Impacts of Gum Arabic and Polyvinylpyrrolidone (PVP) with Salicylic Acid on Peach Fruit (Prunus persica) Shelf Life
Abstract
:1. Introduction
2. Results
2.1. Fourier Transform Infrared Analysis (FT-IR)
2.2. Evaluation of Fruit Properties: Water Loss %, Skin Browning Index (SBI), Hue Angle, and Firmness (N)
2.3. The Fruit Skin Browning Variables: Total Phenol (TPs), Flavonoids (FLs), and Browning Enzyme Activities
2.4. The Activities of Cell-Wall-Degrading Enzymes (CWDEs)
2.5. Cell Membrane: Lipid Peroxidation (MDA; μM mg−1 FW) and Electrolyte Leakage (EL)
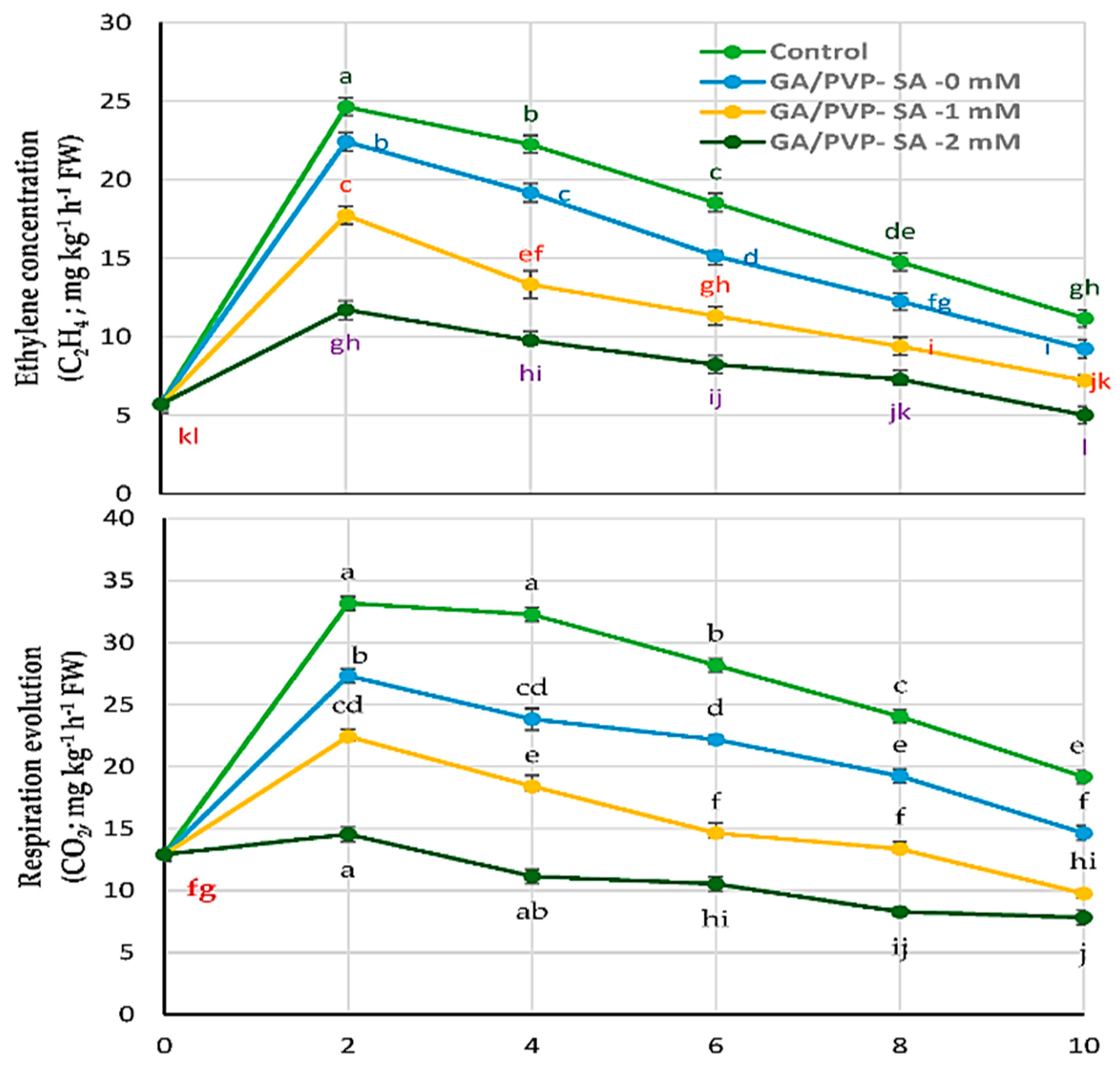
2.6. Fruit Ethylene and Respiration
2.7. Data Modeling
3. Discussion
4. Materials and Methods
4.1. Fruit Material
4.2. Gum Arabic and Coating Protocol
4.3. Fourier Transform Infrared Analysis (FT-IR)
4.4. Water Loss %, Skin Browning Index, and Fruit Color Hue Angle
4.5. The Browning Parameters: Total Phenols (TPs) and Total Flavonoids (TFs)
4.6. Extraction of Browning Enzyme
4.7. The Activities of Cell-Wall-Degradation Enzymes (CWDEs) and Fruit Firmness
4.8. Lipid Peroxidation and Cell Membrane Permeability
4.9. Ethylene and Respiration
4.10. Statistical Analysis and Data Modeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Awad, R.M. Effect of post-harvest salicylic acid treatments on fruit quality of peach cv. “Flordaprince” during cold storage. Aust. J. Basic Appl. Sci. 2013, 7, 920–927. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Gayed, A.A.; Shaarawi, S.A.; Elkhishen, M.A.; Elsherbini, N.M. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of Early Swelling peach during cold storage. Ciência E Agrotecnologia 2017, 41, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. Diverse metabolism of cell wall components of melting and non-melting peach genotypes during ripening after harvest or cold storage. J. Sci. Food Agric. 2006, 86, 243–250. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Horti. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martínez-Romero, D. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol. Technol. 2013, 83, 54–57. [Google Scholar] [CrossRef]
- Hazrati, S.; Beyraghdar, K.A.; Habibzadeh, F.; Tahmasebi-Sarvestani, Z.; Sadeghi, A.R. Evaluation of Aloe vera gel as an alternative edible coating for peach fruits during cold Ssorage period. Gesunde Pflanz. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Forato, L.A.; de Britto, D.; de Rizzo, J.S.; Gastaldi, T.A.; Assis, O.B.G. Effect of cashew gum-carboxymethylcellulose edible coatings in extending the shelf-life of fresh and cut guavas. Food Packag. Shelf Life 2015, 5, 68–74. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Minimize browning incidence of banana by postharvest active chitosan/PVA Combines with oxalic acid treatment to during shelf-life. Sci. Hortic. 2017, 226, 208–215. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Pobiega, K.; Igielska, M.; Włodarczyk, P.; Gniewosz, M. The use of pullulan coatings with propolis extract to extend the shelf life of blueberry (Vaccinium corymbosum) fruit. Int. J. Food Sci. Technol. 2021, 56, 1013–1020. [Google Scholar] [CrossRef]
- Melo, N.F.C.B.; de Lima, M.A.B.; Stamford, T.L.M.; Galembeck, A.; Flores, M.A.; de Campos Takaki, G.M.; da Costa Medeiros, J.A.; Stamford-Arnaud, T.M.; Montenegro Stamford, T.C. In vivo and in vitro antifungal effect of fungal chitosan nanocomposite edible coating against strawberry phytopathogenic fungi. Int. J. Food Sci. Technol. 2020, 55, 3381–3391. [Google Scholar] [CrossRef]
- Zhang, W.; Jing, L.; Chen, H.; Zhang, S. NC-1 coating combined with 1-MCP treatment maintains better fruit qualities in honey peach during low-temperature storage. Int. J. Food Sci. Technol. 2022, 57, 516–524. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P. A combination of gum arabic and chitosan can control anthracnose caused by Colletotrichum musae and enhance the shelf-life of banana fruit. J. Hortic. Sci. Biotechnol. 2010, 85, 432–436. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Pellicanò, T.M.; Giuffrè, A.M.; Poiana, M. Evaluation of Aloe arborescens gel as new coating to maintain the organoleptic and functional properties of strawberry (Fragaria × ananassa cv. Cadonga) fruits. Int. J. Food Sci. Technol. 2020, 55, 861–870. [Google Scholar] [CrossRef]
- Klein, M.; Aserin, A.; Ishai, P.B.; Garti, N. Interactions between whey protein isolate and gum Arabic. Colloids Surf. B Biointerfaces 2010, 79, 377–383. [Google Scholar] [CrossRef]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2001, 17, 25–39. [Google Scholar] [CrossRef]
- Renard, D.; Lavenant-Gourgeon, L.; Ralet, M.-C.; Sanchez, C. Acaciasenegal Gum: Continuum of molecular species differing by their protein to sugar ratio, molecular weight, and charges. Biomacromolecules 2006, 7, 2637–2649. [Google Scholar] [CrossRef]
- Ali, A.; Maqbool, M.; Ramachandran, S.; Alderson, P.G. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biolo. Technol. 2010, 58, 42–47. [Google Scholar] [CrossRef]
- El-Anany, A.M.; Hassan, G.F.A.; Ali, F.M. Effects of Edible Coatings on the Shelf-Life and Quality of Anna Apple (Malus domestica Borkh) During Cold Storage. J. Food Technol. 2009, 7, 5–11. [Google Scholar]
- Idris, Y.M.A.; Ibrahim, Y.A.; Mariod, A.A. Color of dehydrated tomato: Effects of gum arabic. Int. J. Food Prop. 2013, 16, 838–851. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; et al. Recent application developments of water-soluble synthetic polymers. J. Ind. Eng. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Tadda, M.A.; Gouda, M.; Lin, X.; Shitu, A.; Abdullahi, H.S.; Zhu, S.; Li, X.; Liu, D. Impacts of Baobab (Adansonia Digitata) Powder on the Poly(Butylene Succinate) Polymer Degradability to Form an Eco-Friendly Filler-Based Composite. Front. Mater. 2021, 8, 505. [Google Scholar] [CrossRef]
- FDA. Center for Drug Evaluation and Research, Database Update Frequency: Quarterly. CFR—Code of Federal Regulations Title 21: January 06, 2020— Sec. 173.55 Polyvinylpyrrolidone. 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=173.55 (accessed on 26 March 2022).
- WHO. The Ottawa Charter for Health Promotion; Nov 21; WHO: Geneva, Switzerland, 1986; Available online: http://www.who.int/healthpromotion/conferences/previous/ottawa/en/index.html (accessed on 26 March 2022).
- Panda, H. The Complete Book on Gums and Stabilizers for Food Industry; Asia Pacific Business Press Inc.: Delhi, India, 2010; pp. 1–480. [Google Scholar]
- Lo’ay, A.A.; Taher, M.A. Effectiveness salicylic acid blending in chitosan/PVP biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit. Sci. Hortic. 2018, 238, 343–349. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Lo’ay, A.A. Evaluation berries shattering phenomena of ‘Flame seedless’ vines grafted on different rootstocks during shelf life. Sci. Hortic. 2019, 246, 51–56. [Google Scholar] [CrossRef]
- Hoff, J.F.; Singleton, K.I. A method for determination of tannin in foods by means of immobilized enzymes. J. Food Sci. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, S. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Rabie, M.M.; Alhaithloul, H.A.S.; Alghanem, S.M.S.; Ibrahim, A.M.; Abdein, M.A.; Abdelgawad, Z.A. On the biochemical and physiological responses of ‘Crimson seedless’ grapes coated with an edible composite of pectin, polyphenylene alcohol, and salicylic acid. Horticulturae 2021, 7, 498. [Google Scholar] [CrossRef]
- Jiang, Y.; Louce, D.C.; Jayas, W.; Lu, W. Effect of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.-C. Salicylic acid reduces the incidence of Phomopsis longanae Chi infection in harvested longan fruit by affecting the energy status and respiratory metabolism. Postharvest Biol. Technol. 2020, 160, 111035. [Google Scholar] [CrossRef]
- Pathak, N.; Sanwal, G.G. Multiple forms of polygalacturonase from banana fruits. Phytochemistry 1998, 48, 249–255. [Google Scholar] [CrossRef]
- Belyakova, L.A.; Varvarin, A.M.; Lyashenko, D.Y.; Khora, O.V.; Oranskaya, E.I. Complexation in a b-Cyclodextrin–Salicylic Acid System. Colloid J. 2007, 69, 546–551. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Mireles, L.K.; Wu, M.-R.; Saadeh, N.; Yahia, L.H.; Sacher, E. Physicochemical Characterization of Polyvinyl Pyrrolidone: A Tale of Two Polyvinyl Pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olías, R.; Olías, J.M. Lipoxygenase and Hydroperoxide Lyase Activities in Ripening Strawberry Fruits. J. Agric. Food Chem. 1999, 47, 249–253. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Meindrawan, B.; Ofe, O.; Susanto, C.S.; Ayman, A.; Mangindaan, D.; Kasih, T.P. Glucomannan–beeswax–chitosan antimicrobial edible coating to maintain the storage quality of salak fruit. Salacca Zalacca 2020, 391, 1900164. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Doaa, M.H. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Meng, K.; Hou, Y.; Han, Y.; Ban, Q.; He, Y.; Suo, J.; Rao, J. Exploring the Functions of 9-Lipoxygenase (DkLOX3) in Ultrastructural Changes and Hormonal Stress Response during Persimmon Fruit Storage. Int. J. Mol. Sci. 2017, 18, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadda, M.; Gouda, M.; Lin, X.; Shitu, A.; Abdullahi, H.; Zhu, S.; Li, X.; Liu, D. Evaluation of Aloe vera gel as an alternative edible coating for peach fruits during cold storage period. Front. Mater. 2021, 8, 768960. [Google Scholar] [CrossRef]
- Gouda, M.; El-Din Bekhit, A.; Tang, Y.; Huang, Y.; Huang, L.; He, Y.; Li, X. Recent innovations of ultrasound green technology in herbal phytochemistry: A review. Ultrason. Sonochem. 2021, 73, 105538. [Google Scholar] [CrossRef]
- McCann, M.C.; Carpita, N.C. Designing the deconstruction of plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 314–320. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Schröder, R.; Hallett, I.C.; Cohen, D.; MacRae, E.A. Overexpression of Polygalacturonase in Transgenic Apple Trees Leads to a Range of Novel Phenotypes Involving Changes in Cell Adhesion. Plant Physiol. 2002, 129, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Li, F.; Li, F.; Zhang, D.; Zhao, C. Identification and Expression Analysis of Polygalacturonase Family Members during Peach Fruit Softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Ghosh, B.; Eveleigh, D. Characteristics of fungal cellulases. Bioresour. Technol. 1991, 36, 37–50. [Google Scholar] [CrossRef]
- Abu-Goukh, A.-B.A.; Bashir, H.A. Changes in pectic enzymes and cellulase activity during guava fruit ripening. Food Chem. 2003, 83, 213–218. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Wu, F.; Li, T.; Liang, Y.; Duan, X. Modification of Pectin and Hemicellulose Polysaccharides in Relation to Aril Breakdown of Harvested Longan Fruit. Int. J. Mol. Sci. 2013, 14, 23356–23368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, R.K.; Ramana Rao, T.V.; Nandane, A.S. Improvement of post-harvest quality of pear fruit with optimized composite edible coating formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; Elgammal, R.E.; Alhaithloul, H.A.S.; Alghanem, S.M.; Fikry, M.; Abdein, M.A.; Hikal, D.M. Enhance Fruit Ripening Uniformity and Accelerate the Rutab Stage by Using ATP in ‘Zaghloul’ Dates during the Shelf Life. Foods 2021, 10, 2641. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J. Effects of Nitric Oxide on Fatty Acid Composition in Peach Fruits during Storage. J. Agric. Food Chem. 2006, 54, 9447–9452. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; D’Amico, M.; Albanese, C.; Zhou, J.-N.; Brownlee, M.; Lisanti Michael, P.; Chatterjee, V.K.K.; Lazar Mitchell, A.; Pestell Richard, G. Inhibition of Cellular Proliferation through IκB Kinase-Independent and Peroxisome Proliferator-Activated Receptor γ-Dependent Repression of Cyclin D1. Mol. Cell. Biol. 2001, 21, 3057–3070. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, D.F. Lipoxygenases. Physiol. Plant 1989, 76, 249–253. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [Green Version]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Li, N.; Parsons, B.L.; Liu, D.R.; Mattoo, A.K. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol. Biol. 1992, 18, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Mattheis, J.P.; Fellman, J.K. Inhibition of apple fruit 1-aminocyclopropane-1-carboxylic acid oxidase activity and respiration by acetylsalicylic acid. J. Plant Physiol. 1996, 149, 469–471. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Ismail, H.; Kassem, H.S. Postharvest Treatment of ‘Florida Prince’ Peaches with a Calcium Nanoparticle–Ascorbic Acid Mixture during Cold Storage and Its Effect on Antioxidant Enzyme Activities. Horticulturae 2021, 7, 499. [Google Scholar] [CrossRef]
- Lo’ay, A.A. Chilling Injury in Mangoes. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005; pp. 1–224. [Google Scholar]
- Lohani, S.; Triverdi, P.K.; Nath, P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004, 31, 119–126. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Fikry, M.; Aniza Yusof, Y.; Al-Awaadh, A.M.; Abdul Rahman, R.; Ling Chin, N.; Mousa, E.; Sin Chang, L. Kinetics Modelling of the Colour, Hardness, Grinding Energy Consumption and Oil Yield Changes during the Conventional Roasting of Palm Date Seeds. Food Sci. Technol. Res. 2019, 25, 351–362. [Google Scholar] [CrossRef]
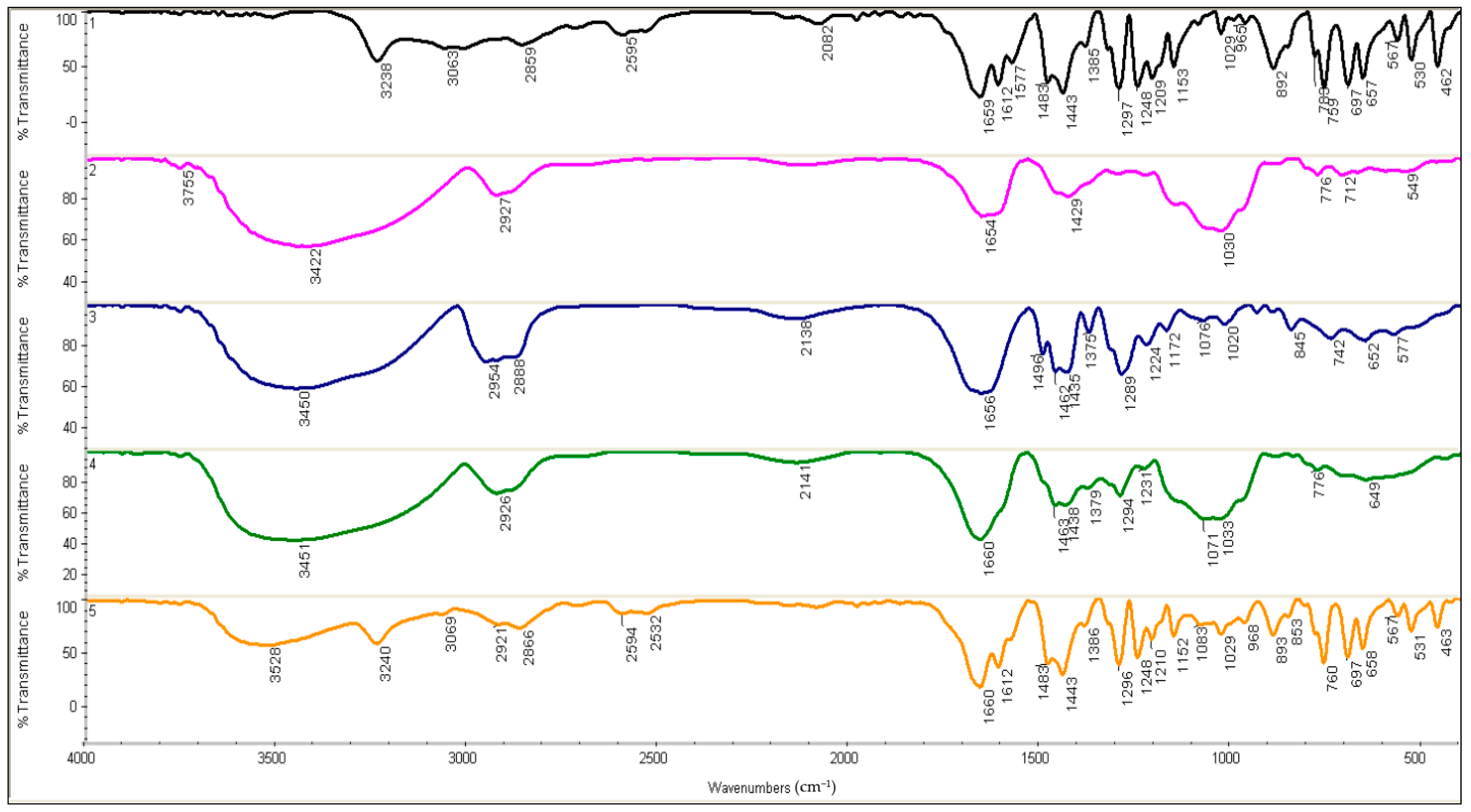
| Treatments | Shelf-Life Period (Days) | ||||||
|---|---|---|---|---|---|---|---|
| D 0 | D 2 | D 4 | D 6 | D 8 | D 10 | ||
| Water loss % | |||||||
| Control | 0.00 ± 0.000 | 3.14 ± 0.017 a | 11.97 ± 1.097 a | 18.47 ± 0.784 a | 27.35 ± 0.784 a | 31.30 ± 0.708 a | |
| GA/PVP–SA 0 mM | 0.00 ± 0.000 | 3.06 ± 0.012 b | 9.46 ± 0.309 b | 12.46 ± 0.652 b | 19.85 ± 0.311 b | 22.41 ± 0.584 b | |
| GA/PVP–SA 1 mM | 0.00 ± 0.000 | 3.02 ± 0.014 c | 7.86 ± 0.236 c | 9.67 ± 0.287 c | 16.71 ± 0.898 c | 18.28 ± 0.545 c | |
| GA/PVP–SA 2 mM | 0.00 ± 0.000 | 2.98 ± 0.008 d | 6.66 ± 0.193 c | 8.68 ± 0.193 d | 12.42 ± 0.506 d | 14.44 ± 0.578 d | |
| Skin browning index | |||||||
| Control | 1.00 ± 0.000 | 1.02 ± 0.006 a | 1.16 ± 0.005 a | 1.60 ± 0.031 a | 1.99 ± 0.003 a | 3.75 ± 0.122 a | |
| GA/PVP–SA 0 mM | 1.00 ± 0.000 | 1.00 ± 0.000 b | 1.02 ± 0.014 b | 1.10 ± 0.008 b | 1.19 ± 0.008 b | 1.93 ± 0.061 b | |
| GA/PVP–SA 1 mM | 1.00 ± 0.000 | 1.00 ± 0.000 b | 1.00 ± 0.000 c | 1.00 ± 0.000 c | 1.01 ± 0.005 c | 1.08 ± 0.015 c | |
| GA/PVP–SA 2 mM | 1.00 ± 0.000 | 1.00 ± 0.000 b | 1.00 ± 0.000 c | 1.00 ± 0.000 c | 1.00 ± 0.000 c | 1.02 ± 0.005 c | |
| Fruit color (hue angle) | |||||||
| Control | 5.81 ± 0.316 a | 8.67 ± 0.299 d | 12.28 ± 0.191 a | 12.80 ± 0.507 d | 9.59 ± 0.485 d | 9.29 ± 0.024 d | |
| GA/PVP–SA 0 mM | 5.81 ± 0.316 a | 9.85 ± 0.141 c | 13.09 ± 0.054 c | 14.19 ± 0.193 c | 13.46 ± 0.277 c | 12.57 ± 0.2137 c | |
| GA/PVP–SA 1 mM | 5.81 ± 0.316 a | 10.38 ± 0.175 b | 13.91 ± 0.200 b | 15.21 ± 0.153 b | 16.07 ± 0.256 b | 15.26 ± 0.021 b | |
| GA/PVP–SA 2 mM | 5.81 ± 0.316 a | 11.43 ± 0.158 a | 15.61 ± 0.300 a | 16.41 ± 0.515 a | 16.88 ± 0.106 a | 17.96 ± 0.229 a | |
| Fruit firmness (N) | |||||||
| Control | 47.68 ± 0.655 a | 39.35 ± 0.498 ef | 32.38 ± 1.387 i | 29.50 ± 0.329 j | 25.71 ± 0.472 k | 19.30 ± 0.572 l | |
| GA/PVP–SA 0 mM | 47.68 ± 0.655 a | 40.63 ± 0.304 de | 38.03 ± 0.342 f | 35.18 ± 0.536 h | 32.19 ± 0.562 i | 29.59 ± 0.367 j | |
| GA/PVP–SA 1 mM | 47.68 ± 0.655 a | 44.25 ± 0.351 b | 42.81 ± 0.682 c | 39.43 ± 0.625 e | 36.55 ± 0.664 g | 31.62 ± 0.888 i | |
| GA/PVP–SA 2 mM | 47.68 ± 0.655 a | 46.70 ± 0.894 a | 44.41 ± 0.385 b | 42.59 ± 0.628 c | 40.80 ± 0.572 d | 39.47 ± 0.398 de | |
| Treatments | Shelf-Life Period (Days) | ||||||
|---|---|---|---|---|---|---|---|
| D 0 | D 2 | D 4 | D 6 | D 8 | D 10 | ||
| Total phenol content (TP; mg 100 g−1 FW) | |||||||
| Control | 86.58 ± 0.858 ab | 79.84 ± 0.896 de | 77.67 ± 0.360 fg | 66.80 ± 0.378 j | 60.86 ± 0.473 k | 55.90 ± 1.815 l | |
| GA/PVP–SA 0 mM | 86.58 ± 0.858 ab | 83.55 ± 0.619 c | 79.07 ± 0.330 ef | 75.39 ± 0.641 h | 70.99 ± 0.291 i | 67.56 ± 0.636 j | |
| GA/PVP–SA 1 mM | 86.58 ± 0.858 ab | 85.57 ± 0.569 b | 81.57 ± 0.715 d | 79.56 ± 0.402 ef | 77.63 ± 0.498 fg | 76.74 ± 0.531 gh | |
| GA/PVP–SA 2 mM | 86.58 ± 0.858 ab | 87.68 ± 0.200 a | 86.43 ± 0.646 ab | 81.66 ± 0.536 cd | 80.12 ± 0.360 de | 79.89 ± 0.564 de | |
| Flavonoids content (FL; mg 100 g−1 FW) | |||||||
| Control | 33.69 ± 1.035 a | 29.47 ± 0.691 d | 26.44 ± 0.543 e | 17.34 ± 0.906 j | 15.40 ± 0.601 k | 12.38 ± 0.590 l | |
| GA/PVP–SA 0 mM | 33.69 ± 1.035 a | 30.91 ± 0.131 b | 29.38 ± 0.306 d | 21.41 ± 0.694 h | 19.61 ± 0.528 i | 16.92 ± 0.344 j | |
| GA/PVP–SA 1 mM | 33.69 ± 1.035 a | 31.53 ± 0.117 b | 30.57 ± 0.129 bc | 26.44 ± 0.543 e | 24.85 ± 0.626 f | 21.83 ± 0.294 h | |
| GA/PVP–SA 2 mM | 33.69 ± 1.035 a | 33.03 ± 0.389 a | 31.36 ± 0.317 b | 29.59 ± 0.442 cd | 26.40 ± 0.528 e | 23.41 ± 0.463 g | |
| Phenylalanine ammonia-lyase activity (PAL; EC: 4.3.1.24 U min−1 mg−1 protein−1) | |||||||
| Control | 7.47 ± 0.269 j | 8.95 ± 0.076 h | 12.35 ± 0.513 ef | 15.71 ± 0.473 d | 16.95 ± 0.404 c | 24.31 ± 0.629 a | |
| GA/PVP–SA 0 mM | 7.47 ± 0.269 j | 8.55 ± 0.026 hi | 10.49 ± 0.348 g | 12.86 ± 0.566 e | 15.31 ± 0.294 d | 18.50 ± 0.440 b | |
| GA/PVP–SA 1 mM | 7.47 ± 0.269 j | 8.03 ± 0.030 ij | 8.92 ± 0.092 h | 10.58 ± 0.461 g | 11.86 ± 0.263 f | 12.68 ± 0.556 e | |
| GA/PVP–SA 2 mM | 7.47 ± 0.269 j | 7.85 ± 0.097 ij | 8.29 ± 0.023 hi | 9.03 ± 0.040 h | 9.85 ± 0.156 g | 10.22 ± 0.190 g | |
| Polyphenol oxidase activity (PPO; EC: 1.14.18.1 U min−1 mg−1 protein−1) | |||||||
| Control | 0.26 ± 0.005 n | 0.34 ± 0.008 j | 0.44 ± 0.008 f | 0.52 ± 0.012 d | 0.58 ± 0.008 b | 0.65 ± 0.011 a | |
| GA/PVP–SA 0 mM | 0.26 ± 0.005 n | 0.31 ± 0.005 l | 0.39 ± 0.005 h | 0.46 ± 0.005 e | 0.54 ± 0.005 c | 0.59 ± 0.005 b | |
| GA/PVP–SA 1 mM | 0.26 ± 0.005 n | 0.29 ± 0.005 m | 0.33 ± 0.005 k | 0.37 ± 0.005 i | 0.40 ± 0.005 h | 0.43 ± 0.005 g | |
| GA/PVP–SA 2 mM | 0.26 ± 0.005 n | 0.26 ± 0.003 n | 0.29 ± 0.005 m | 0.31 ± 0.005 l | 0.33 ± 0.005 k | 0.35 ± 0.005 j | |
| Treatments | Shelf-Life Period (Days) | ||||||
|---|---|---|---|---|---|---|---|
| D 0 | D 2 | D 4 | D 6 | D 8 | D 10 | ||
| Lipoxygenase activity (LOX; EC:1.13.11, U min−1 mg−1 protein−1) | |||||||
| Control | 0.84 ± 0.008 m | 0.93 ± 0.023 ij | 0.96 ± 0.021 hi | 1.31 ± 0.020 e | 1.94 ± 0.032 b | 2.28 ± 0.015 a | |
| GA/PVP–SA 0 mM | 0.84 ± 0.008 m | 0.88 ± 0.003 l | 0.92 ± 0.005 jk | 1.00 ± 0.012 g | 1.73 ± 0.043 c | 1.94 ± 0.023 b | |
| GA/PVP–SA 1 mM | 0.84 ± 0.008 m | 0.86 ± 0.008 lm | 0.88 ± 0.006 kl | 0.94 ± 0.005 ij | 1.11 ± 0.017 f | 1.35 ± 0.017 d | |
| GA/PVP–SA 2 mM | 0.84 ± 0.008 m | 0.84 ± 0.005 m | 0.86 ± 0.003 lm | 0.89 ± 0.005 kl | 0.93 ± 0.008 ij | 0.99 ± 0.005 gh | |
| Cellulase activity (CEL; EC: 3.2.1.4 U min−1 mg−1 protein−1) | |||||||
| Control | 8.00 ± 0.063 i | 13.03 ± 0.777 f | 15.42 ± 0.666 e | 22.67 ± 1.353 a | 19.65 ± 0.619 b | 17.85 ± 0.543 c | |
| GA/PVP–SA 0 mM | 8.00 ± 0.063 i | 10.65 ± 0.450 g | 12.66 ± 0.538 f | 16.67 ± 0.390 d | 14.76 ± 0.459 e | 12.72 ± 0.592 f | |
| GA/PVP–SA 1 mM | 8.00 ± 0.063 i | 9.61 ± 0.363 h | 10.76 ± 0.453 g | 14.71 ± 0.509 e | 12.43 ± 0.738 f | 10.96 ± 0.383 g | |
| GA/PVP–SA 2 mM | 8.00 ± 0.063 i | 8.50 ± 0.100 i | 9.52 ± 0.272 h | 11.00 ± 0.455 g | 10.41 ± 0.057 gh | 9.64 ± 0.048 h | |
| Pectinase activity (PT; EC: 3.2.1.15, U min−1 mg−1 protein−1) | |||||||
| Control | 0.27 ± 0.005 o | 0.36 ± 0.005 klm | 1.46 ± 0.014 d | 1.57 ± 0.005 c | 1.81 ± 0.033 b | 2.86 ± 0.067 a | |
| GA/PVP–SA 0 mM | 0.27 ± 0.005 o | 0.33 ± 0.005 mn | 0.40 ± 0.003 jk | 0.50 ± 0.005 i | 1.03 ± 0.040 f | 1.22 ± 0.063 e | |
| GA/PVP–SA 1 mM | 0.27 ± 0.005 o | 0.30 ± 0.003 no | 0.38 ± 0.003 jkl | 0.44 ± 0.005 j | 0.64 ± 0.008 h | 0.72 ± 0.005 g | |
| GA/PVP–SA 2 mM | 0.27 ± 0.005 o | 0.28 ± 0.003 no | 0.34 ± 0.005 lmn | 0.39 ± 0.006 jk | 0.42 ± 0.005 j | 0.43 ± 0.005 j | |
| Treatments | Shelf-Life Period (Days) | ||||||
|---|---|---|---|---|---|---|---|
| D 0 | D 2 | D 4 | D 6 | D 8 | D 10 | ||
| Lipid peroxidation (MDA; µM g−1 FW) | |||||||
| Control | 0.19 ± 0.005 n | 0.29 ± 0.012 j | 0.45 ± 0.008 e | 0.56 ± 0.020 c | 0.59 ± 0.011 b | 0.68 ± 0.014 a | |
| GA/PVP–SA 0 mM | 0.19 ± 0.005 n | 0.26 ± 0.005 k | 0.46 ± 0.005 e | 0.40 ± 0.005 f | 0.46 ± 0.005 e | 0.50 ± 0.005 d | |
| GA/PVP–SA 1 mM | 0.19 ± 0.005 n | 0.24 ± 0.005 l | 0.29 ± 0.005 j | 0.31 ± 0.012 ij | 0.37 ± 0.005 g | 0.39 ± 0.005 f | |
| GA/PVP–SA 2 mM | 0.19 ± 0.005 n | 0.21 ± 0.005 m | 0.22 ± 0.005 m | 0.25 ± 0.005 kl | 0.30 ± 0.005 j | 0.32 ± 0.005 i | |
| Electrolyte leakage (EL%) | |||||||
| Control | 6.67 ± 0.273 p | 12.45 ± 0.631 kl | 18.75± 0.548 g | 24.89 ± 1.132 e | 35.20 ± 0.273 b | 52.88 ± 1.475 a | |
| GA/PVP–SA 0 mM | 6.67 ± 0.273 p | 11.09± 0.147 mn | 15.91 ± 0.419 i | 18.79 ± 0.275 g | 26.28 ± 0.273 d | 30.44 ± 0.419 c | |
| GA/PVP–SA 1 mM | 6.67 ± 0.273 p | 10.08 ± 0.037 n | 14.34 ± 0.328 j | 17.13 ± 0.273 h | 20.63 ± 0.273 f | 26.76 ± 0.539 d | |
| GA/PVP–SA 2 mM | 6.67 ± 0.273 p | 7.59 ± 0.117 p | 8.86 ± 0.150 o | 10.45± 0.273 mn | 11.62± 0.273 lm | 13.24± 0.541 jk | |
| Treatments | Shelf-Life Period (Days) | ||||||
|---|---|---|---|---|---|---|---|
| D 0 | D 2 | D 4 | D 6 | D 8 | D 10 | ||
| Ethylene concentration (C2H4; mg kg−1 h−1 FW) | |||||||
| Control | 5.71 ± 0.340 kl | 24.64 ± 0.583 a | 22.25 ± 0571 b | 18.52 ± 0.586 c | 14.76 ± 0.583 de | 11.16 ± 0.554 gh | |
| GA/PVP–SA 0 mM | 5.71 ± 0.340 kl | 22.43 ± 0.571 b | 19.17 ± 0.591 c | 15.14 ± 0.318 d | 12.25 ± 0.554 fg | 9.23 ± 0.560 i | |
| GA/PVP–SA 1 mM | 5.71 ± 0.340 kl | 17.73 ± 0.574 c | 13.32 ± 0.886 ef | 11.32 ± 0.571 gh | 9.38 ± 0.562 i | 7.21 ± 0.333 jk | |
| GA/PVP–SA 2 mM | 5.71 ± 0.340 kl | 11.17 ± 0.606 gh | 9.76 ± 0.307 hi | 8.23 ± 0.571 ij | 7.28 ± 0.355 jk | 5.00 ± 0.330 l | |
| Respiration evolution (CO2; mg kg−1 h−1 FW) | |||||||
| Control | 12.91 ± 0.597 fg | 33.16 ± 0.586 a | 32.26 ± 0.560 a | 28.17 ± 0.640 b | 24.03 ± 0.331 c | 19.16 ± 0.552 e | |
| GA/PVP–SA 0 mM | 12.91 ± 0.597 fg | 27.32 ± 0.568 b | 23.82 ± 0.869 cd | 22.17 ± 0.346 d | 19.23 ± 0.568 e | 14.62 ± 0.586 f | |
| GA/PVP–SA 1 mM | 12.91 ± 0.597 fg | 22.42 ± 0.577 cd | 18.41 ± 0.902 e | 14.63 ± 0.853 f | 13.36 ± 0.586 f | 9.76 ± 0.336 hi | |
| GA/PVP–SA 2 mM | 12.91 ± 0.597 fg | 14.54 ± 0.588 f | 11.14 ± 0.586 gh | 10.52 ± 0.574 hi | 8.28 ± 0.353 ij | 7.83 ± 0.568 j | |
| Properties | Linear Model (Y = a ± bX) * | ||
|---|---|---|---|
| a (p Value) | b (p Value) | R2 | |
| C2H4 | 8.99 (0.00) | 0.224 (0.23) | 0.101 |
| CO2 | 14.11(0.00) | −0.64 (0.00) | 0.753 |
| WL% | 0.287 (0.231) | 1.42 (0.00) | 0.988 |
| SBI | 0.997 (0.00) | 0.0009 (0.015) | 0.357 |
| Hue | 8.28 (0.00) | 1.19 (0.00) | 0.814 |
| Firmness | 47.86(0.00) | −0.82(0.00) | 0.901 |
| TP | 88.00(0.00) | −0.82(0.00) | 0.755 |
| TF | 34.61(0.00) | −0.95(0.00) | 0.892 |
| PAL | 7.43(0.00) | 0.27(0.00) | 0.949 |
| PPO | 0.26(0.00) | 0.01(0.00) | 0.933 |
| LOX | 0.82(0.00) | 0.01(0.00) | 0.865 |
| CEL | 8.35 (0.00) | 0.242(0.002) | 0.512 |
| PT | 0.27(0.00) | 0.02(0.00) | 0.931 |
| MDA | 0.18(0.00) | 0.01(0.00) | 0.929 |
| EL% | 6.53(0.00) | 0.61(0.00) | 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taher, M.A.; Lo’ay, A.A.; Gouda, M.; Limam, S.A.; Abdelkader, M.F.M.; Osman, S.O.; Fikry, M.; Ali, E.F.; Mohamed, S.Y.; Khalil, H.A.; et al. Impacts of Gum Arabic and Polyvinylpyrrolidone (PVP) with Salicylic Acid on Peach Fruit (Prunus persica) Shelf Life. Molecules 2022, 27, 2595. https://doi.org/10.3390/molecules27082595
Taher MA, Lo’ay AA, Gouda M, Limam SA, Abdelkader MFM, Osman SO, Fikry M, Ali EF, Mohamed SY, Khalil HA, et al. Impacts of Gum Arabic and Polyvinylpyrrolidone (PVP) with Salicylic Acid on Peach Fruit (Prunus persica) Shelf Life. Molecules. 2022; 27(8):2595. https://doi.org/10.3390/molecules27082595
Chicago/Turabian StyleTaher, Mohamed A., A. A. Lo’ay, Mostafa Gouda, Safaa A. Limam, Mohamed F. M. Abdelkader, Samah O. Osman, Mohammad Fikry, Esmat F. Ali, Sayed. Y. Mohamed, Hoda A. Khalil, and et al. 2022. "Impacts of Gum Arabic and Polyvinylpyrrolidone (PVP) with Salicylic Acid on Peach Fruit (Prunus persica) Shelf Life" Molecules 27, no. 8: 2595. https://doi.org/10.3390/molecules27082595
APA StyleTaher, M. A., Lo’ay, A. A., Gouda, M., Limam, S. A., Abdelkader, M. F. M., Osman, S. O., Fikry, M., Ali, E. F., Mohamed, S. Y., Khalil, H. A., El-Ansary, D. O., El-Gioushy, S. F., Ghazzawy, H. S., Ibrahim, A. M., Maklad, M. F., Abdein, M. A., & Hikal, D. M. (2022). Impacts of Gum Arabic and Polyvinylpyrrolidone (PVP) with Salicylic Acid on Peach Fruit (Prunus persica) Shelf Life. Molecules, 27(8), 2595. https://doi.org/10.3390/molecules27082595














