Mechanisms of Penetration Enhancement and Transport Utilizing Skin Keratine Liposomes for the Topical Delivery of Licochalcone A
Abstract
:1. Introduction
2. Results and Discussion
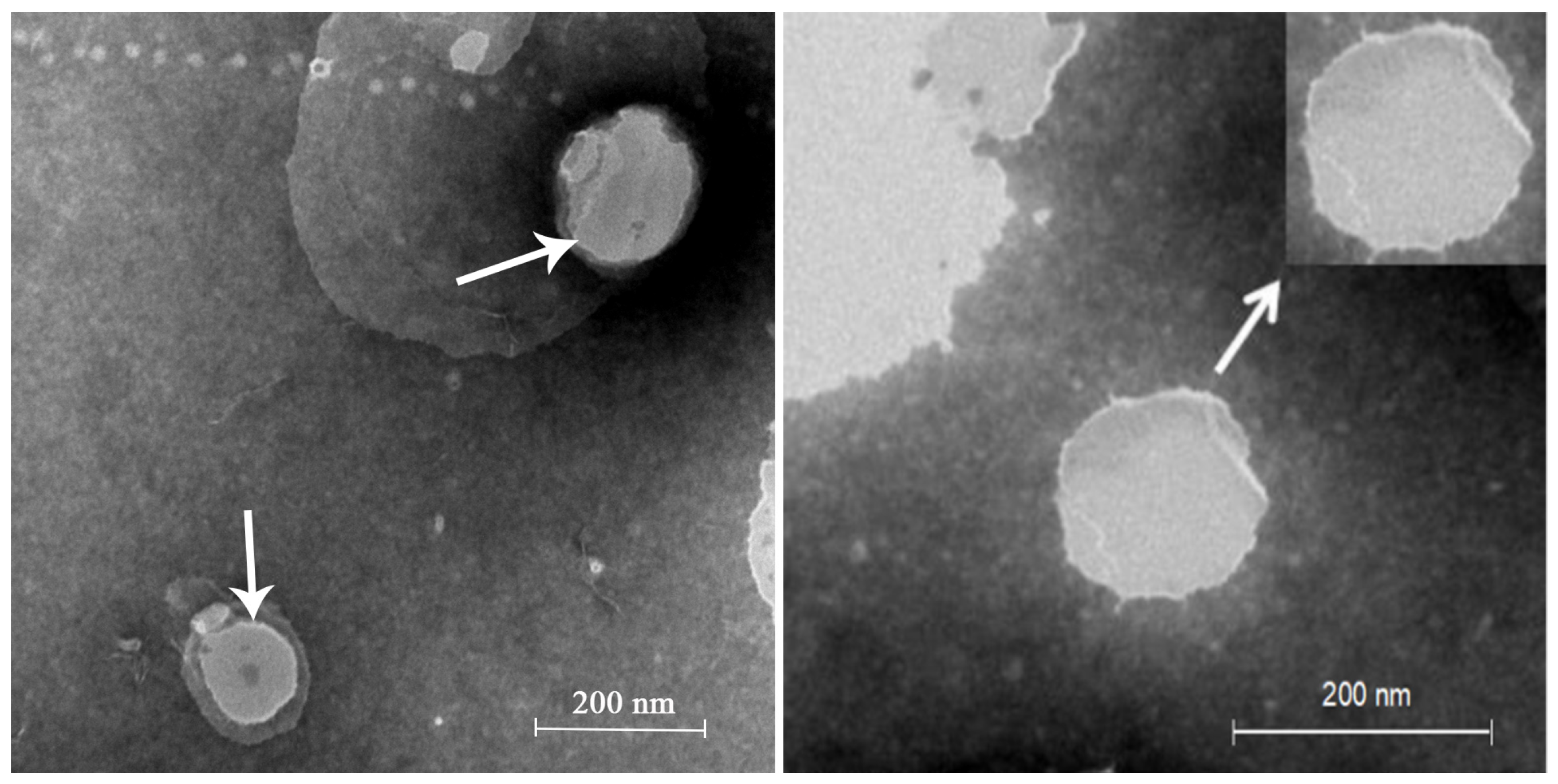
2.1. LA Skin Keratin Liposome (LAL) Characteristics
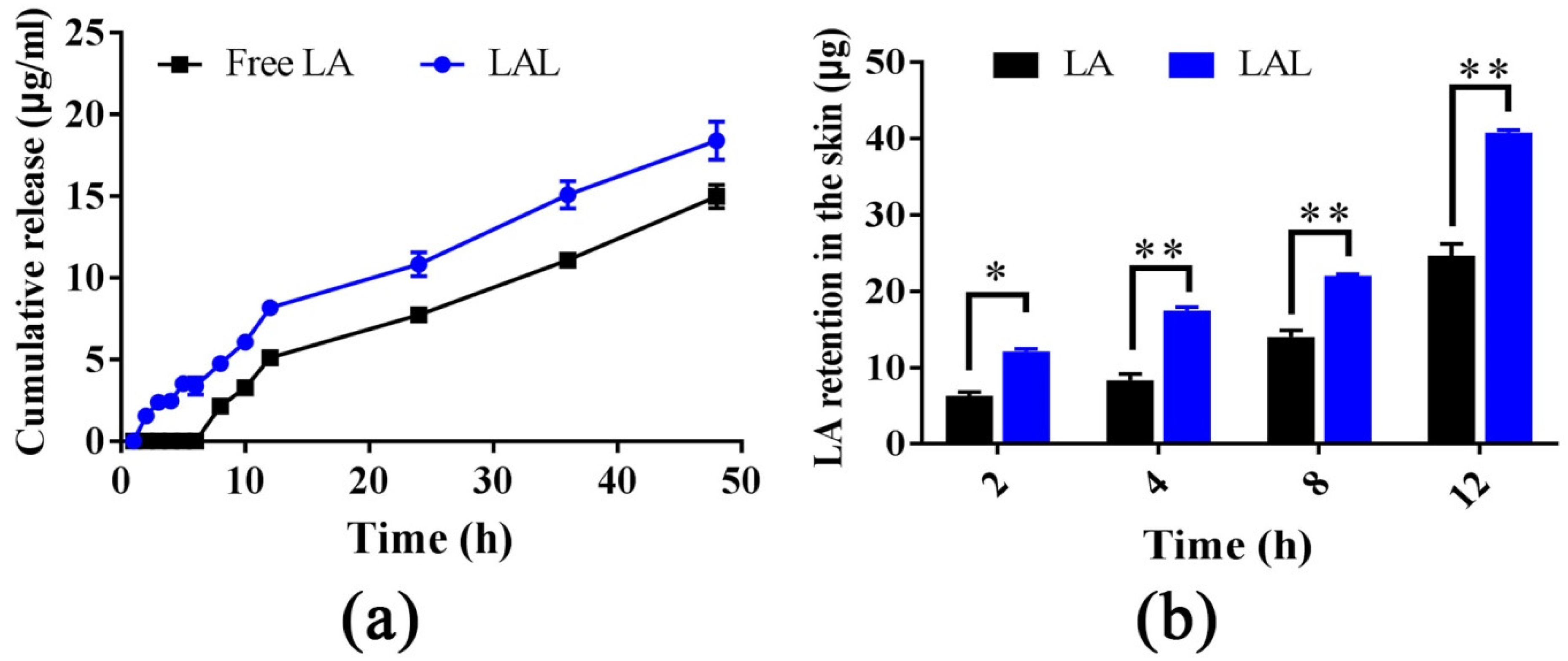
2.2. Skin Retention and Dialysis Bag Experiments
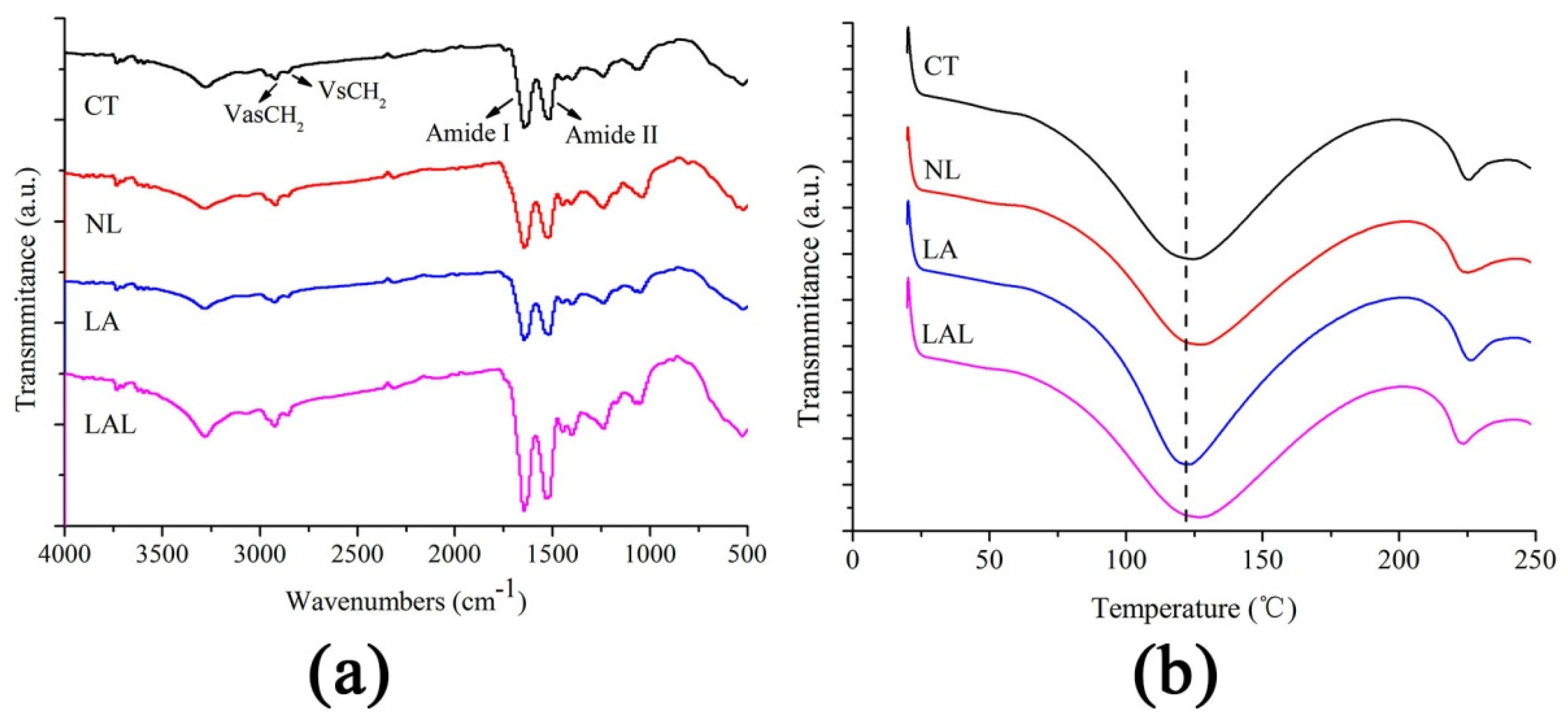
2.3. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Test of SC Components
2.4. Differential Scanning Calorimetry (DSC) Study of SC Thermotropic Properties
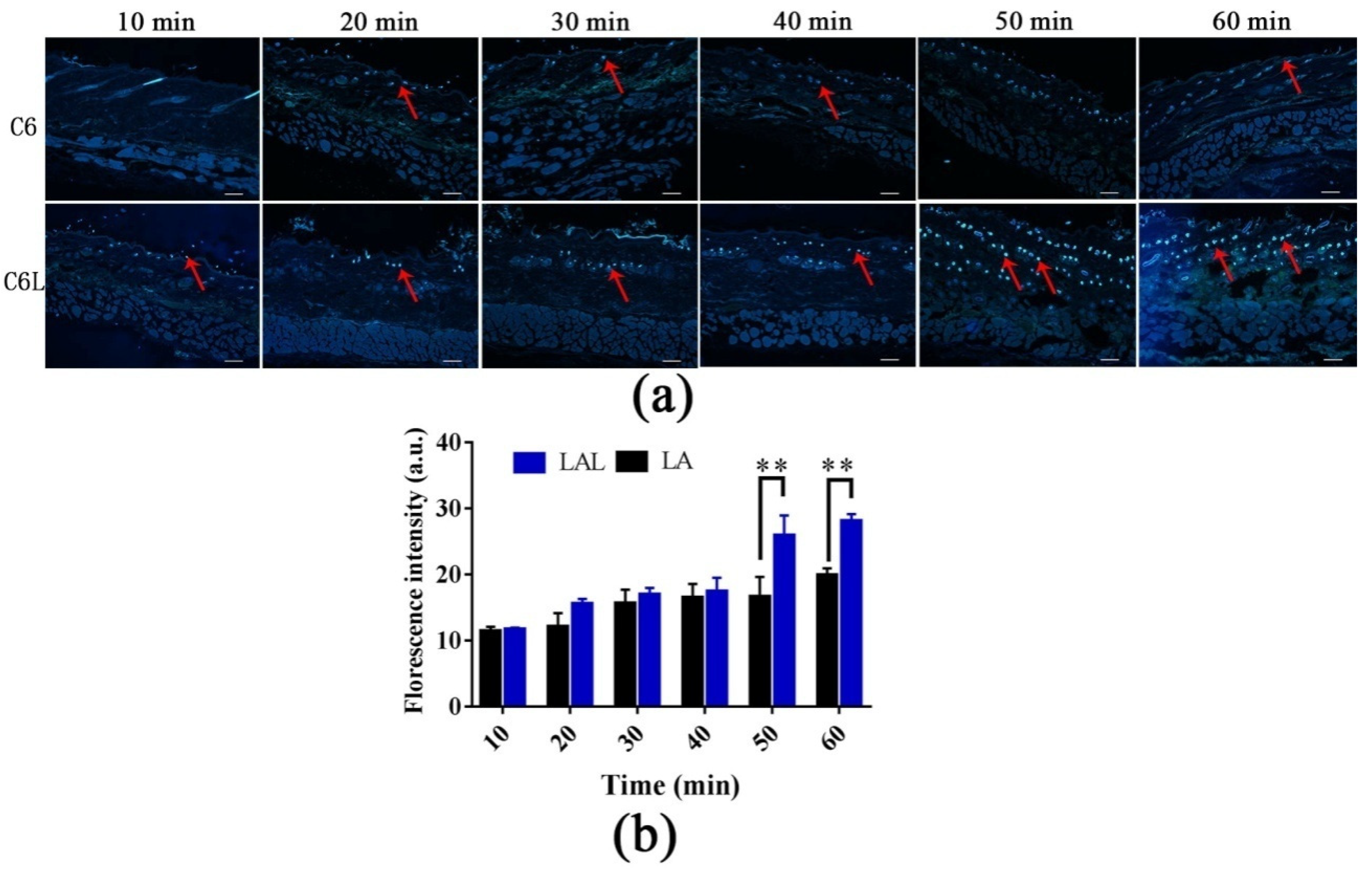
2.5. Skin Distribution of Nanoparticles
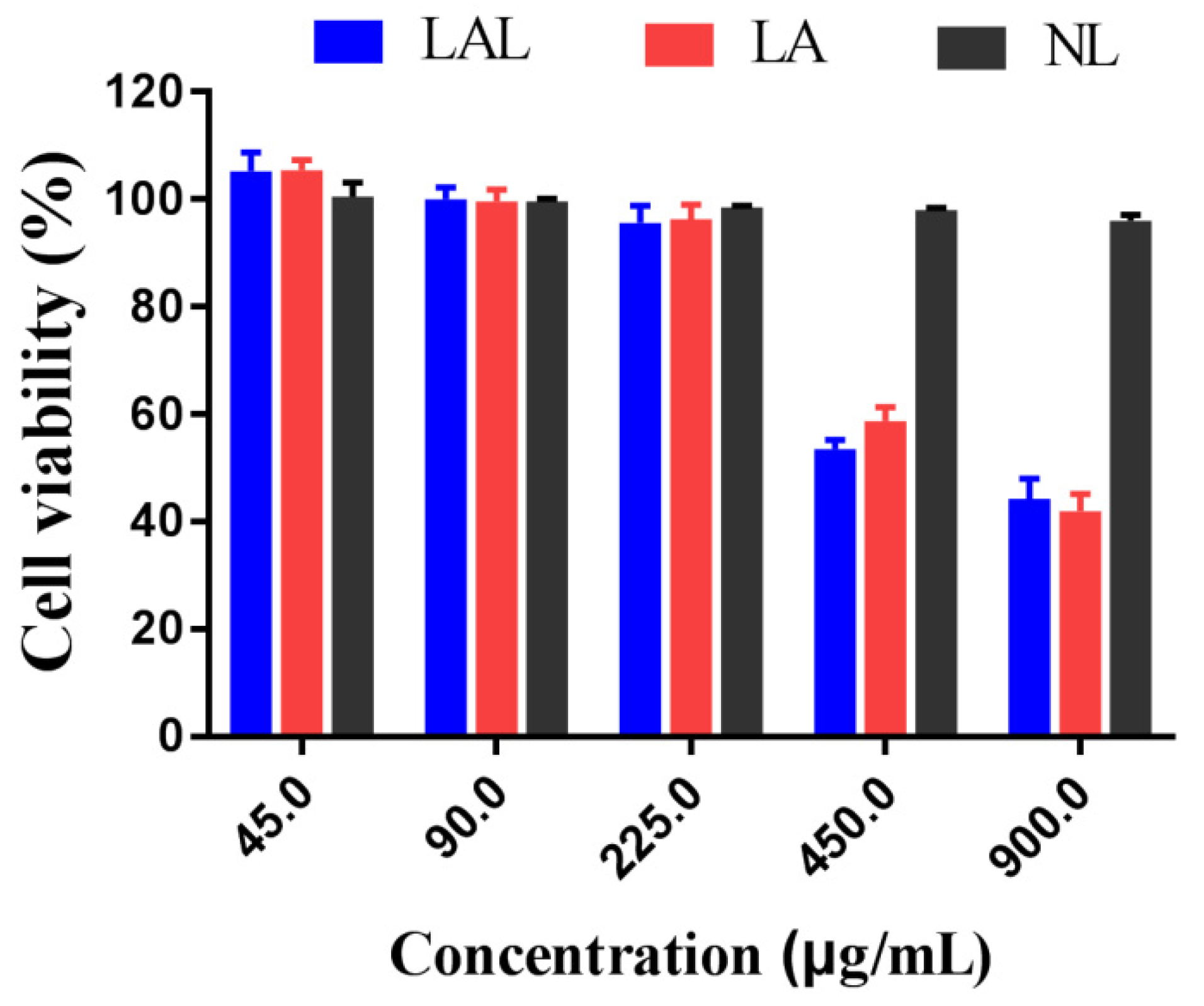
2.6. Cytotoxicity Experiments
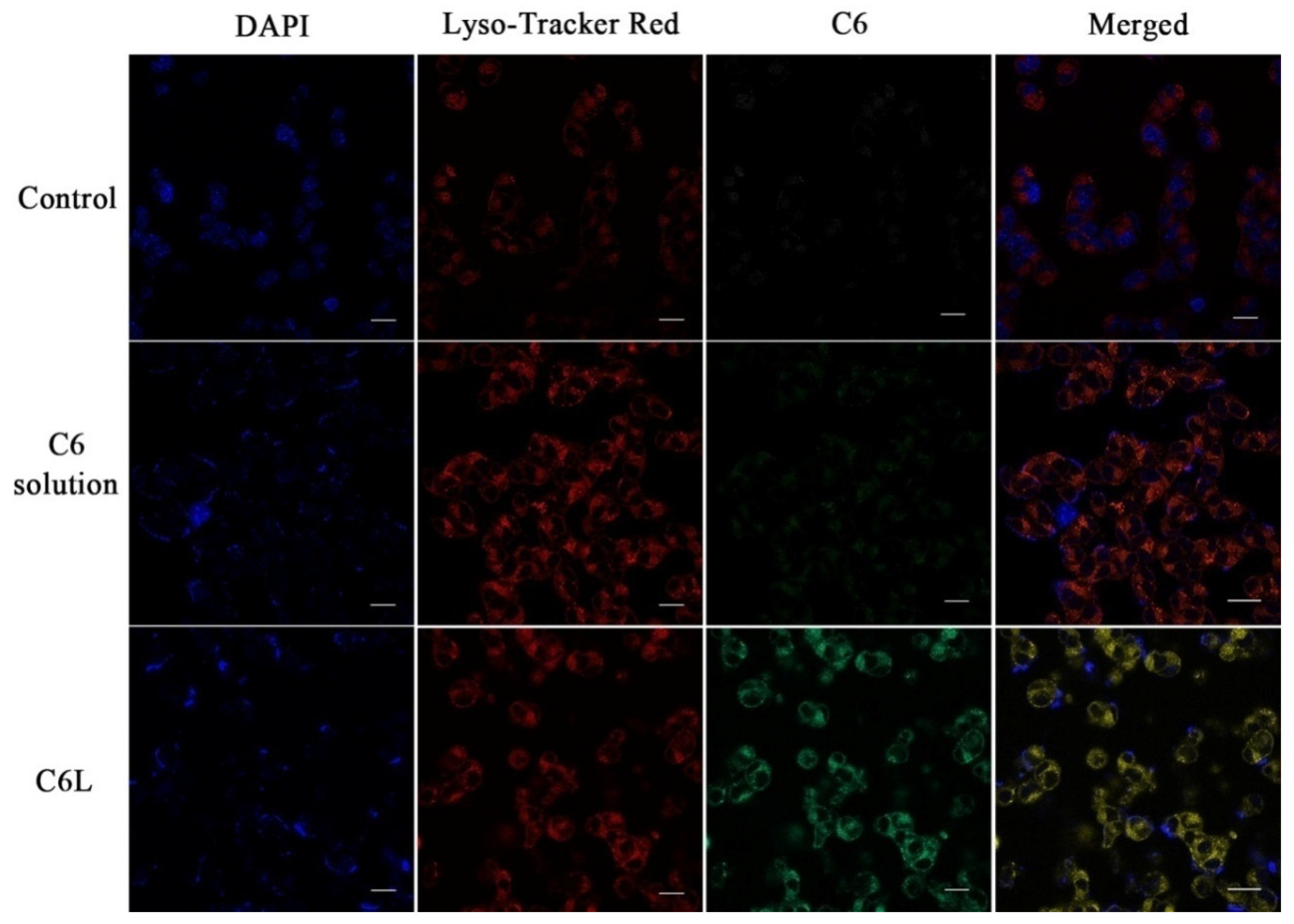
2.7. Intracellular Distribution of LAL Nanoparticles
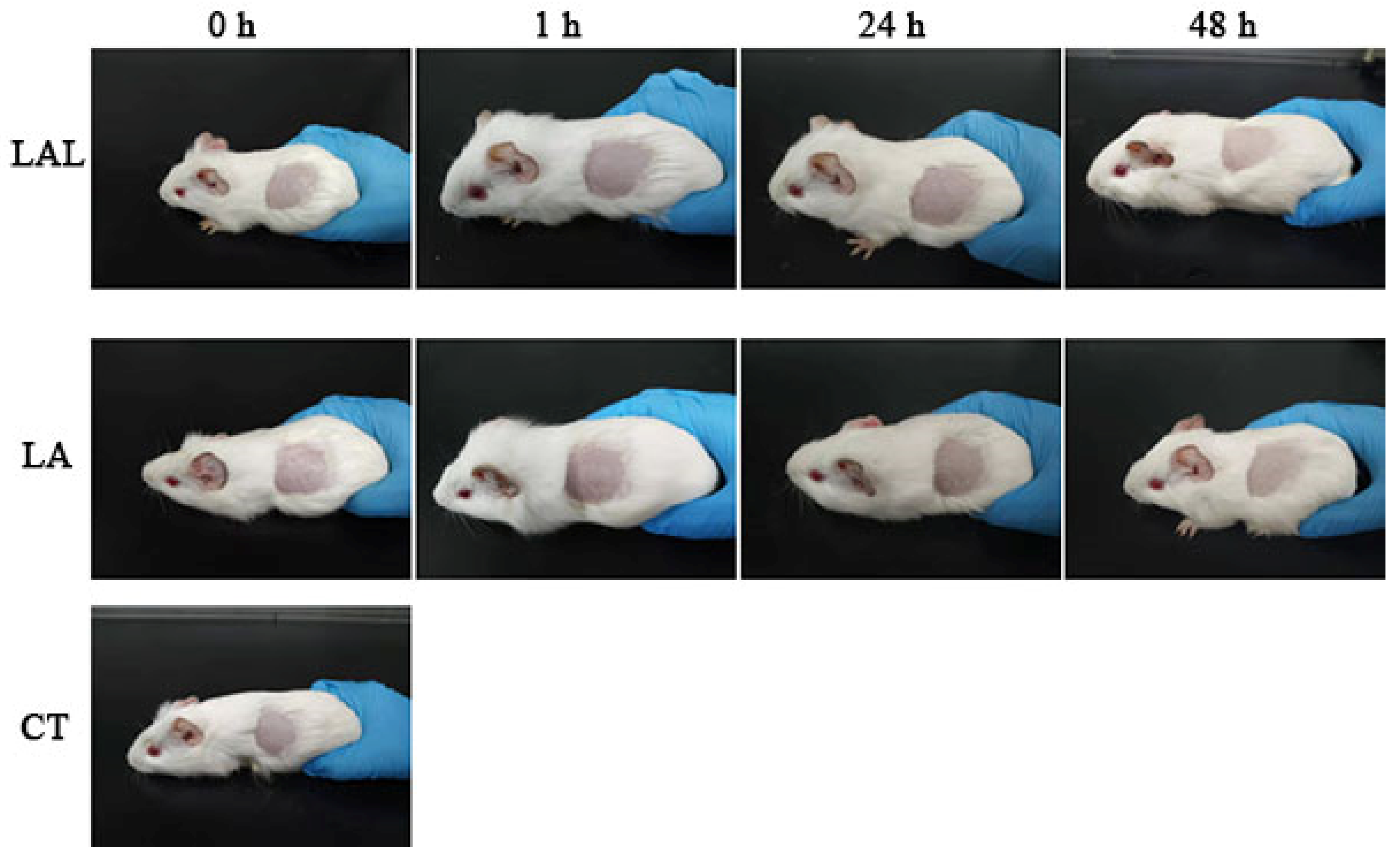
2.8. Skin Irritation
3. Materials and Methods
3.1. Materials
3.2. Quantification of LA
3.3. Optimizing the Preparation Condition of LAL
3.3.1. Preparation of LAL
3.3.2. Encapsulation Efficiency of the LALs
3.3.3. The Zeta Potential, Poly Dispersity Index (PdI), and Particle Size
3.4. In Vitro Permeation Studies of LALs
3.4.1. Skin Deposition Studies
3.4.2. In Vitro Dialysis Experiments
3.4.3. Differential Scanning Calorimetry (DSC)
3.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.5. Skin Distribution Analysis
3.4.6. Intracellular Distribution and Cellular Uptake of Lipomes
3.5. In Vitro Cytotoxicity Experiments
3.6. Skin Irritation
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, P.; Yu, C.; Zeng, F.S.; Fu, X.Y.; Yuan, X.J.; Wang, Q.; Fan, C.D.; Sun, B.L.; Sun, Q.S. Licochalcone A Attenuates Chronic Neuropathic Pain in Rats by Inhibiting Microglia Activation and Inflammation. Neurochem. Res. 2021, 46, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, K.S.; Squarisi, I.S.; Acesio, N.O.; Nicolella, H.D.; Ozelin, S.D.; de Melo, M.R.S.; Guissone, A.P.P.; Fernandes, G.; Silva, L.M.; da Silva, A.A.; et al. Licochalcone A, a licorice flavonoid: Antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J. Toxicol. Environ. Health-Part A-Curr. Issues 2020, 83, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.W.; Jiang, X.M.; Xu, Y.L.; Huang, M.Y.; Chen, Y.C.; Yu, W.B.; Su, M.X.; Ye, Z.H.; Chen, X.P.; Wang, Y.T.; et al. Licochalcone A inhibits interferon-gamma-induced programmed death-ligand 1 in lung cancer cells. Phytomedicine 2021, 80. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.H.; Fu, C.F.; Li, T.; Gao, Q.Q.; Miao, D.Y.; Xu, J.; Zhao, Y.Q. Hypoglycemic Effects of Licochalcone A on the Streptozotocin-Induced Diabetic Mice and Its Mechanism Study. J. Agric. Food Chem. 2021, 69, 2444–2456. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Du, Q.; Zhu, Z.; Chen, T.; Xue, Y.; Wang, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Pharmacological Effects and Underlying Mechanisms of Licorice-Derived Flavonoids. Evid. -Based Complementary Altern. Med. Ecam 2022, 2022, 9523071. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, Y.; Chen, T.; Du, Q.; Zhu, Z.; Wang, Y.; Wu, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Glycyrrhiza acid micelles loaded with licochalcone A for topical delivery: Co-penetration and anti-melanogenic effect. Eur. J. Pharm. Sci. 2021, 167, 106029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Xue, Y.Q.; Zhu, Z.M.; Hu, Y.; Zeng, Q.F.; Wu, Y.F.; Wang, Y.; Shen, C.Y.; Jiang, C.P.; Liu, L.; et al. Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel. Pharmaceutics 2022, 14, 262. [Google Scholar] [CrossRef]
- Hong, J.H.; Cao, S.W.; Xiang, S.J.; Ruan, S.F.; An, B.C.; Wang, Z.X.; Wu, W.F.; Chen, H.J.; Weng, L.D.; Zhang, L.; et al. Glycyrrhiza flavonoids and its major component, licochalcone A, inhibit melanogenesis through MAPK/ERIC pathway by activating ERIC phosphorylation. J. Dermatol. Sci. 2018, 91, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, L.; Xiang, S.; Jiang, C.; Wu, W.; Ruan, S.; Du, Q.; Chen, T.; Xue, Y.; Chen, H.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. AAPS PharmSciTech 2020, 21, 159. [Google Scholar] [CrossRef]
- Vovesna, A.; Zhigunov, A.; Balouch, M.; Zbytovska, J. Ceramide liposomes for skin barrier recovery: A novel formulation based on natural skin lipids. Int. J. Pharm. 2021, 596, 120264. [Google Scholar] [CrossRef]
- Guy, R.H. Transdermal drug delivery. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, pp. 399–410. [Google Scholar]
- Zhao, Z.M.; Ukidve, A.; Dasgupta, A.; Mitragotri, S. Transdermal immunomodulation: Principles, advances and perspectives. Adv. Drug Deliv. Rev. 2018, 127, 3–19. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Design of liposomal formulations for cell targeting. Colloids Surf. B-Biointerfaces 2015, 136, 514–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, T.; Chen, H.; Xiang, S.; Hong, J.; Cao, S.; Weng, L.; Zhang, L.; Liu, L.; Li, H.; Zhu, H.; et al. Cryptotanshinone-Loaded Cerasomes Formulation: In Vitro Drug Release, in Vivo Pharmacokinetics, and in Vivo Efficacy for Topical Therapy of Acne. ACS Omega 2016, 1, 1326–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, C.N.; Ding, M.L.; Zhang, C.Y.; Wang, W.; Cao, Z. Indocyanine green (ICG)-Menthol loaded cerasomal nanoparticles for ultrasound imaging and photothermal therapy against tumor. Mater. Lett. 2019, 255, 126524. [Google Scholar] [CrossRef]
- Carbone, C.; Arena, E.; Pepe, V.; Prezzavento, O.; Cacciatore, I.; Turkez, H.; Marrazzo, A.; Di Stefano, A.; Puglisi, G. Nanoencapsulation strategies for the delivery of novel bifunctional antioxidant/sigma 1 selective ligands. Colloids Surf. B-Biointerfaces 2017, 155, 238–247. [Google Scholar] [CrossRef]
- Guo, D.Y.; Liu, J.; Fan, Y.; Cheng, J.X.; Shi, Y.J.; Zou, J.B.; Zhang, X.F. Optimization, characterization and evaluation of liposomes from Malus hupehensis (Pamp.) Rehd. extracts. J. Liposome Res. 2020, 30, 366–376. [Google Scholar] [CrossRef]
- Souto, E.B.; Macedo, A.S.; Dias-Ferreira, J.; Cano, A.; Zielinska, A.; Matos, C.M. Elastic and Ultradeformable Liposomes for Transdermal Delivery of Active Pharmaceutical Ingredients (APIs). Int. J. Mol. Sci. 2021, 22, 9743. [Google Scholar] [CrossRef]
- Bondi, M.L.; Craparo, E.F.; Giammona, G.; Cervello, M.; Azzolina, A.; Diana, P.; Martorana, A.; Cirrincione, G. Nanostructured lipid carriers-containing anticancer compounds: Preparation, characterization, and cytotoxicity studies. Drug Deliv. 2007, 14, 61–67. [Google Scholar] [CrossRef]
- Smejkalova, D.; Muthny, T.; Nesporova, K.; Hermannova, M.; Achbergerova, E.; Huerta-Angeles, G.; Svoboda, M.; Cepa, M.; Machalova, V.; Luptakova, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Kasetvatin, C.; Rujivipat, S.; Tiyaboonchai, W. Combination of elastic liposomes and low frequency ultrasound for skin permeation enhancement of hyaluronic acid. Colloids Surf. B-Biointerfaces 2015, 135, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Yang, M.; Tang, X.M.; Wang, T.; Yang, D.S.; Zhai, G.X.; Liu, J.Y. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vavrova, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef]
- Gonullu, U.; Uner, M.; Yener, G.; Karaman, E.F.; Aydogmus, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Salimi, A.; Hedayatipour, N.; Moghimipour, E. The Effect of Various Vehicles on the Naproxen Permeability through Rat Skin: A Mechanistic Study by DSC and FT-IR Techniques. Adv. Pharm. Bull. 2016, 6, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Vitorino, C.; Taylor, K.M.G. How can lipid nanocarriers improve transdermal delivery of olanzapine? Pharm. Dev. Technol. 2017, 22, 587–596. [Google Scholar] [CrossRef]
- Kasongo, K.W.; Pardeike, J.; Muller, R.H.; Walker, R.B. Selection and Characterization of Suitable Lipid Excipients for use in the Manufacture of Didanosine-Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Pharm. Sci. 2011, 100, 5185–5196. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Shafiq, S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J. Nanobiotechnol. 2008, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xue, Y.; Zeng, Q.; Zhu, Z.; Wang, Y.; Wu, Y.; Shen, C.; Zhu, H.; Jiang, C.; Liu, L.; et al. Glycyrrhiza acid-Licochalcone A complexes for enhanced bioavailability and anti-melanogenic effect of Licochalcone A: Cellular uptake and in vitro experiments. J. Drug Deliv. Sci. Technol. 2022, 68, 103037. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sci. 2013, 92, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Yang, Z.L.; Luo, J.B.; Zhu, Q.H.; Zhao, H.N. Effects of cinnamene enhancers on transdermal delivery. of ligustrazine hydrochloride. Eur. J. Pharm. Biopharm. 2007, 67, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Huang, Z.; Zuo, T.T.; Lu, Q.; Wu, G.Y.; Shen, Q. Reducing Interstitial Fluid Pressure and Inhibiting Pulmonary Metastasis of Breast Cancer by Gelatin Modified Cationic Lipid Nanoparticles. Acs Appl. Mater. Interfaces 2017, 9, 29457–29468. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thomas, N.S.; Panchagnula, R. Transdermal drug delivery of imipramine hydrochloride. I. Effect of terpenes. J. Control. Release Off. J. Control. Release Soc. 2002, 79, 93–101. [Google Scholar] [CrossRef]
- Li, Y.P.; Hong, W.Y.; Zhang, H.N.; Zhang, T.T.; Chen, Z.Y.; Yuan, S.L.; Peng, P.; Xiao, M.; Xu, L. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control. Release 2020, 317, 232–245. [Google Scholar] [CrossRef]
| Sample | Lipids | Keratin | Keratin | |||
|---|---|---|---|---|---|---|
| VasCH2 | VsCH2 | Amide I | Amide II | Melting Temperature (°C) | Enthaly (J/g) | |
| LA | 2923.12 | 2856.22 | 1585.93 | 1531.20 | 120.71 | 322.13 |
| LAL | 2928.43 | 2859.27 | 1584.04 | 1535.92 | 115.24 | 351.60 |
| NL | 2925.68 | 2856.14 | 1577.66 | 1542.62 | 111.65 | 336.78 |
| CT | 2922.36 | 2858.31 | 1588.15 | 1518.49 | 118.96 | 335.39 |
| Occurrence Rate (%) | Level | Strength | Skin Irritation Definition |
|---|---|---|---|
| 0–8 | I | None | No erythema and no hydroderma |
| 9–28 | II | Mild | Mild erythema and mild hydroderma (barely visible) |
| 29–64 | III | Moderate | Moderate erythema and moderate hydroderma (obviously raised) |
| 65–80 | IV | Severe | Severe erythema and severe hydroderma (the skin humps 1 mm with the outline clear) |
| 81–100 | V | Extreme | Purplish red erythema or mild eschar hydroderma, extreme hydroderma (skin humps of more than 1 mm with the outline expansile) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Wang, Z.; Wu, Y.; Wu, H.; Chen, T.; Xue, Y.; Wang, Y.; Jiang, C.; Shen, C.; Liu, L.; et al. Mechanisms of Penetration Enhancement and Transport Utilizing Skin Keratine Liposomes for the Topical Delivery of Licochalcone A. Molecules 2022, 27, 2504. https://doi.org/10.3390/molecules27082504
Wu W, Wang Z, Wu Y, Wu H, Chen T, Xue Y, Wang Y, Jiang C, Shen C, Liu L, et al. Mechanisms of Penetration Enhancement and Transport Utilizing Skin Keratine Liposomes for the Topical Delivery of Licochalcone A. Molecules. 2022; 27(8):2504. https://doi.org/10.3390/molecules27082504
Chicago/Turabian StyleWu, Wenfeng, Zhuxian Wang, Yufan Wu, Huiyi Wu, Tingting Chen, Yaqi Xue, Yuan Wang, Cuiping Jiang, Chunyan Shen, Li Liu, and et al. 2022. "Mechanisms of Penetration Enhancement and Transport Utilizing Skin Keratine Liposomes for the Topical Delivery of Licochalcone A" Molecules 27, no. 8: 2504. https://doi.org/10.3390/molecules27082504
APA StyleWu, W., Wang, Z., Wu, Y., Wu, H., Chen, T., Xue, Y., Wang, Y., Jiang, C., Shen, C., Liu, L., Zhu, H., & Liu, Q. (2022). Mechanisms of Penetration Enhancement and Transport Utilizing Skin Keratine Liposomes for the Topical Delivery of Licochalcone A. Molecules, 27(8), 2504. https://doi.org/10.3390/molecules27082504





