Effects of Sphingomyelin-Containing Milk Phospholipids on Skin Hydration in UVB-Exposed Hairless Mice
Abstract
:1. Introduction
2. Results
2.1. Effects of Milk Phospholipids on Body Weight Changes and Plasma Biochemical Parameter
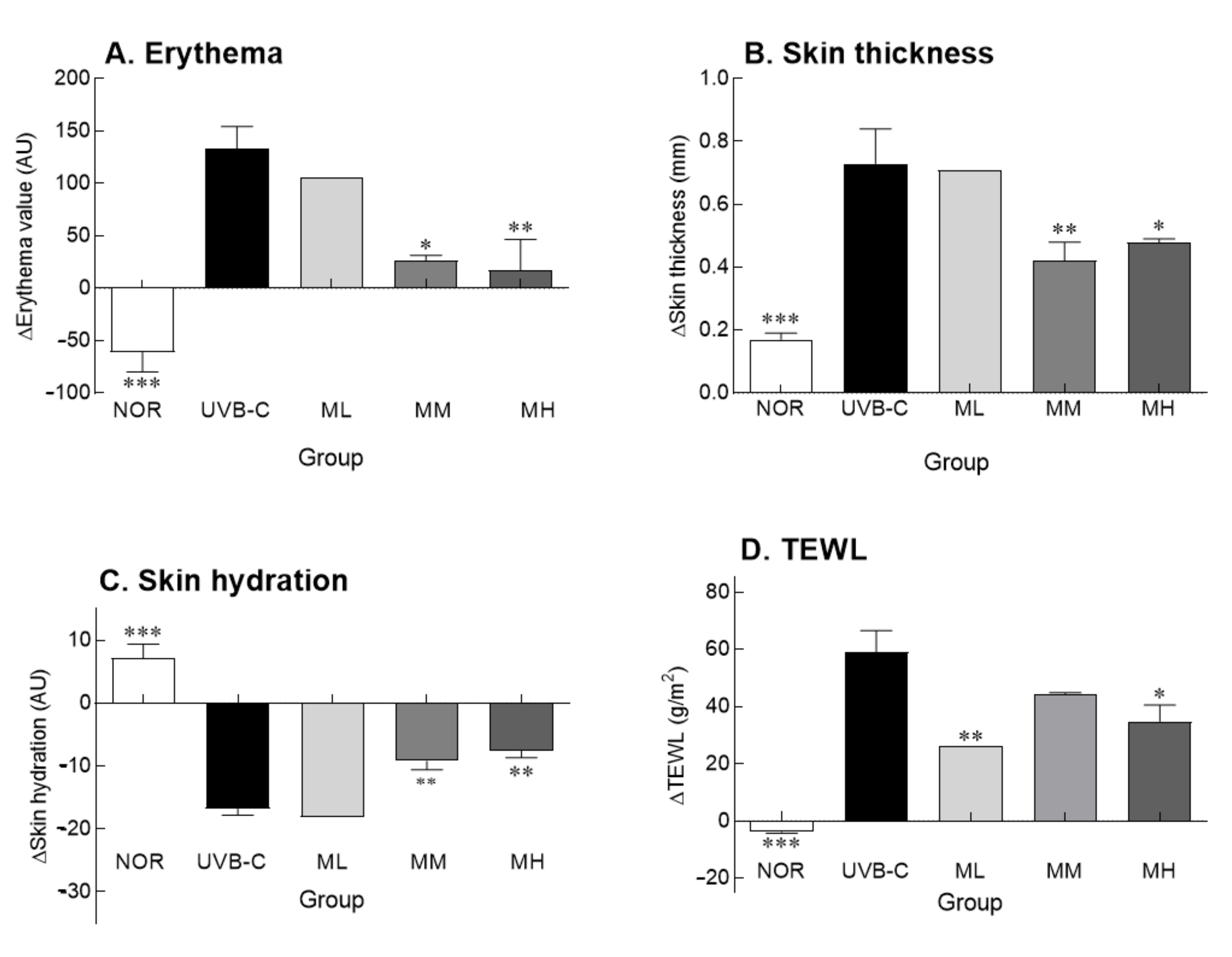
2.2. Effects of Milk Phospholipids on Skin Parameters
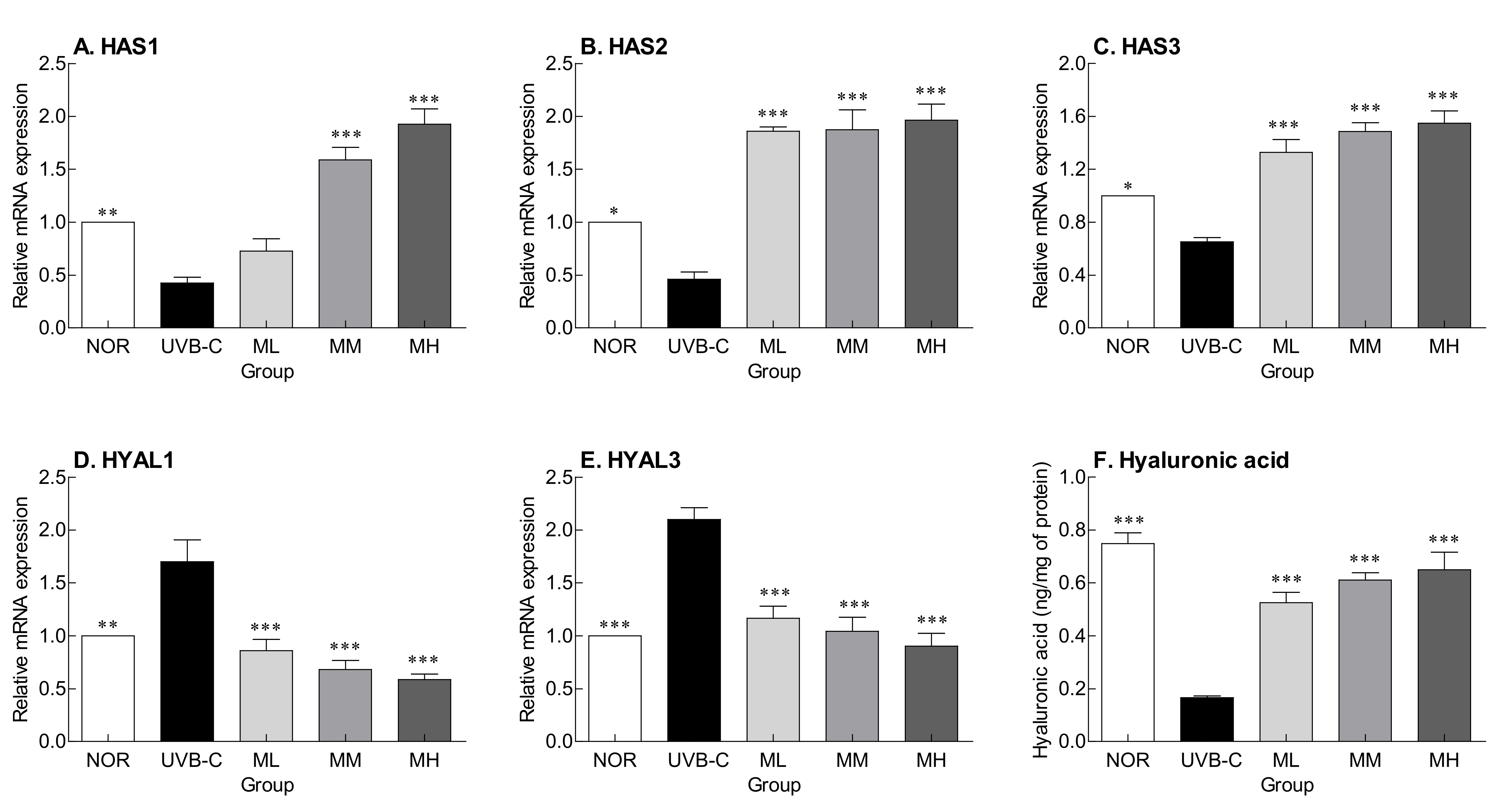
2.3. Effects of Milk Phospholipids on HA Synthesis and Degradation
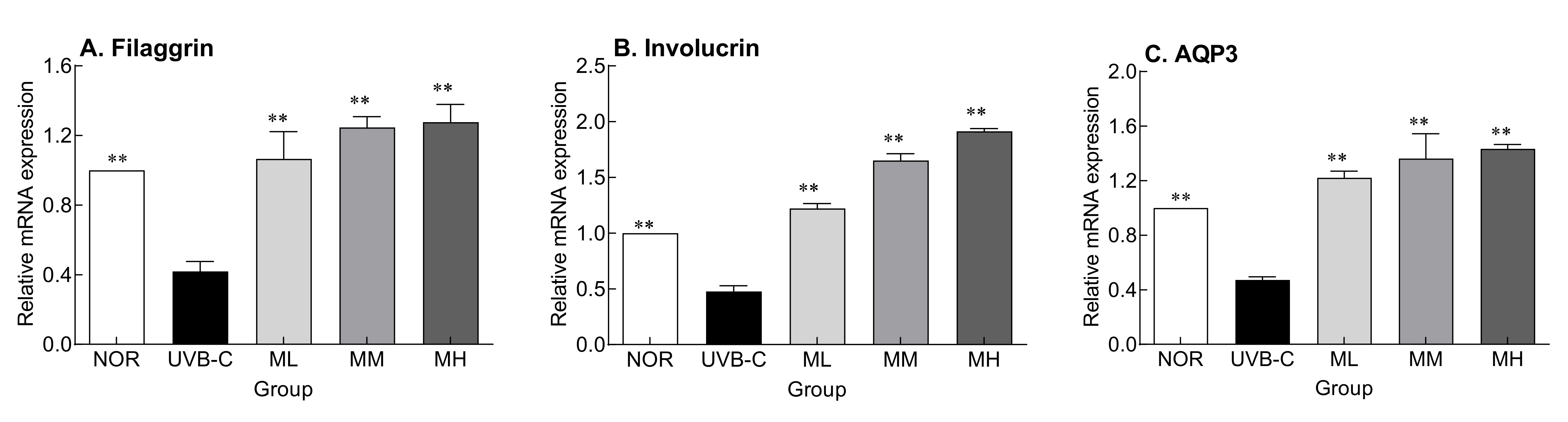
2.4. Effects of Milk Phospholipids on the Expression of Skin Moisture-Related Factors
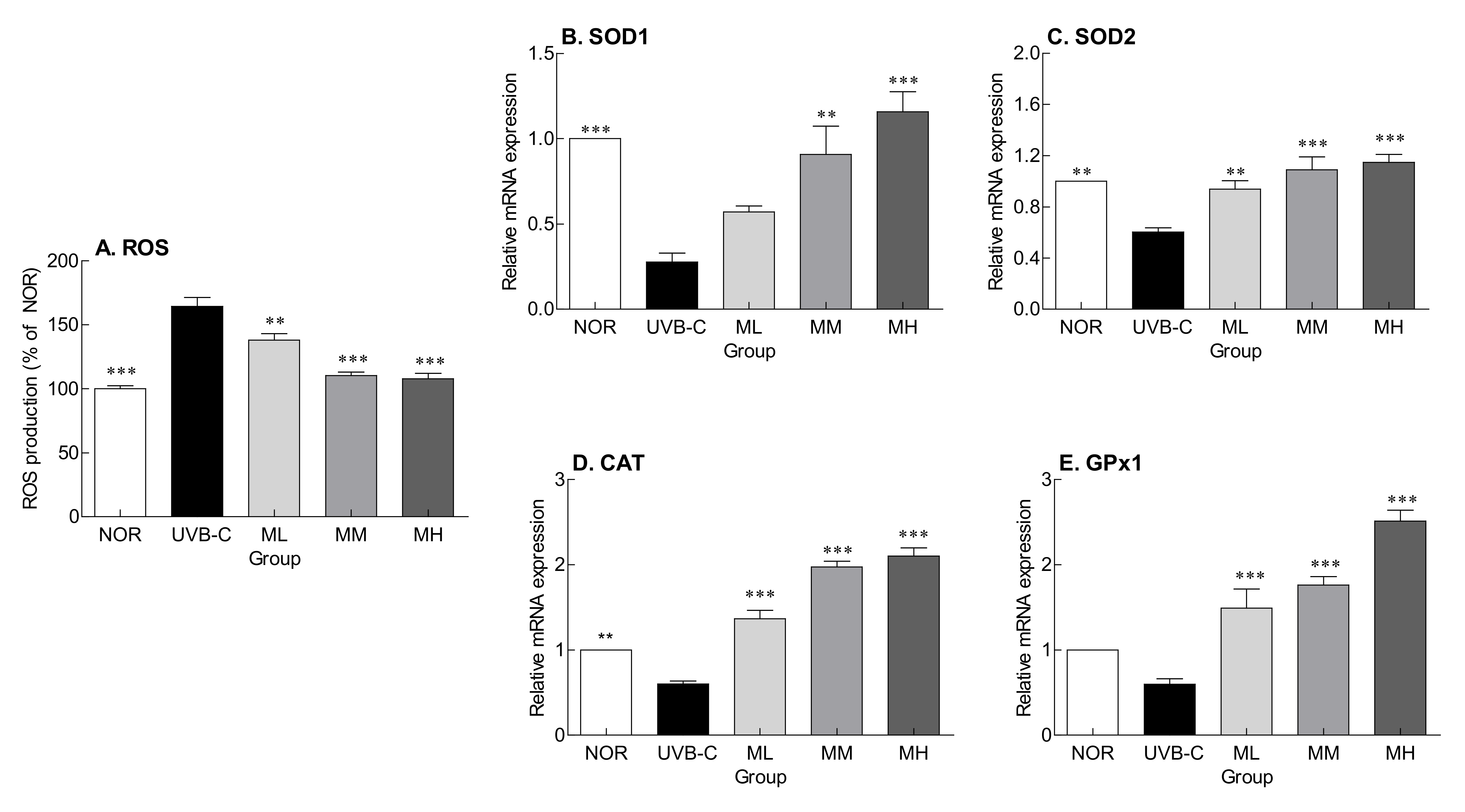
2.5. Effects of Milk Phospholipids on ROS Production and Expression of Genes Encoding Antioxidant Enzymes
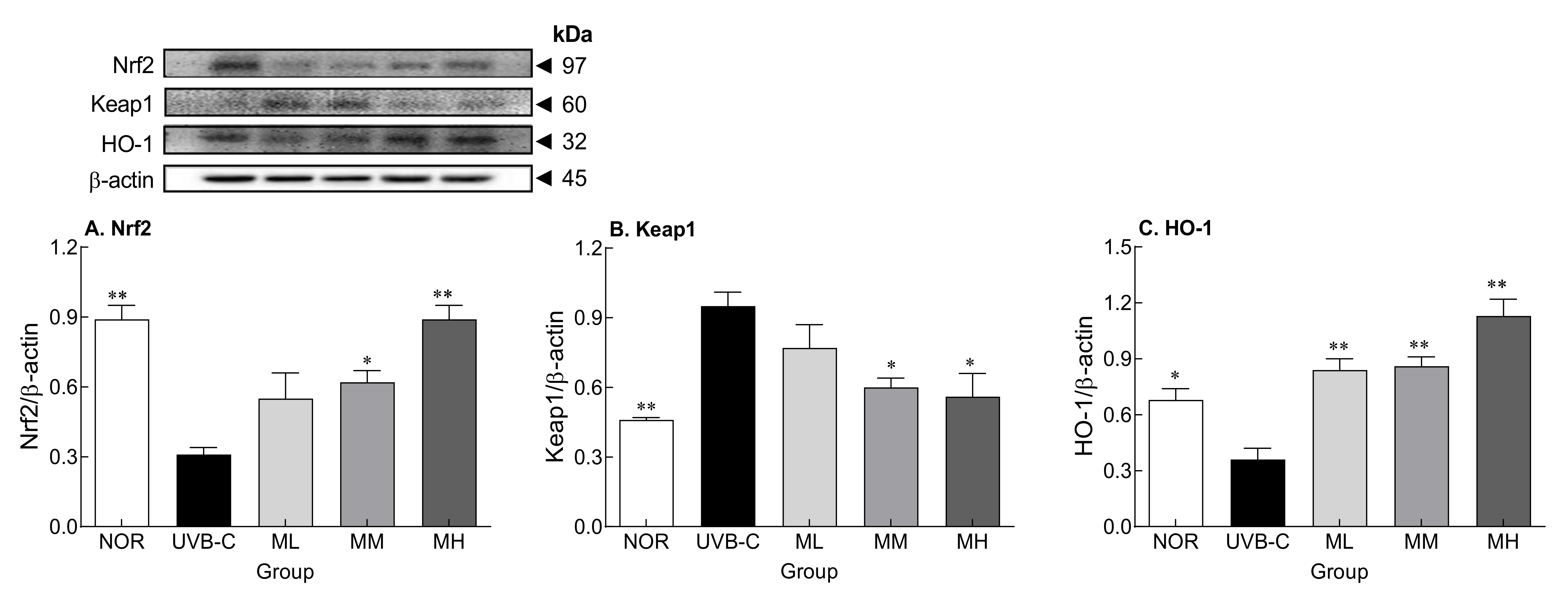
2.6. Effects of Milk Phospholipids on Nrf2-Keap1-Related Protein Expression
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Measurement of Skin Parameters
4.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.4. Measurement of Protein Expression by Western Blot Analysis
4.5. Measurement of ROS
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Parke, M.A.; Perez-Sanchez, A.; Zamil, D.H.; Katta, R. Diet and skin barrier: The role of dietary interventions on skin barrier function. Dermatol. Pract. Concept 2021, 11, e2021132. [Google Scholar] [CrossRef] [PubMed]
- Fahad, D.; Mohammed, M.T. Oxidative stress: Implications on skin diseases. Plant Arch. 2020, 20, 4150–4157. [Google Scholar]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmetic Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chavoshnejad, P.; Foroughi, A.H.; Dhandapani, N.; German, G.K.; Razavi, M.J. Effect of collagen degradation on the mechanical behavior and wrinkling of skin. Phys. Rev. E 2021, 104, 034406. [Google Scholar] [CrossRef]
- Jang, H.H.; Lee, S.N.; Jang, H.H.; Lee, S.N. Epidermal skin barrier. Asian J. Beauty Cosmetol. 2016, 14, 339–347. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthet. Dermatol. 2011, 4, 22. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Structural characteristics of the aging skin: A review. Cutan. Ocul. Toxicol. 2007, 26, 343–357. [Google Scholar] [CrossRef]
- Lipozencić, J.; Pastar, Z.; Marinović-Kulisić, S. Moisturizers. Acta Dermatovenerol. Croat. 2006, 14, 104–108. [Google Scholar]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, A.; Hong, K.B.; Suh, H.J.; Jo, K. Preparation and characterization of a polar milk lipid-enriched component from whey powder. Food Sci. Anim. Resour. 2020, 40, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parodi, P.W. Cows’ milk fat components as potential anticarcinogenic agents. J. Nutr. 1997, 127, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S.; Kim, A.; Suh, H.J.; Hong, K.B. Sphingolipid identification and skin barrier recovery capacity of a milk sphingolipid-enriched fraction (MSEF) from buttermilk powder. Int. J. Cosmet. Sci. 2020, 42, 270–276. [Google Scholar] [CrossRef]
- Oh, M.J.; Nam, J.J.; Lee, E.O.; Kim, J.W.; Park, C.S. A synthetic C16 omega-hydroxyphytoceramide improves skin barrier functions from diversely perturbed epidermal conditions. Arch. Dermatol. Res. 2016, 308, 563–574. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Ahn, G. Fucoidan refined by Sargassum confusum indicate protective effects suppressing photo-oxidative stress and skin barrier perturbation in UVB-induced human keratinocytes. Int. J. Biol. Macromol. 2020, 164, 149–161. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. -Endocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.W.; Rhim, D.B.; Kim, C.; Hwang, J.K. Effect of standardized boesenbergia pandurata extract and its active compound panduratin a on skin hydration and barrier function in human epidermal keratinocytes. Prev. Nutr. Food Sci. 2015, 20, 15–21. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Cheong, Y.; Kim, C.; Kim, M.B.; Hwang, J.K. The anti-photoaging and moisturizing effects of Bouea macrophylla extract in UVB-irradiated hairless mice. Food Sci. Biotechnol. 2018, 27, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: Holistic approach to its origin and functions. Br. J. Dermatol. 2008, 159, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, H.; Hu, X.; Chen, M.; Xie, H. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas. J. Dermatol. 2010, 51, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, H.I.; Hwang, J.K. Oral administration of fingerroot (Boesenbergia pandurata) extract reduces ultraviolet b-induced skin aging in hairless mice. Food Sci. Biotechnol. 2012, 21, 1753–1760. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, M.G.; Choi, Y.J.; Lee, S.J.; Suh, H.J.; Jo, K. Photoprotective effects of sphingomyelin-containing milk phospholipids in ultraviolet B-irradiated hairless mice by suppressing nuclear factor-kappaB expression. J. Dairy Sci. 2022, 105, 1929–1939. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Shen, X.; Guo, M.; Yu, H.; Liu, D.; Lu, Z.; Lu, Y. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci. Biotechnol. Biochem. 2019, 83, 561–568. [Google Scholar] [CrossRef]
- Haiyuan, Y.U.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The protective effects of beta-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Cienc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Agren, U.M.; Tammi, R.H.; Tammi, M.I. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic. Biol. Med. 1997, 23, 996–1001. [Google Scholar] [CrossRef]
- Wat, E.; Tandy, S.; Kapera, E.; Kamili, A.; Chung, R.W.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar] [CrossRef]
- Bissett, D.L.; Hannon, D.P.; Orr, T.V. An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem. Photobiol. 1987, 46, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Oba, C.; Morifuji, M.; Ichikawa, S.; Ito, K.; Kawahata, K.; Yamaji, T.; Asami, Y.; Itou, H.; Sugawara, T. Dietary milk sphingomyelin prevents disruption of skin barrier function in hairless mice after UV-B irradiation. PLoS ONE 2015, 10, e0136377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Assaf, S.; Navaratnam, S.; Parsons, B.J.; Phillips, G.O. Chain scission of hyaluronan by peroxynitrite. Arch. Biochem. Biophys. 2003, 411, 73–82. [Google Scholar] [CrossRef]
- Kennett, E.C.; Davies, M.J. Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: Evidence for a hydroxyl-radical-like mechanism. Free Radic. Biol. Med. 2007, 42, 1278–1289. [Google Scholar] [CrossRef]
- Kim, S.-H.; Nam, G.-W.; Kang, B.-Y.; Lee, H.-K.; Moon, S.-J.; Chang, I.-S. The effect of kaempferol, guercetin on hyaluronan-synthesis stimulation in human keratinocytes (HaCaT). J. Soc. Cosmet. Sci. Korea 2005, 31, 97–102. [Google Scholar]
- Ghersetich, I.; Lotti, T.; Campanile, G.; Grappone, C.; Dini, G. Hyaluronic acid in cutaneous intrinsic aging. Int. J. Dermatol. 1994, 33, 119–122. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Derm. -Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [Green Version]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci 2021, 22, 10799. [Google Scholar] [CrossRef]
- Davies, K.J.A. Protein damage and degradation by oxygen radicals.1. General-aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [CrossRef]
- Podda, M.; Traber, M.G.; Weber, C.; Yan, L.-J.; Packer, L. UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Won, J.S.; Singh, I. Sphingolipid signaling and redox regulation. Free Radic. Biol. Med. 2006, 40, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment. Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway an indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Elias, P.M.; Oda, Y.; Mackenzie, D.; Mauro, T.; Holleran, W.M.; Uchida, Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin d receptor-independent pathway. J. Biol. Chem. 2011, 286, 34121–34130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gupta, R.; Dubey, D.K.; Kannan, G.M.; Flora, S.J.S. Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol. Int. 2007, 31, 44–56. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Kim, M.G.; Jo, K.; Hong, K.-B.; Suh, H.J. Effects of Sphingomyelin-Containing Milk Phospholipids on Skin Hydration in UVB-Exposed Hairless Mice. Molecules 2022, 27, 2545. https://doi.org/10.3390/molecules27082545
Ahn Y, Kim MG, Jo K, Hong K-B, Suh HJ. Effects of Sphingomyelin-Containing Milk Phospholipids on Skin Hydration in UVB-Exposed Hairless Mice. Molecules. 2022; 27(8):2545. https://doi.org/10.3390/molecules27082545
Chicago/Turabian StyleAhn, Yejin, Min Guk Kim, Kyungae Jo, Ki-Bae Hong, and Hyung Joo Suh. 2022. "Effects of Sphingomyelin-Containing Milk Phospholipids on Skin Hydration in UVB-Exposed Hairless Mice" Molecules 27, no. 8: 2545. https://doi.org/10.3390/molecules27082545
APA StyleAhn, Y., Kim, M. G., Jo, K., Hong, K. -B., & Suh, H. J. (2022). Effects of Sphingomyelin-Containing Milk Phospholipids on Skin Hydration in UVB-Exposed Hairless Mice. Molecules, 27(8), 2545. https://doi.org/10.3390/molecules27082545






