A Molecular Study of Aspirin and Tenofovir Using Gold/Dextran Nanocomposites and Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Results
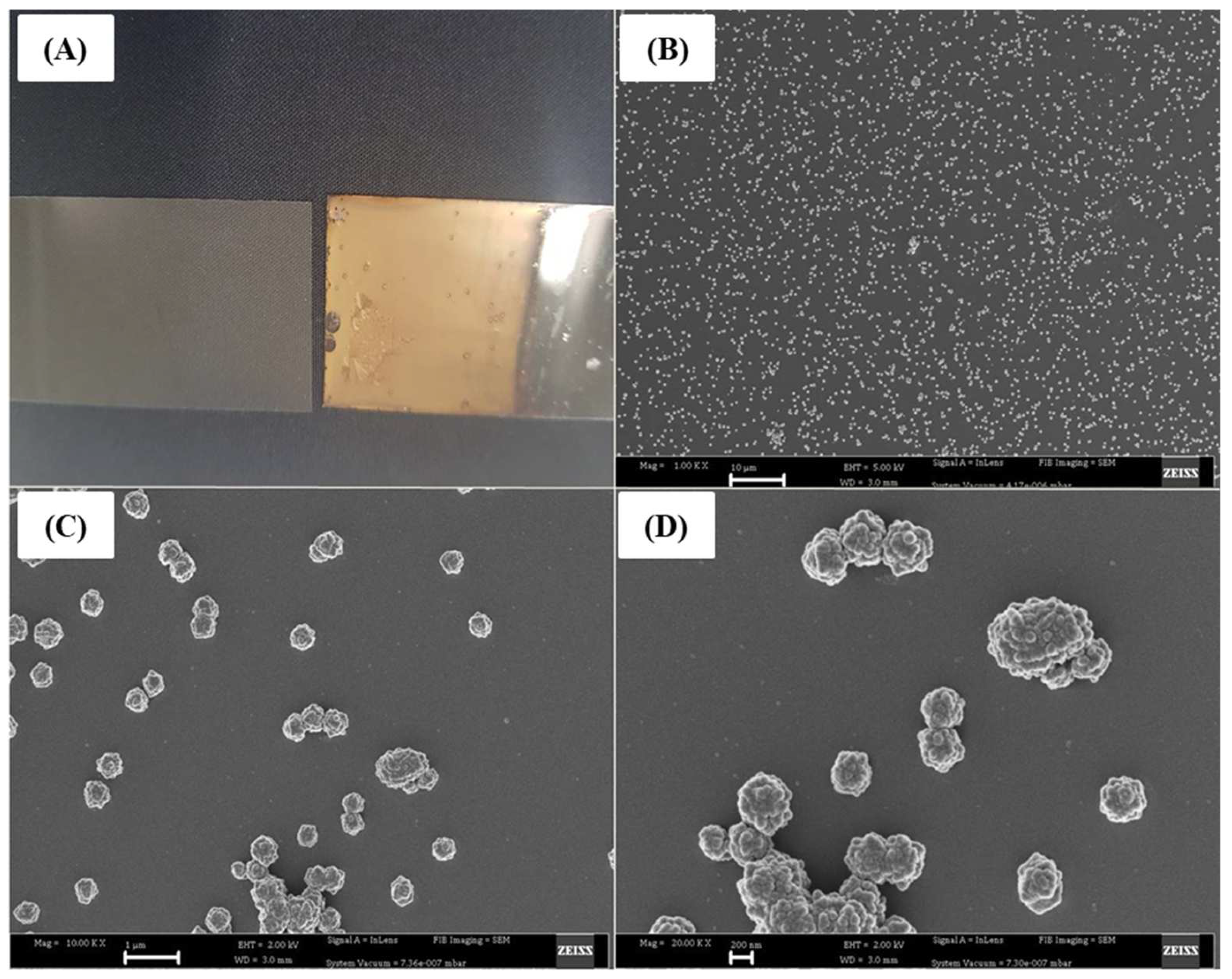
2.1. SEM of Gold-Coated Glass Slides Using Self-Assembly Method
2.2. UV-Vis Spectroscopy of Au/Dextran Nanocomposites
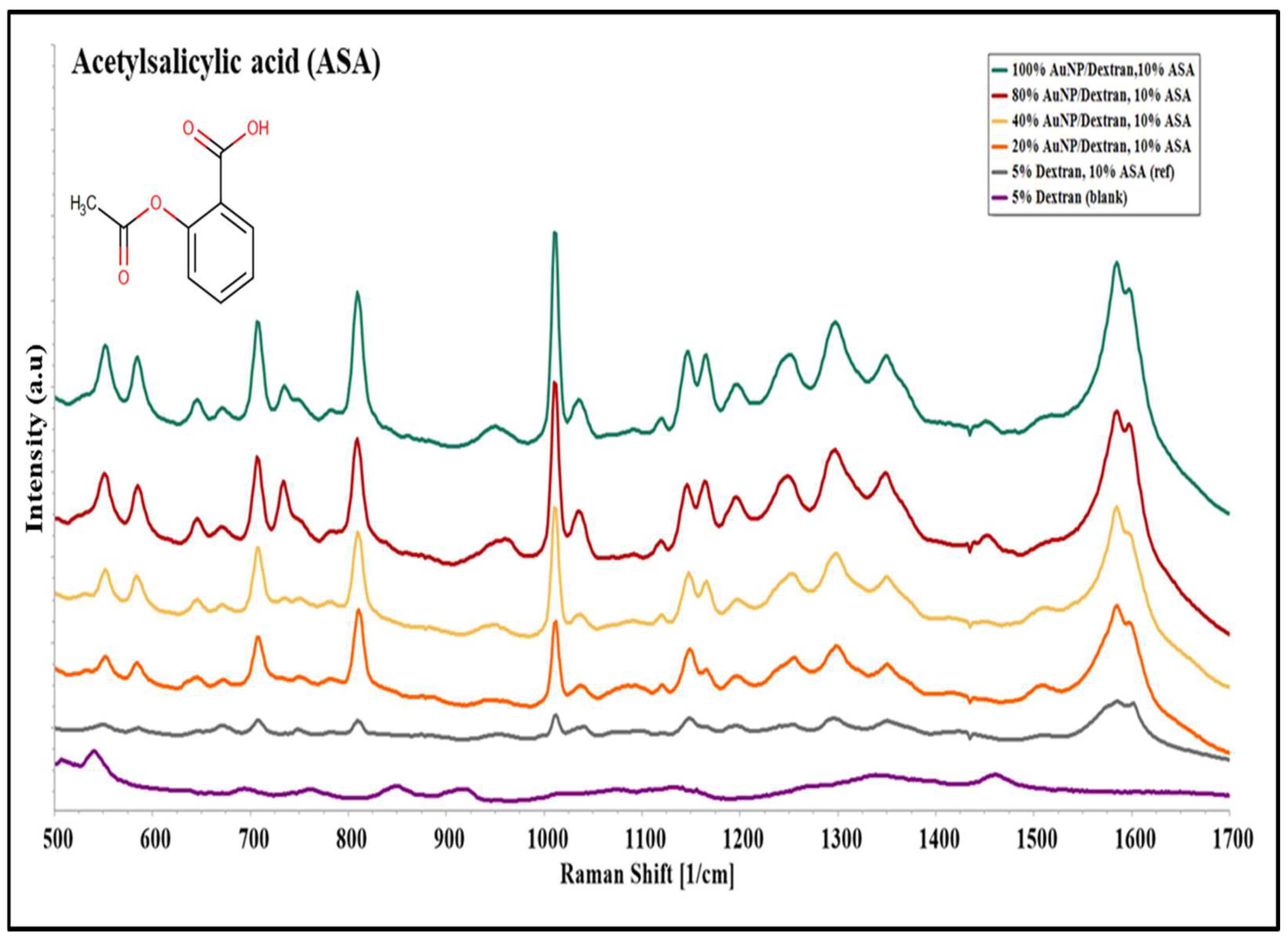
2.3. Surface Enhanced Raman Spectroscopy on Acetylsalicylic Acid (ASA)
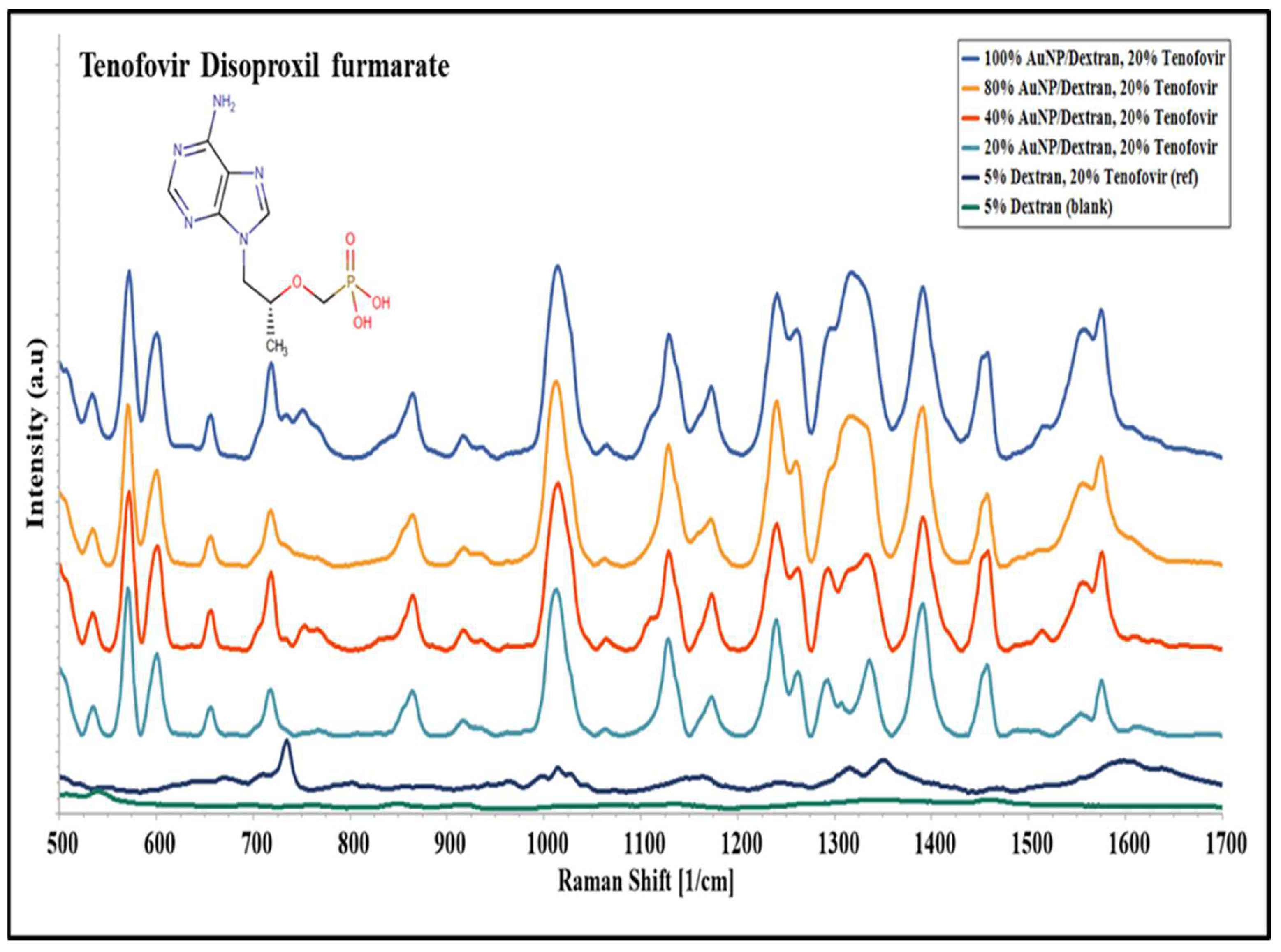
2.4. Surface Enhanced Raman Spectroscopy on Tenofovir Disoproxil Fumarate
2.5. Qualitative and Statistical Analysis of SERS Peaks on ASA
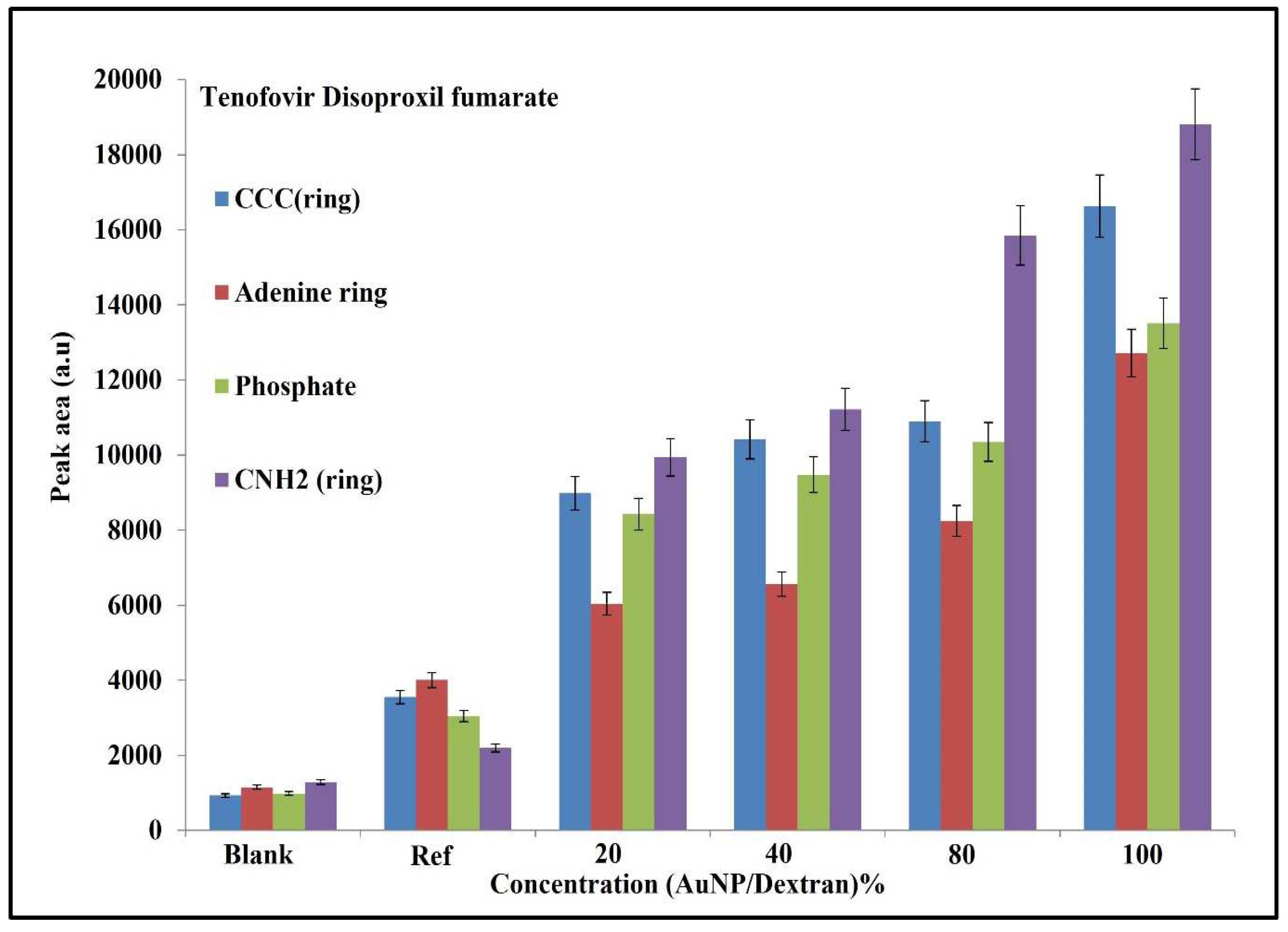
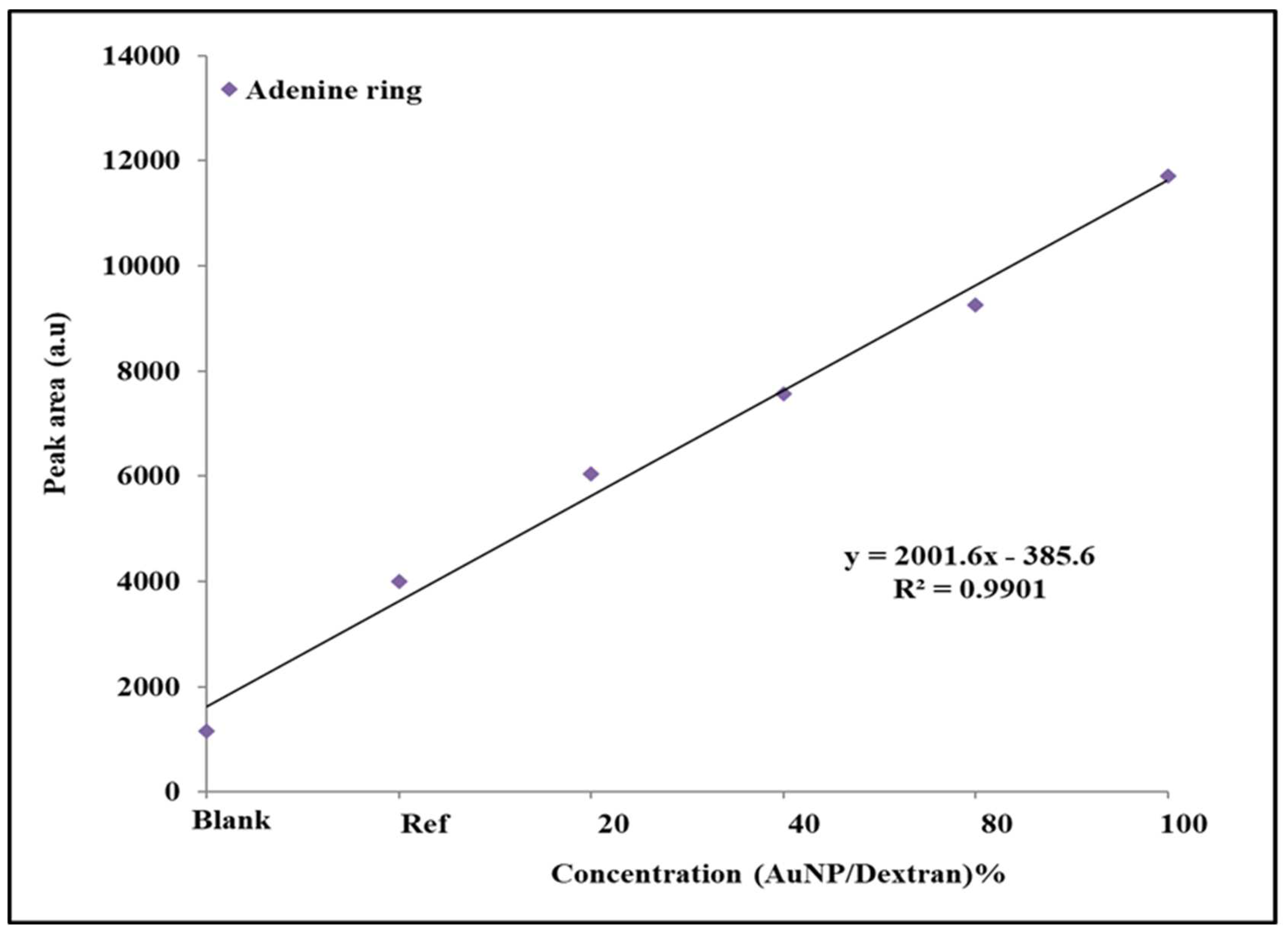
2.6. Qualitative and Statistical Analysis of SERS Peaks on Tenofovir
3. Discussion
3.1. Molecular Evaluation of Acetylsalicylic Acid
3.2. Molecular Evaluation of Tenofovir
4. Materials and Methods
4.1. Coating Gold Nanoparticles on Glass Using the Self-Assembly Method
4.2. Synthesis of Gold/Dextran (DEAE) Nanocomposites
4.3. Sample Preparation and SERS Signal Acquisition
4.4. Spectral Processing and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skoog, A.; Holler, J.; Crouch, S. Principles of Instrumental Analysis; ISE: Balmont, CA, USA, 2007. [Google Scholar]
- Chen, S.; Lin, X.; Yuen, C.; Padmanabhan, S.; Beuerman, R.W.; Liu, Q. Recovery of Raman spectra with low signal-to-noise ratio using Wiener estimation. Opt. Express 2014, 22, 12102. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced Raman spectroscopy–based biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.; Kim, N.J.; Kim, H.; Yi, G.C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of Chemical Enhancement Mechanism in Non-plasmonic Surface Enhanced Raman Spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- Dab, C.; Thomas, R.; Ruediger, A. Design of a Plasmonic Platform to Improve the SERS Sensitivity for Molecular Detection. Biosensors 2020, 10, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Fleger, Y.; Rosenbluh, M. Surface Plasmons and Surface Enhanced Raman Spectra of Aggregated and Alloyed Gold-Silver Nanoparticles. Res. Lett. Opt. 2009, 2009, 475941. [Google Scholar] [CrossRef] [Green Version]
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. R. Soc. Chem. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Liu, Y.; Liu, G.; Chen, Q.; Li, Y.; Shi, L.; Liu, Z.; Liu, X. A Novel SERS Substrate Platform: Spatially Stacking Plasmonic Hotspots Films. Nanoscale Res. Lett. 2019, 14, 94. [Google Scholar] [CrossRef]
- Meyer, S.A.; Le Ru, E.C.; Etchegoin, P.G. Quantifying resonant Raman cross sections with SERS. AIP Conf. Proc. 2010, 1267, 204–205. [Google Scholar]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, M.; Pastoriza-Santos, I.; Huang, C.-C.; Kneipp, J.; Szekeres, G.P. SERS Probing of Proteins in Gold Nanoparticle Agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Paris, J.L.; Roumani, F.; Diéguez, L.; Prado, M.; Espiña, B.; Abalde-Cela, S.; Garrido-Maestu, A.; Rodriguez-Lorenzo, L. Multifuntional gold nanoparticles for the SERS detection of pathogens combined with a LAMP-in-microdroplets approach. Materials 2020, 13, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.R.; Kim, J.; Tran, V.T.; Suzuki, T.; Neethirajan, S.; Lee, J.; Park, E.Y. In situ self-assembly of gold nanoparticles on hydrophilic and hydrophobic substrates for influenza virus-sensing platform. Sci. Rep. 2017, 7, 44495. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Shen, Y.; Fu, Y.; Muroski, M.E.; Zhang, P.; Wang, Q.; Xu, C.; Lesniak, M.S.; Li, G.; Cheng, Y. Self-Assembly of Gold Nanoparticles Shows Microenvironment-Mediated Dynamic Switching and Enhanced Brain Tumor Targeting. Theranostics 2017, 7, 1875–1889. [Google Scholar] [CrossRef]
- Pengo, P.; Şologan, M.; Pasquato, L.; Guida, F.; Pacor, S.; Tossi, A.; Stellacci, F.; Marson, D.; Boccardo, S.; Pricl, S.; et al. Gold nanoparticles with patterned surface monolayers for nanomedicine: Current perspectives. Eur. Biophys. J. 2017, 46, 749–771. [Google Scholar] [CrossRef] [Green Version]
- Sherpa, L.; Tripathi, A.; Singh, M.; Mandal, R.; Tiwari, A. Self-assembly of arsenic nanoparticles into magnetic nanotubules and their SERS activity. Appl. Phys. A Mater. Sci. Process. 2020, 126, 581. [Google Scholar] [CrossRef]
- Stetsenko, M.; Margitych, T.; Kryvyi, S.; Maksimenko, L.; Hassan, A.; Filonenko, S.; Li, B.; Qu, J.; Scheer, E.; Snegir, S. Gold Nanoparticle Self-Aggregation on Surface with 1,6-Hexanedithiol Functionalization. Nanomaterials 2020, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Buttry Walker, J.; Minic, Z.; Liu, F.; Goshgarian, H.; Mao, G. Transporter Protein and Drug-Conjugated Gold Nanoparticles Capable of Bypassing the Blood-Brain Barrier. Sci. Rep. 2016, 6, 25794. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.S.; Ozaki, Y.; Itoh, T. Recent progress and frontiers in the electromagnetic mechanism of surface-enhanced Raman scattering. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 81–104. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013, 2013, 49. [Google Scholar] [CrossRef]
- Wang, R.; George, J.; Potts, S.K.; Kremer, M.; Dronskowski, R.; Englert, U. The many flavours of halogen bonds-message from experimental electron density and Raman spectroscopy. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 1190–1201. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Shrestha, B.K.; Haes, A.J. Promoting Intra-and Intermolecular Interactions in Surface-Enhanced Raman Scattering. Anal. Chem. 2018, 90, 128–143. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Panwar, N.; Yap, S.H.K.; Wu, Q.; Zeng, S.; Xu, J.; Tjin, S.C.; Song, J.; Qu, J.; Yong, K.-T. SERS-based ultrasensitive sensing platform: An insight into design and practical applications. Coord. Chem. Rev. 2017, 337, 1–33. [Google Scholar] [CrossRef]
- Tu, Q.; Chang, C. Diagnostic applications of Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 545–558. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef] [Green Version]
- Salvo, P.; Vivaldi, F.M.; Bonini, A.; Biagini, D.; Bellagambi, F.G.; Miliani, F.M.; di Francesco, F.; Lomonaco, T. Biosensors for Detecting Lymphocytes and Immunoglobulins. Biosensors 2020, 10, 155. [Google Scholar] [CrossRef]
- Mäntele, W.; Deniz, E. UV–VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Muthuselvi, C.; Dhavachitra, M.; Pandiarajan, S. Growth and Characterization of Aspirin Crystal in the Phosphoric acid Medium. J. Chem. Pharm. Res. 2016, 8, 804–814. [Google Scholar]
- Adomavičiūtė, S.; Velička, M.; Šablinskas, V. Detection of aspirin traces in blood by means of surface-enhanced Raman scattering spectroscopy. J. Raman Spectrosc. 2020, 51, 919–931. [Google Scholar] [CrossRef]
- Chuchuen, O.; Henderson, M.H.; Sykes, C.; Kim, M.S.; Kashuba, A.D.M. Quantitative Analysis of Microbicide Concentrations in Fluids, Gels and Tissues Using Confocal Raman Spectroscopy. PLoS ONE 2013, 8, 85124. [Google Scholar] [CrossRef] [Green Version]
- Presnell, A.L.; Chuchuen, O.; Simons, M.G.; Maher, J.R.; Katz, D.F. Full depth measurement of tenofovir transport in rectal mucosa using confocal Raman spectroscopy and optical coherence tomography. Drug Deliv. Transl. Res. 2018, 8, 843–852. [Google Scholar] [CrossRef]
- De Vito, F.; Veytsman, B.; Painter, P.; Kokini, J.L. Simulation of the effect of hydrogen bonds on water activity of glucose and dextran using the Veytsman model. Carbohydr. Polym. 2015, 117, 236–246. [Google Scholar] [CrossRef]
- Wade, L. Organic Chemistry, 4th ed.; Prentice Hall: Hoboken, NJ, USA, 1999. [Google Scholar]



| Raman Shift (cm−1) | Literature | Peak Assignment [33,34] |
|---|---|---|
| 552 | 551 | r CO2 |
| 707 | 705 | δ CH |
| 809 | 837 | δ CH |
| 1011 | 1014 | δ CH, ν CO (ester) |
| 1036 | 1045 | Aromatic ring breathing |
| 1146 | 1146 | δ CH |
| 1197 | 1191 | δ CH |
| 1251 | 1223 | ν CO, acid |
| 1297 | 1293 | ν CO, acid |
| 1349 | 1367 | ν CC |
| 1585 | 1576 | ν CC, Aromatic |
| Raman Shift (cm−1) | Literature | Peak Assignment [35,36] |
|---|---|---|
| 533 | 537 | δ CCC |
| 600 | 614 | δ NCC |
| 718 | 725 | Adenine ring |
| 864 | 841 | Skeletal breathing |
| 1011 | 1014 | δ CH, ν CO (ester) |
| 1014 | 1007 | Phosphate |
| 1014 | 1045 | Aromatic ring breathing |
| 1260 | 1255 | ν CN, CNH2 |
| 1318 | 1311 | ν CN |
| 1391 | 1370 | δ CH3 |
| 1557 | 1517 | δ CNH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thobakgale, S.L.; Ombinda-Lemboumba, S.; Mthunzi-Kufa, P. A Molecular Study of Aspirin and Tenofovir Using Gold/Dextran Nanocomposites and Surface-Enhanced Raman Spectroscopy. Molecules 2022, 27, 2554. https://doi.org/10.3390/molecules27082554
Thobakgale SL, Ombinda-Lemboumba S, Mthunzi-Kufa P. A Molecular Study of Aspirin and Tenofovir Using Gold/Dextran Nanocomposites and Surface-Enhanced Raman Spectroscopy. Molecules. 2022; 27(8):2554. https://doi.org/10.3390/molecules27082554
Chicago/Turabian StyleThobakgale, Setumo Lebogang, Saturnin Ombinda-Lemboumba, and Patience Mthunzi-Kufa. 2022. "A Molecular Study of Aspirin and Tenofovir Using Gold/Dextran Nanocomposites and Surface-Enhanced Raman Spectroscopy" Molecules 27, no. 8: 2554. https://doi.org/10.3390/molecules27082554
APA StyleThobakgale, S. L., Ombinda-Lemboumba, S., & Mthunzi-Kufa, P. (2022). A Molecular Study of Aspirin and Tenofovir Using Gold/Dextran Nanocomposites and Surface-Enhanced Raman Spectroscopy. Molecules, 27(8), 2554. https://doi.org/10.3390/molecules27082554







