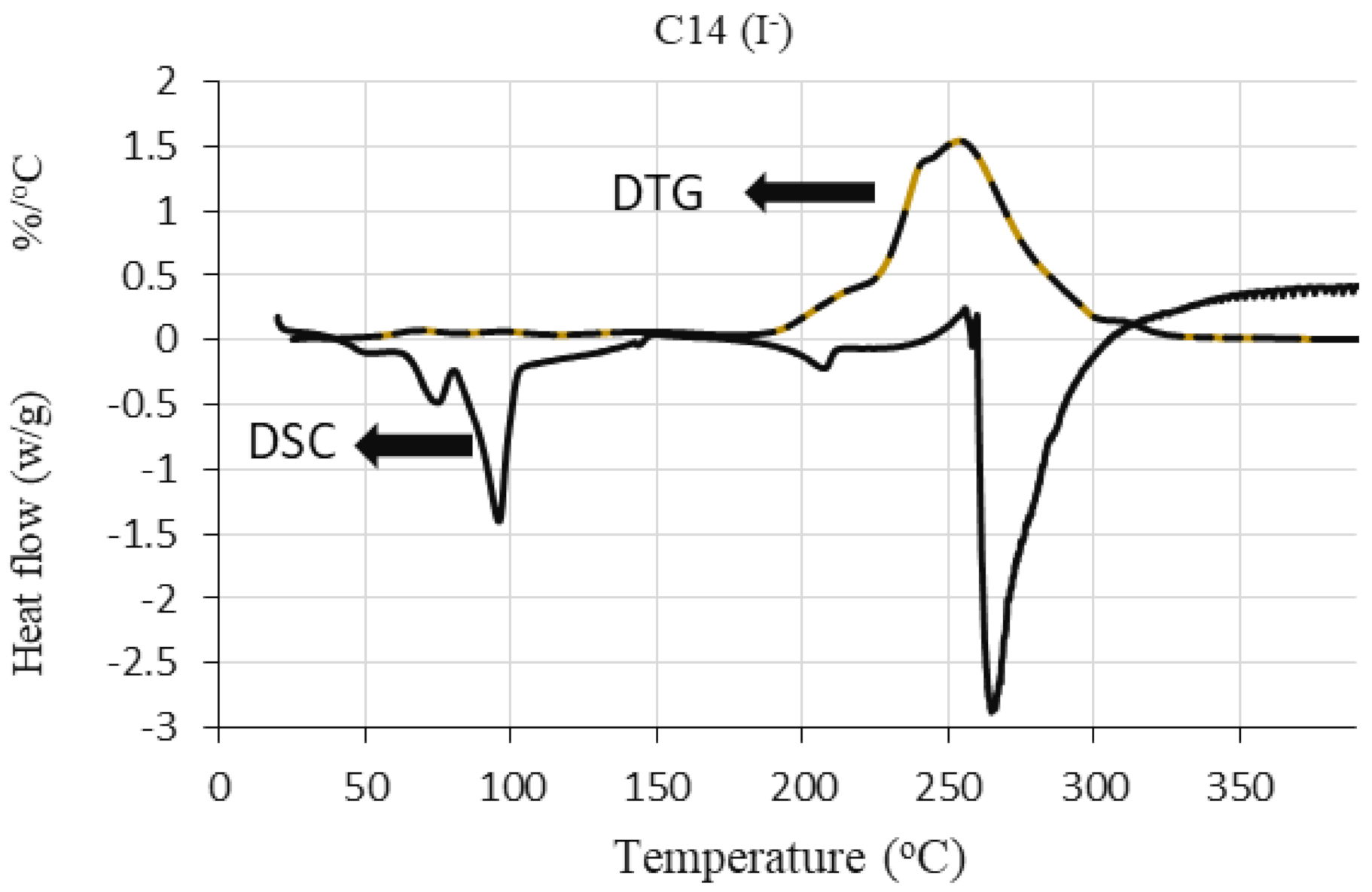
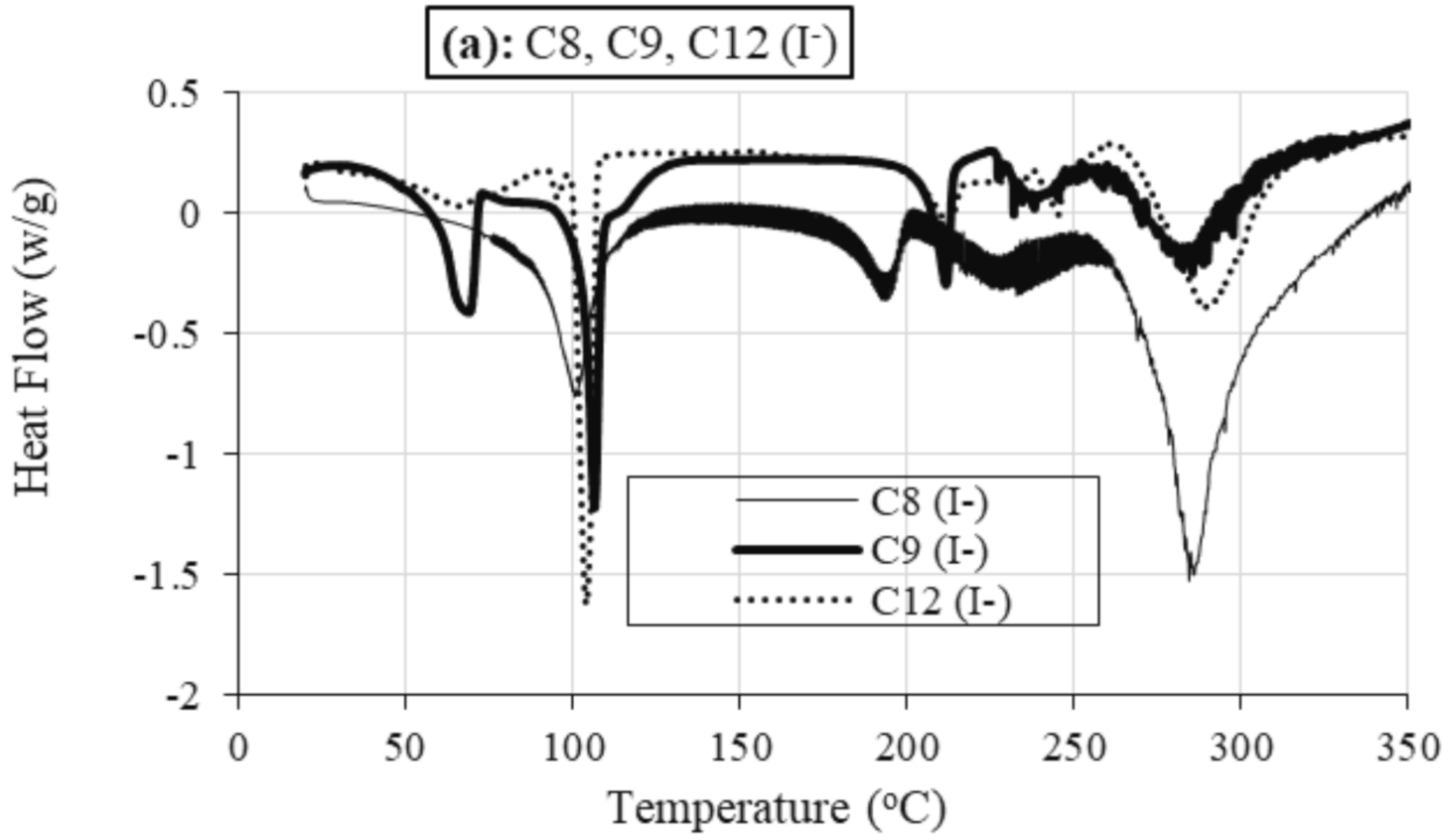
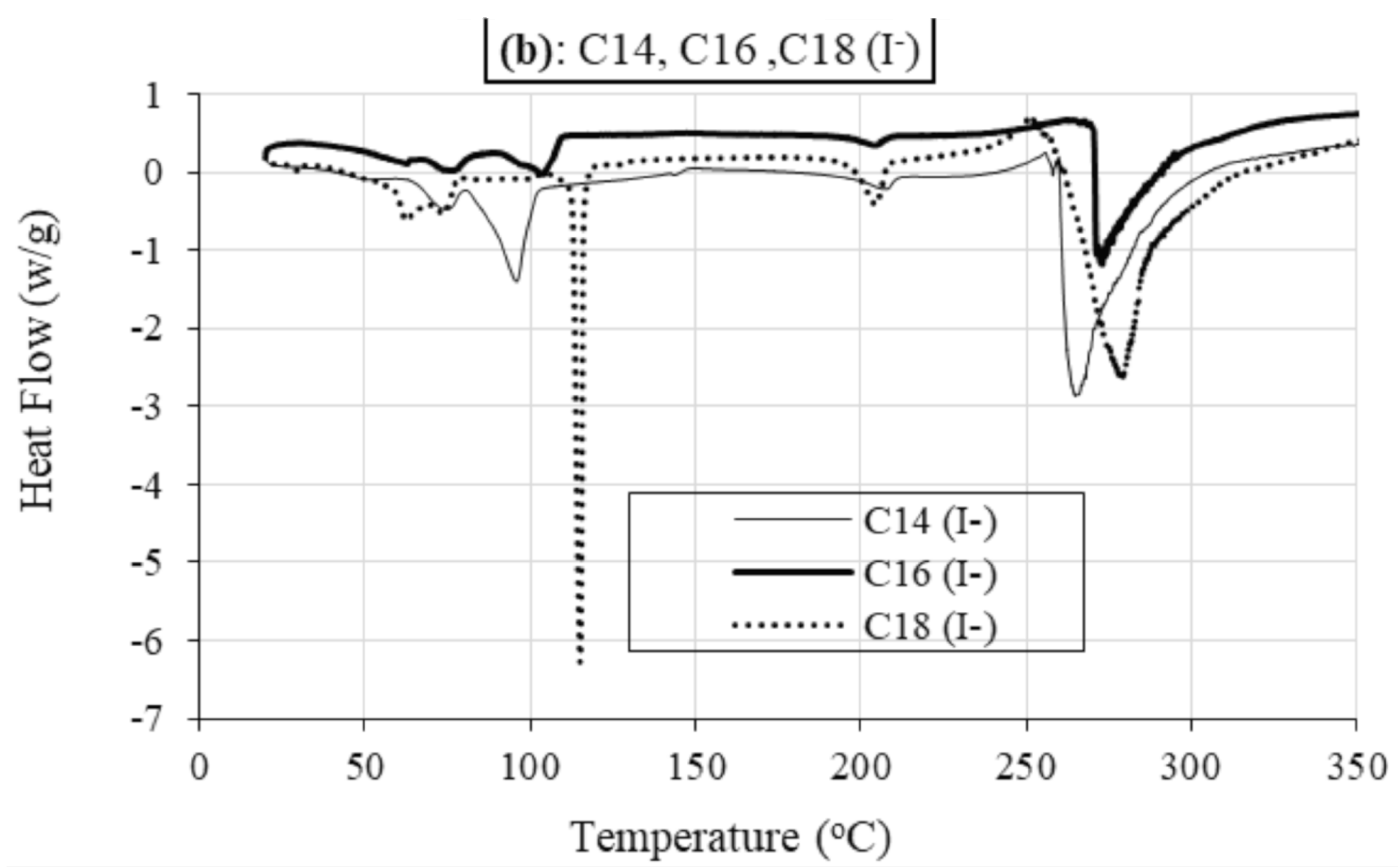
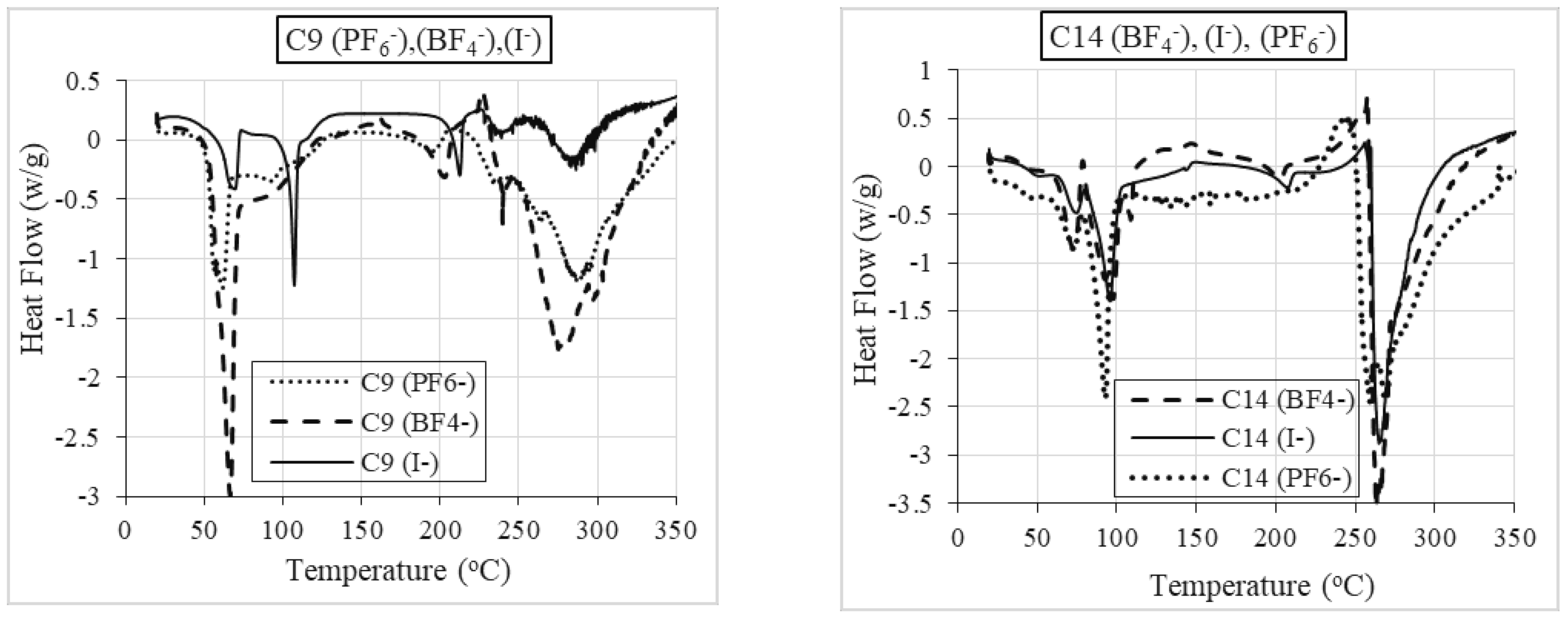
2.2.2. Differential Scanning Calorimetry (DSC)
Role of Alkyl Chain
Role of Anion
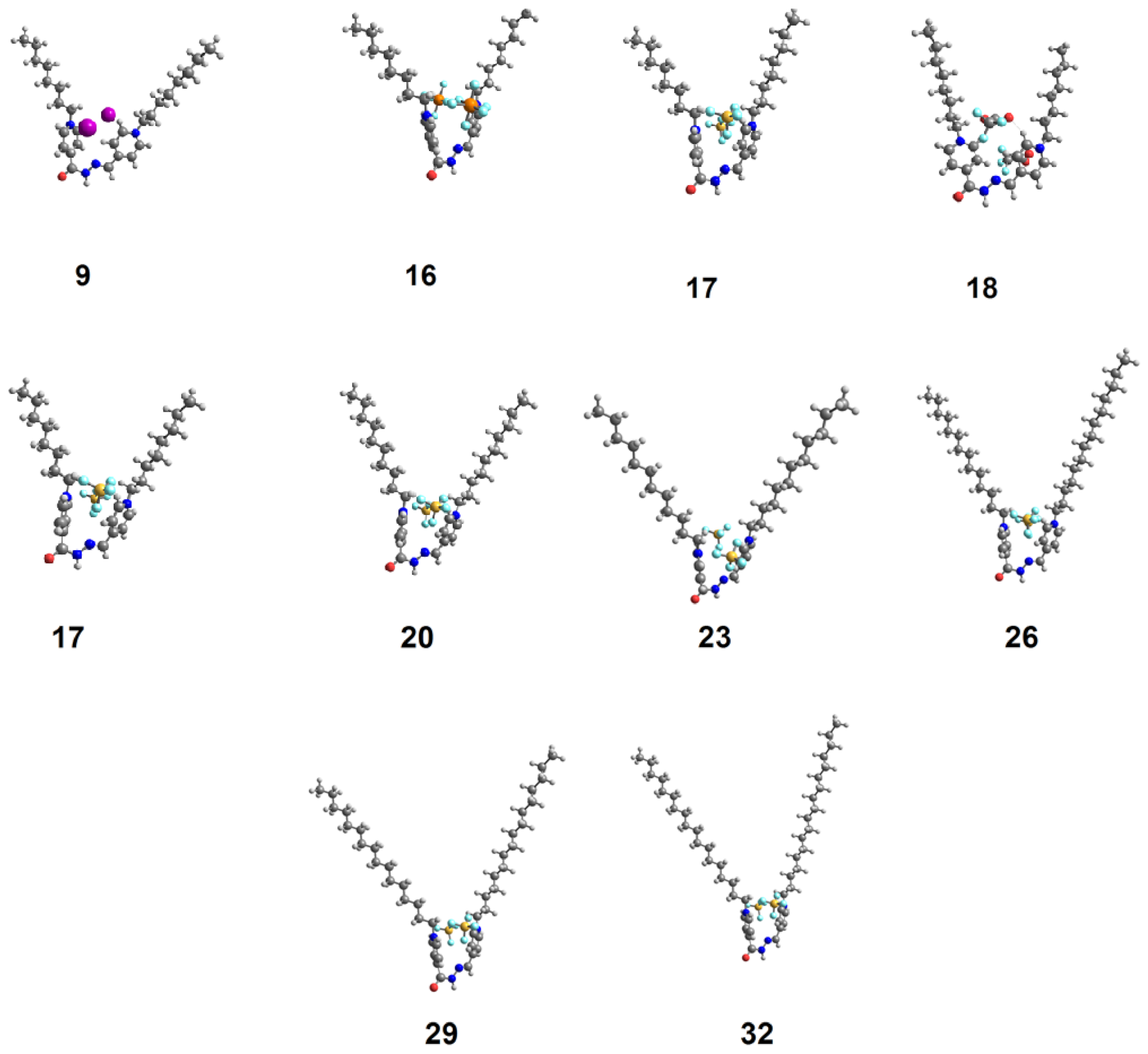
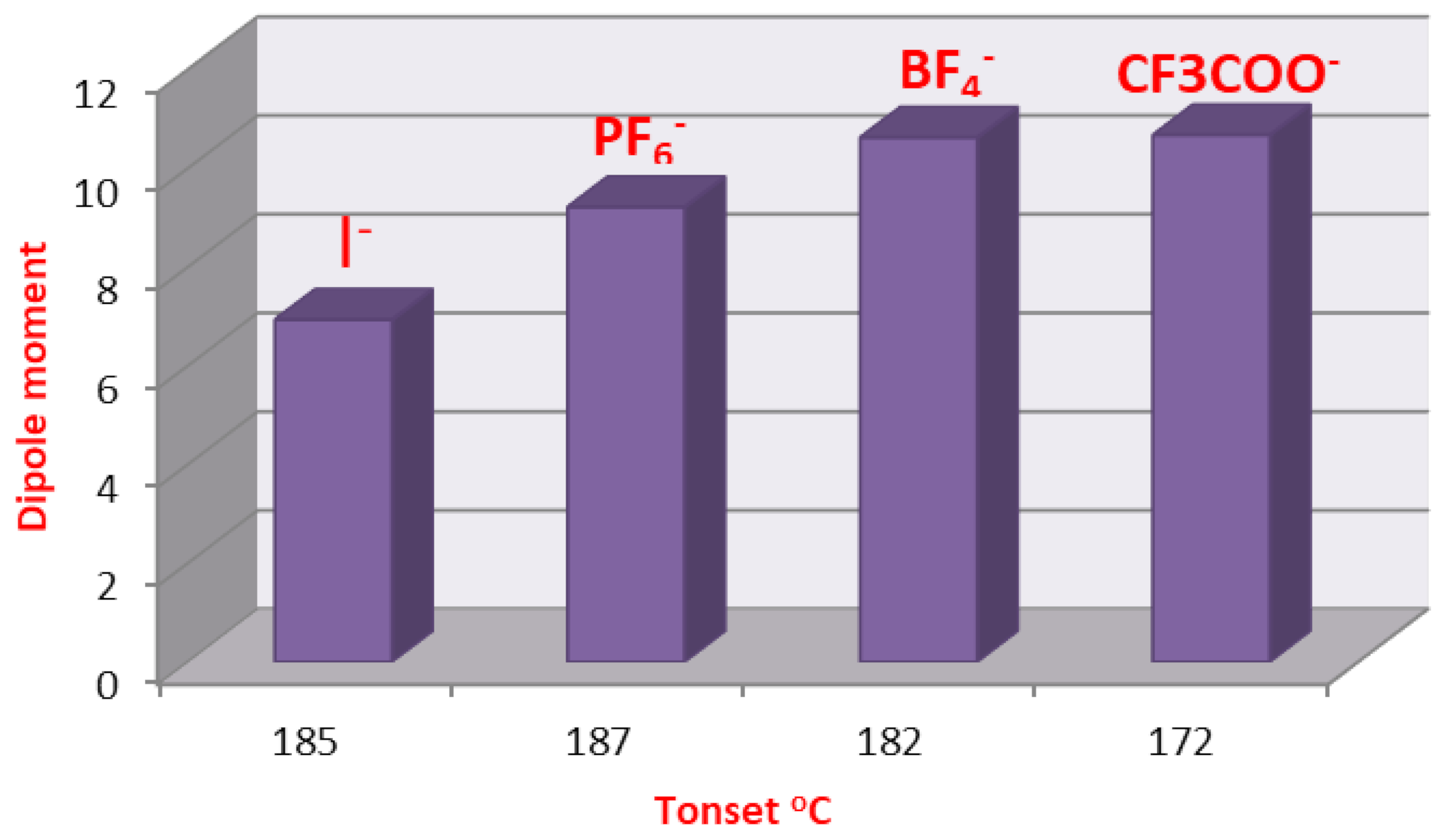
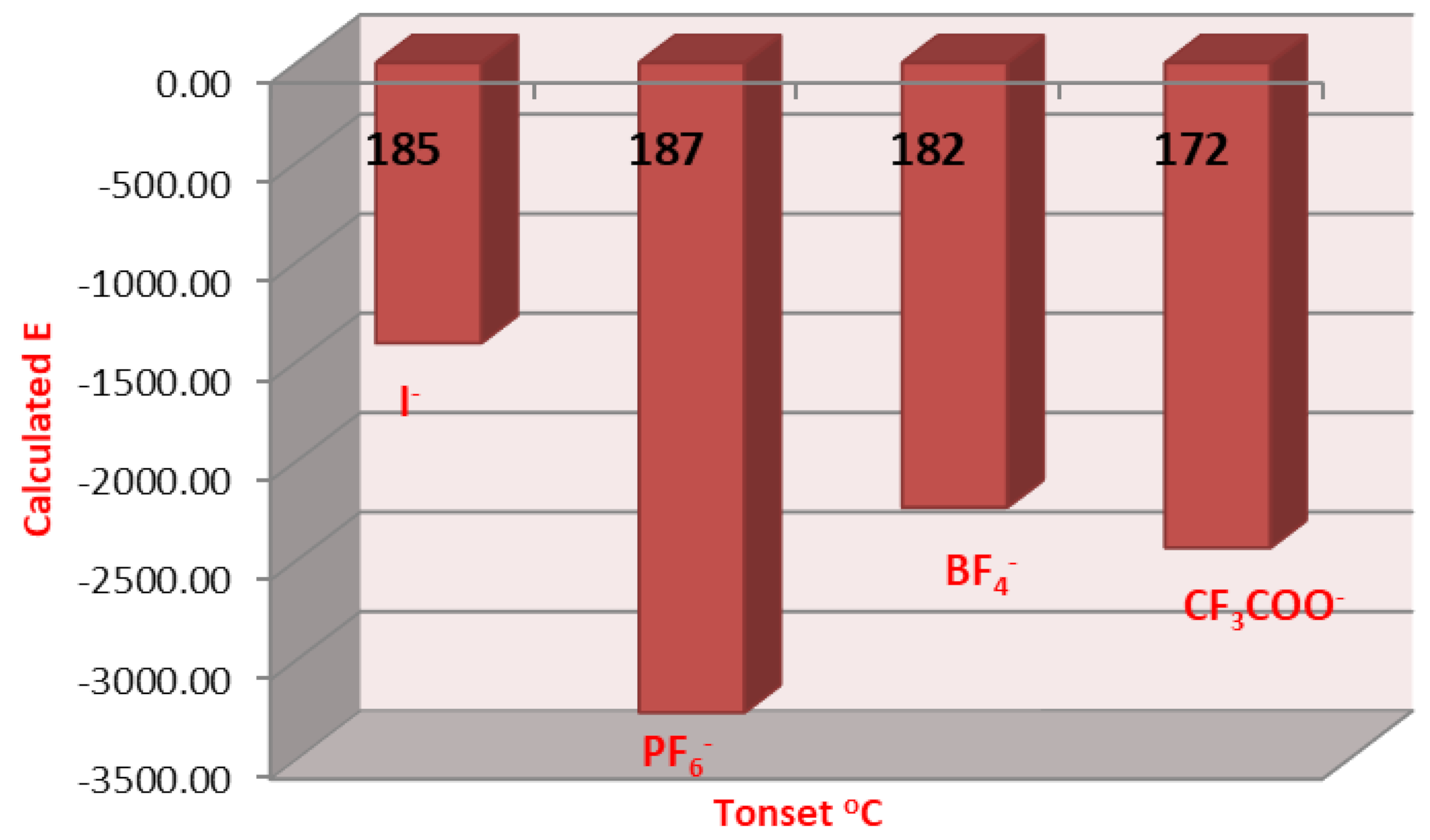
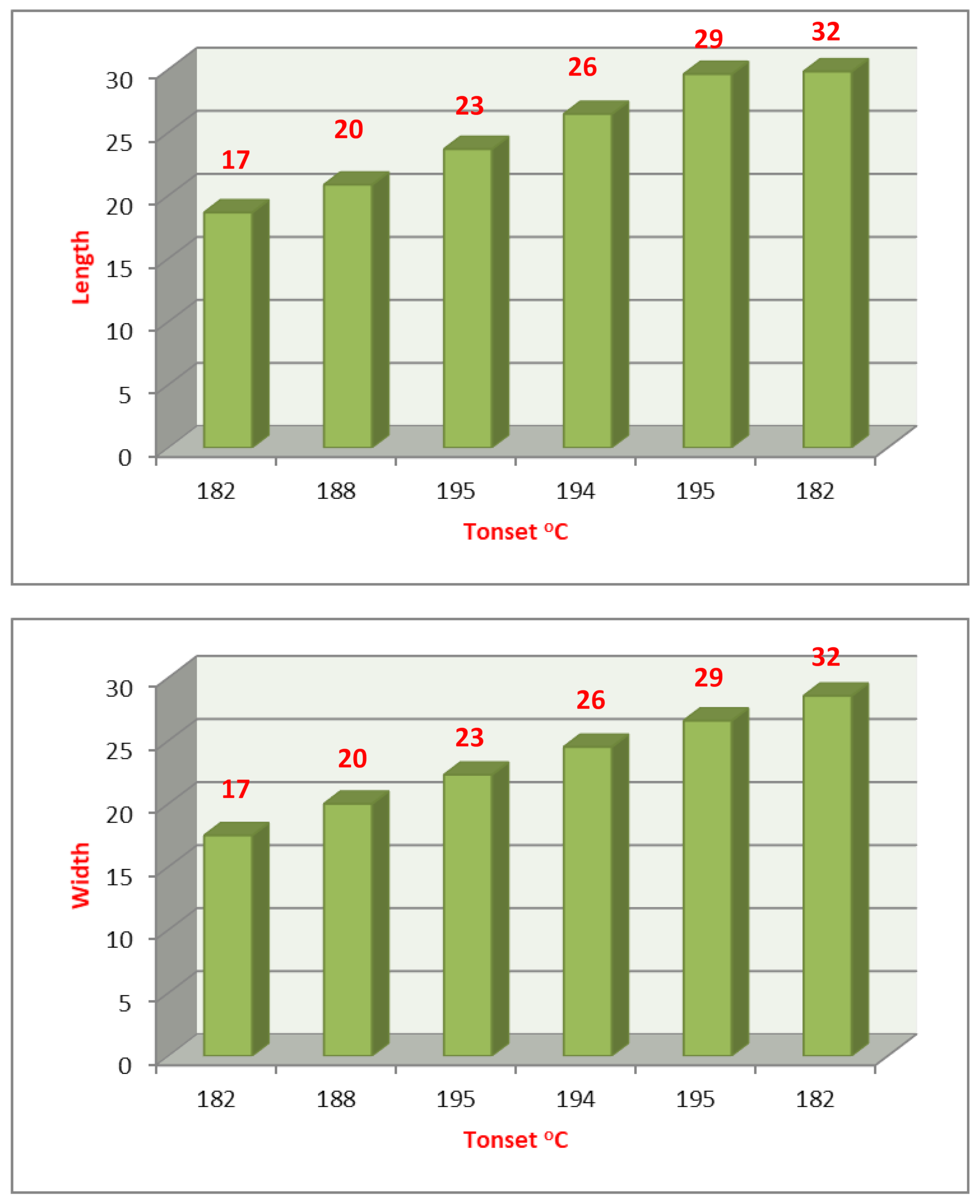
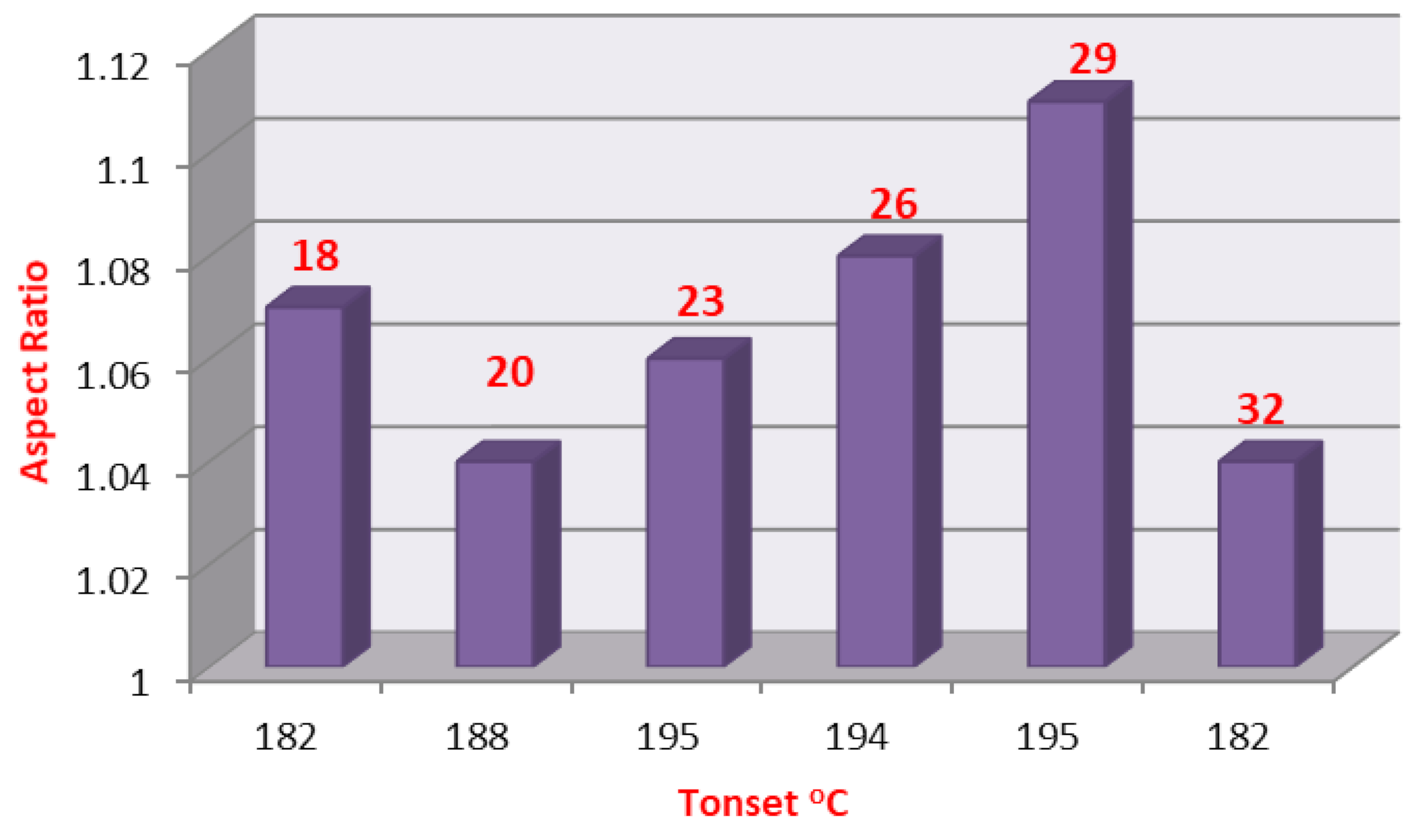
2.3. DFT Theoretical Calculations
3. Experimental Section
3.1. General Procedures for Synthesis of Amphiphilic Dicationic Pyridinium Hydrazone with Iodide Counter Anions 9–15
3.2. General Procedures for Synthesis of Amphiphilic Dicationic Bis-Pyridinium Hydrazone with Fluorinated Counter Anions 16–36
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yue, C.; Fang, D.; Liu, L.; Yi, T.-F. Synthesis and application of task-specific ionic liquids used as catalysts and/or solvents in organic unit reactions. J. Mol. Liq. 2011, 163, 99–121. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Muhammad, N.; Man, Z.; Khan, A.S. Synthesis and thermophysical properties of hydrogensulfate based acidic ionic liquids. J. Solut. Chem. 2015, 44, 875–889. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Hu, X.; Ngwa, C.; Zheng, Q. A simple and efficient procedure for Knoevenagel reaction promoted by imidazolium-based ionic liquids. Curr. Org. Synth. 2016, 13, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Mester, P.; Wagner, M.; Rossmanith, P. Ionic liquids designed as chaotrope and surfactant for use in protein chemistry. Sep. Purif. Technol. 2012, 97, 211–215. [Google Scholar] [CrossRef]
- Velleman, L.; Shapter, J.; Losic, D. Gold nanotube membranes functionalised with fluorinated thiols for selective molecular transport. J. Membr. Sci. 2009, 328, 121–126. [Google Scholar] [CrossRef]
- Tröger-Müller, S.; Brandt, J.; Antonietti, M.; Liedel, C. Green Imidazolium Ionics–From Truly Sustainable Reagents to Highly Functional Ionic Liquids. Chem.–Eur. J. 2017, 23, 11810–11817. [Google Scholar] [CrossRef]
- Ganapathi, P.; Ganesan, K. Synthesis and characterization of 1,2-dimethyl imidazolium ionic liquids and their catalytic activities. Synth. Commun. 2015, 45, 2135–2141. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Earle, M.J.; Esperança, J.M.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Pieer, B.; Nicholus, A. Highly Conductive Ambient Temperature Molten Salts. Inorg. Chem. 1996, 35, 1178–1186. [Google Scholar]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Xiao, J.-C.; Shreeve, J.N.M. Synthesis of 2,2′-biimidazolium-based ionic liquids: Use as a new reaction medium and ligand for palladium-catalyzed suzuki cross-coupling reactions. J. Org. Chem. 2005, 70, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Cai, J.; Liu, R. Research on water evaporation in the process of biomass pyrolysis. Energy Fuels 2007, 21, 3695–3697. [Google Scholar] [CrossRef]
- Senneca, O.; Chirone, R.; Salatino, P. A thermogravimetric study of nonfossil solid fuels. 2. Oxidative pyrolysis and char combustion. Energy Fuels 2002, 16, 661–668. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 44–53, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Dharaskar Swapnil, A. Ionic liquids (a review): The green solvents for petroleum and hydrocarbon industries. Res. J. Chem. Sci. 2012, 2231, 606X. [Google Scholar]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas hydrate inhibition: A review of the role of ionic liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Kim, K.-S.; Kang, S.-P. Kinetic promotion and inhibition of methane hydrate formation by morpholinium ionic liquids with chloride and tetrafluoroborate anions. Energy Fuels 2016, 30, 3879–3885. [Google Scholar] [CrossRef]
- Tariq, M.; Connor, E.; Thompson, J.; Khraisheh, M.; Atilhan, M.; Rooney, D. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv. 2016, 6, 23827–23836. [Google Scholar] [CrossRef]
- Del Villano, L.; Kelland, M.A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure II gas hydrate. Chem. Eng. Sci. 2010, 65, 5366–5372. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Kim, K.-S.; Kang, S.-P. Synergetic effect of ionic liquids on the kinetic inhibition performance of poly (N-vinylcaprolactam) for natural gas hydrate formation. Energy Fuels 2016, 30, 9162–9169. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Cha, J.-H.; Kim, K.-S.; Kang, S.-P. Inhibition effect of ionic liquids and their mixtures with poly (N-vinylcaprolactam) on methane hydrate formation. J. Ind. Eng. Chem. 2016, 38, 211–216. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.-S.; Kang, S.-P.; Kim, J.-N. Synergetic performance of the mixture of poly (n-vinylcaprolactam) and a pyrrolidinium-based ionic liquid for kinetic hydrate inhibition in the presence of the mineral oil phase. Energy Fuels 2018, 32, 4932–4941. [Google Scholar] [CrossRef]
- Kang, S.-P.; Jung, T.; Lee, J.-W. Macroscopic and spectroscopic identifications of the synergetic inhibition of an ionic liquid on hydrate formations. Chem. Eng. Sci. 2016, 143, 270–275. [Google Scholar] [CrossRef]
- Phillips, N.; Kelland, M. The application of surfactants in preventing gas hydrate formation. In Industrial Applications of Surfactants IV; Elsevier: Amsterdam, The Netherlands, 1999; pp. 244–259. [Google Scholar]
- Gupta, P.; Sakthivel, S.; Sangwai, J.S. Effect of aromatic/aliphatic based ionic liquids on the phase behavior of methane hydrates: Experiments and modeling. J. Chem. Thermodyn. 2018, 117, 9–20. [Google Scholar] [CrossRef]
- Saikia, T.; Mahto, V. Evaluation of 1-Decyl-3-Methylimidazolium Tetrafluoroborate as clathrate hydrate crystal inhibitor in drilling fluid. J. Nat. Gas Sci. Eng. 2016, 36, 906–915. [Google Scholar] [CrossRef]
- Bittner, B.; Wrobel, R.J.; Milchert, E. Physical properties of pyridinium ionic liquids. J. Chem. Thermodyn. 2012, 55, 159–165. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Blewi, F.F.; Al-Sodies, S.A.; Alnuzha, A.K.; Messali, M.; Ali, I.; Aouad, M.R. Synthesis, characterization, DNA binding, anticancer, and molecular docking studies of novel imidazolium-based ionic liquids with fluorinated phenylacetamide tethers. ACS Omega 2020, 5, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Rezki, N.; Al-Sodies, S.A.; Ahmed, H.E.; Ihmaid, S.; Messali, M.; Ahmed, S.; Aouad, M.R. A novel dicationic ionic liquids encompassing pyridinium hydrazone-phenoxy conjugates as antimicrobial agents targeting diverse high resistant microbial strains. J. Mol. Liq. 2019, 284, 431–444. [Google Scholar] [CrossRef]
- Aljuhani, A.; Aouad, M.R.; Rezki, N.; Aljaldy, O.A.; Al-Sodies, S.A.; Messali, M.; Ali, I. Novel pyridinium based ionic liquids with amide tethers: Microwave assisted synthesis, molecular docking and anticancer studies. J. Mol. Liq. 2019, 285, 790–802. [Google Scholar] [CrossRef]
- Al-Blewi, F.; Rezki, N.; Naqvi, A.; Qutb Uddin, H.; Al-Sodies, S.; Messali, M.; Aouad, M.R.; Bardaweel, S. A profile of the in vitro anti-tumor activity and in silico ADME predictions of novel benzothiazole amide-functionalized imidazolium ionic liquids. Int. J. Mol. Sci. 2019, 20, 2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Blewi, F.; Rezki, N.; Al-Sodies, S.; Bardaweel, S.; Sabbah, D.; Messali, M.; Aouad, M. Novel cationic amphiphilic fluorinated pyridinium hydrazones: Conventional versus green ultrasound assisted synthesis, characterization, molecular docking, and anticancer evaluation. Chem. Cent. J. 2018, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezki, N.; Al-Sodies, S.A.; Aouad, M.R.; Bardaweel, S.; Messali, M.; El Ashry, E.S.H. An eco-friendly ultrasound-assisted synthesis of novel fluorinated pyridinium salts-based hydrazones and antimicrobial and antitumor screening. Int. J. Mol. Sci. 2016, 17, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezki, N.; Al-Sodies, S.A.; Shreaz, S.; Shiekh, R.A.; Messali, M.; Raja, V.; Aouad, M.R. Green ultrasound versus conventional synthesis and characterization of specific task pyridinium ionic liquid hydrazones tethering fluorinated counter anions: Novel inibitors of fungal Ergosterol biosynthesis. Molecules 2017, 22, 1532. [Google Scholar] [CrossRef] [Green Version]
- Aljuhani, A.; El-Sayed, W.S.; Sahu, P.K.; Rezki, N.; Aouad, M.R.; Salghi, R.; Messali, M. Microwave-assisted synthesis of novel imidazolium, pyridinium and pyridazinium-based ionic liquids and/or salts and prediction of physico-chemical properties for their toxicity and antibacterial activity. J. Mol. Liq. 2018, 249, 747–753. [Google Scholar] [CrossRef]
- Rezki, N.; Messali, M.; Al-Sodies, S.A.; Naqvi, A.; Bardaweel, S.K.; Al-blewi, F.F.; Aouad, M.R.; El Sayed, H. Design, synthesis, in-silico and in-vitro evaluation of di-cationic pyridinium ionic liquids as potential anticancer scaffolds. J. Mol. Liq. 2018, 265, 428–441. [Google Scholar] [CrossRef]
- Rezki, N.; Al-Sodies, S.A.; Messali, M.; Bardaweel, S.K.; Sahu, P.K.; Al-blewi, F.F.; Sahu, P.K.; Aouad, M.R. Identification of new pyridinium ionic liquids tagged with Schiff bases: Design, synthesis, in silico ADMET predictions and biological evaluations. J. Mol. Liq. 2018, 264, 367–374. [Google Scholar] [CrossRef]
- Al-Sodies, S.A.; Aouad, M.R.; Ihmaid, S.; Aljuhani, A.; Messali, M.; Ali, I.; Rezki, N. Microwave and conventional synthesis of ester based dicationic pyridinium ionic liquids carrying hydrazone linkage: DNA binding, anticancer and docking studies. J. Mol. Struct. 2020, 1207, 127756. [Google Scholar] [CrossRef]
- Ratti, R. Ionic liquids: Synthesis and applications in catalysis. Adv. Chem. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Lima, P.C.; Lima, L.M.; da Silva, K.C.M.; Léda, P.H.O.; de Miranda, A.L.P.; Fraga, C.A.; Barreiro, E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000, 35, 187–203. [Google Scholar] [CrossRef]
- Cammarata, L.; Kazarian, S.; Salter, P.; Welton, T. Molecular states of water in room temperature ionic liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef] [Green Version]
- Al-Sodies, S.; Rezki, N.; Albelwi, F.F.; Messali, M.; Aouad, M.R.; Bardaweel, S.K.; Hagar, M. Novel Dipyridinium Lipophile-Based Ionic Liquids Tethering Hydrazone Linkage: Design, Synthesis and Antitumorigenic Study. Int. J. Mol. Sci. 2021, 22, 10487. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Neves, C.M.; Marrucho, I.M.; Coutinho, J.A.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Steudte, S.; Neumann, J.; Bottin-Weber, U.; Diedenhofen, M.; Arning, J.; Stepnowski, P.; Stolte, S. Hydrolysis study of fluoroorganic and cyano-based ionic liquid anions–consequences for operational safety and environmental stability. Green Chem. 2012, 14, 2474–2483. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Scammells, P.J.; Scott, J.L.; Singer, R.D. Ionic liquids: The neglected issues. Aust. J. Chem. 2005, 58, 155–169. [Google Scholar] [CrossRef]
- Kosmulski, M.M.; Gustafsson, J.; Rosenholm, J.B. Thermal Stability of Low. Temperature Ionic Liquids Revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar]
- Prasad, M.; Krishnamurthy, V. Thermal decomposition and pyrolysis-GC studies on tetraalkyl-substituted ammonium hexafluorophosphates. Thermochim. Acta 1991, 185, 1–10. [Google Scholar] [CrossRef]
- Arellano, I.H.J.; Guarino, J.G.; Paredes, F.U.; Arco, S.D. Thermal stability and moisture uptake of 1-alkyl-3-methylimidazolium bromide. J. Therm. Anal. Calorim. 2011, 103, 725–730. [Google Scholar] [CrossRef]
- Song, Y.; Xia, Y.; Liu, Z. Influence of cation structure on physicochemical and antiwear properties of hydroxyl-functionalized imidazolium bis (trifluoromethylsulfonyl) imide ionic liquids. Tribol. Trans. 2012, 55, 738–746. [Google Scholar] [CrossRef]
- Dominguez-Vidal, A.; Kaun, N.; Ayora-Cañada, M.J.; Lendl, B. Probing intermolecular interactions in water/ionic liquid mixtures by far-infrared spectroscopy. J. Phys. Chem. B 2007, 111, 4446–4452. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and properties of high stability geminal dicationic ionic liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Montanino, M.; Carewska, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. The role of the cation aliphatic side chain length in piperidinium bis (trifluoromethansulfonyl) imide ionic liquids. Electrochim. Acta 2011, 57, 153–159. [Google Scholar] [CrossRef]




| Sample | TStart (°C) | Tonset (°C) | TEnd (°C) | % | TPeak (°C) | m.p (°C) | Lit. m.p (°C) of Analogue Compounds [47] | M.Wt (g) |
|---|---|---|---|---|---|---|---|---|
| 9 | 25 150 455 | 185 | 150 450 600 | 3.85 86.39 9.94 | 55–95 250 500 | 211–212 | 82–84 | 704 |
| 16 | 25 185 450 | 187 | 180 440 745 | 4.05 88.10 8.7 | 75 255 520 | 200–201 | 58–59 | 742 |
| 17 | 25 155 445 | 182 | 150 425 590 | 3.35 87.29 9.05 | 55–70 265 (270), 295 sh 510 | 205–206 | Syrup | 626 |
| 18 | 25 145 495 | 172 | 140 440 710 | 2.5 88.72 8.52 | 60 260 565 | 196–197 | Syrup | 678 |
| 19 | 25 150 400 | 185 | 145 400 745 | 4.38 86.13 9.20 | 50 250 490 | 202–203 | 49–50 | 770 |
| 20 | 25 170 465 | 188 | 165 460 635 | 4.97 87 7.95 | 60 255 565 | 210–211 | Syrup | 654 |
| 21 | 25 130 500 | 185 | 125 500 700 | 1.00 56.34 4.13 | 45 260 570 | 188–189 | Syrup | 706 |
| 11 | 25 160 450 | 195 | 160 330 590 | 1.70 88.8 9.05 | 55 255 525 | 217–218 | 70–72 | 762 |
| 22 | 25 190 445 | 198 | 185 440 600 | 5.56 86.33 7.95 | 65–95 255 510 | 135–136 | 42–44 | 798 |
| 23 | 25 170 385 | 195 | 165 385 555 | 2.5 86.84 10.26 | 90 255, 205 sh 510 | 189–190 | Syrup | 682 |
| 12 | 25 160 410 | 190 | 155 400 680 | 3.0 86.61 8.9 | 50 250 505 | 209–210 | 63–65 | 818 |
| 25 | 25 185 410 | 193 | 180 410 695 | 4.43 87.48 8.0 | 65, 100 245, 250, 305 sh 515 | 184–185 | 37–38 | 854 |
| 26 | 25 185 385 | 194 | 180 385 700 | 4.27 86.24 9.49 | 65, 95 250, 300 sh 500 | 193–194 | Syrup | 738 |
| 27 | 25 140 410 | 185 | 140 305 580 | 2.5 88.61 9.0 | 50 250 505 | 179–180 | Syrup | 790 |
| 13 | 25 190 440 | 195 | 185 440 605 | 7.99 83.95 8.06 | 75, 150 255 (220) 525 | 194–195 | Syrup | 874 |
| 28 | 25 180 405 | 185 | 180 405 590 | 6.86 85.93 7.75 | 100 245, 210 sh 505 | 180–181 | Syrup | 910 |
| 29 | 25 190 440 | 195 | 190 440 605 | 5.5 86.95 8.06 | 100 250 515 | 183–184 | Syrup | 794 |
| 30 | 25 165 400 | 175 | 140 400 600 | 5.45 86.1 8.63 | 95 250, 320 sh, 530 | 171–172 | Syrup | 846 |
| 14 | 25 110 475 | 170 | 105 475 755 | 3.35 87.0 9.6 | 40 250 535 | 190–191 | Syrup | 930 |
| 31 | 25 125 455 | 175 | 120 455 600 | 4.00 87.34 9.03 | 70 250 510 | 169–170 | Syrup | 966 |
| 32 | 25 135 470 | 182 | 130 470 745 | 3.51 86.85 9.98 | 65, 150 250, 225 sh 550 | 177–178 | Syrup | 850 |
| 33 | 25 125 460 | 145 | 120 460 600 | 3.5 87.41 9.59 | 70 250, 180 sh, 520 | 173–174 | Syrup | 902 |
| 15 | 25 125 455 | 135 | 120 455 605 | 2.50 89.93 8.37 | 40 250 510 | 192–193 | Syrup | 986 |
| 35 | 25 130 455 | 145 | 125 455 580 | 3.5 87.89 9.81 | 70 175, 250, 225 sh 540 | 189–190 | Syrup | 908 |
| 36 | 25 125 405 | 135 | 120 405 745 | 1.75 91.8 7.0 | 55 250, 165 sh 555 | 165–166 | Syrup | 958 |
| Cmpds No. | Calculated Energy, E | Dipole Moment, μ | Dimension Ǻ | Aspect Ratio (L/D) | Tonset | |
|---|---|---|---|---|---|---|
| Length (L) | Width (D) | |||||
| 9 | −1410.85 | 7.82 | 21.95 | 16.18 | 1.38 | 185 |
| 16 | −1409.81 | 6.94 | 22.05 | 16.00 | 1.38 | 182 |
| 17 | −3267.23 | 9.22 | 20.55 | 16.91 | 1.22 | 187 |
| 18 | −2235.31 | 10.63 | 18.67 | 17.50 | 1.07 | 182 |
| 19 | −2438.60 | 10.68 | 18.12 | 17.44 | 1.04 | 172 |
| 20 | −2392.40 | 7.22 | 20.87 | 20.01 | 1.04 | 188 |
| 23 | −2549.50 | 7.1 9 | 23.69 | 22.31 | 1.06 | 195 |
| 26 | 2706−.60 | 7.17 | 26.48 | 24.52 | 1.08 | 194 |
| 29 | −2863.69 | 7.16 | 29.64 | 26.60 | 1.11 | 195 |
| 32 | −3020.79 | 7.15 | 29.85 | 28.57 | 1.04 | 182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljuhani, A.; Rezki, N.; Al-Sodies, S.; Messali, M.; ElShafei, G.M.S.; Hagar, M.; Aouad, M.R. Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations. Molecules 2022, 27, 2492. https://doi.org/10.3390/molecules27082492
Aljuhani A, Rezki N, Al-Sodies S, Messali M, ElShafei GMS, Hagar M, Aouad MR. Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations. Molecules. 2022; 27(8):2492. https://doi.org/10.3390/molecules27082492
Chicago/Turabian StyleAljuhani, Ateyatallah, Nadjet Rezki, Salsabeel Al-Sodies, Mouslim Messali, Gamal M. S. ElShafei, Mohamed Hagar, and Mohamed R. Aouad. 2022. "Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations" Molecules 27, no. 8: 2492. https://doi.org/10.3390/molecules27082492
APA StyleAljuhani, A., Rezki, N., Al-Sodies, S., Messali, M., ElShafei, G. M. S., Hagar, M., & Aouad, M. R. (2022). Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations. Molecules, 27(8), 2492. https://doi.org/10.3390/molecules27082492







