Induction of Osseointegration by Nacre in Pigs
Abstract
:1. Introduction
2. Results
2.1. Micro-CT Imaging
2.2. Micro-CT Analysis
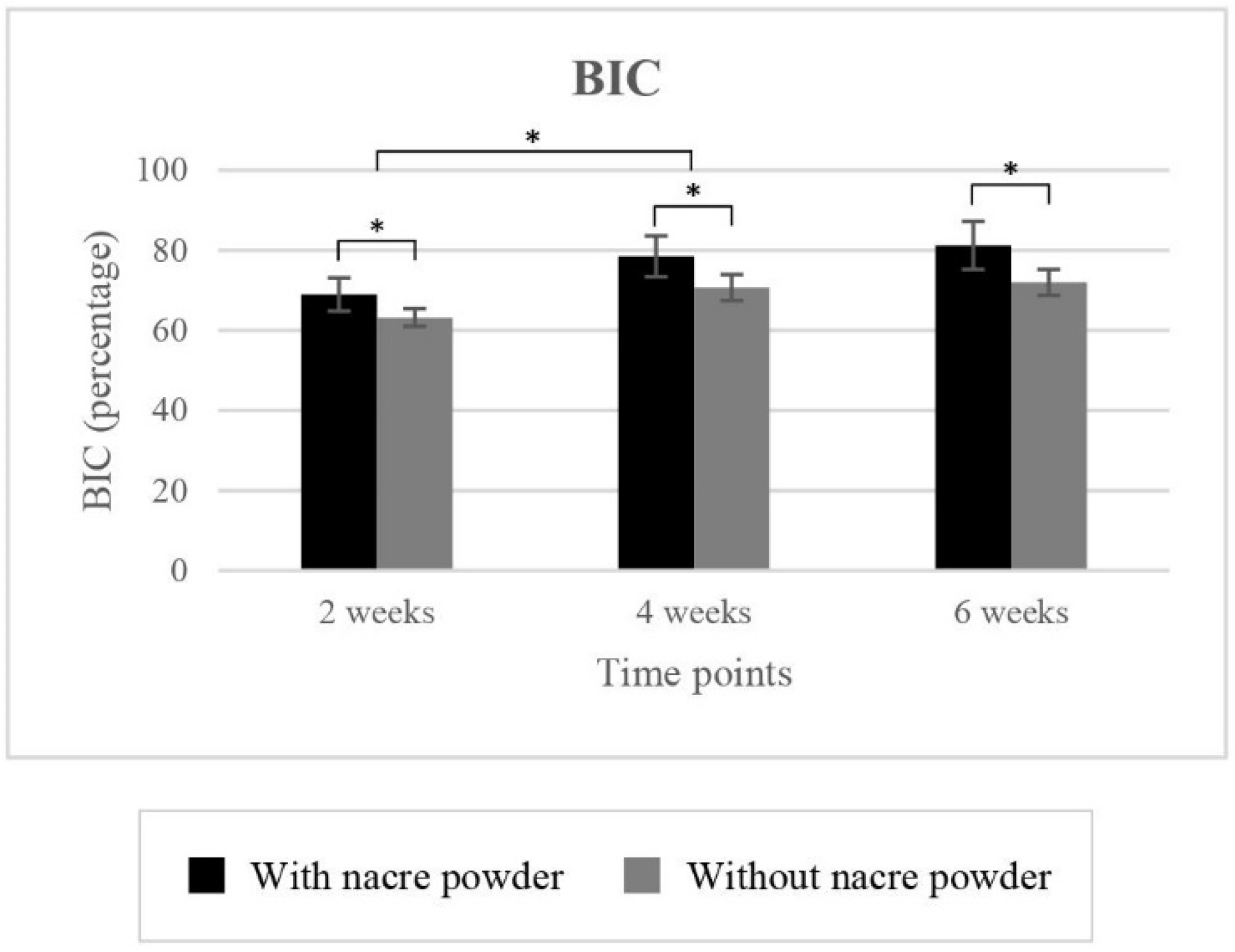
2.2.1. Bone-to-Implant Contact (BIC)
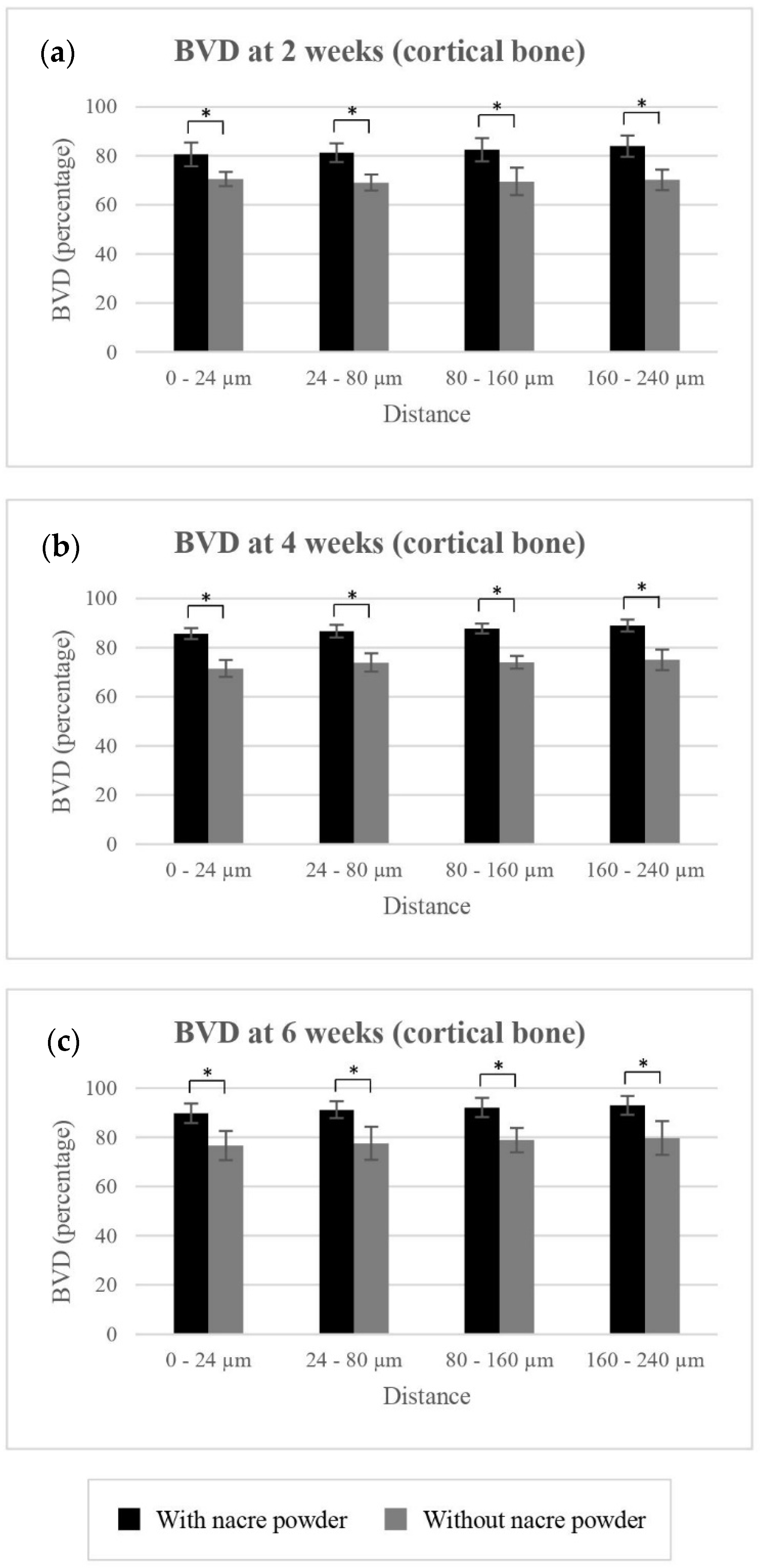
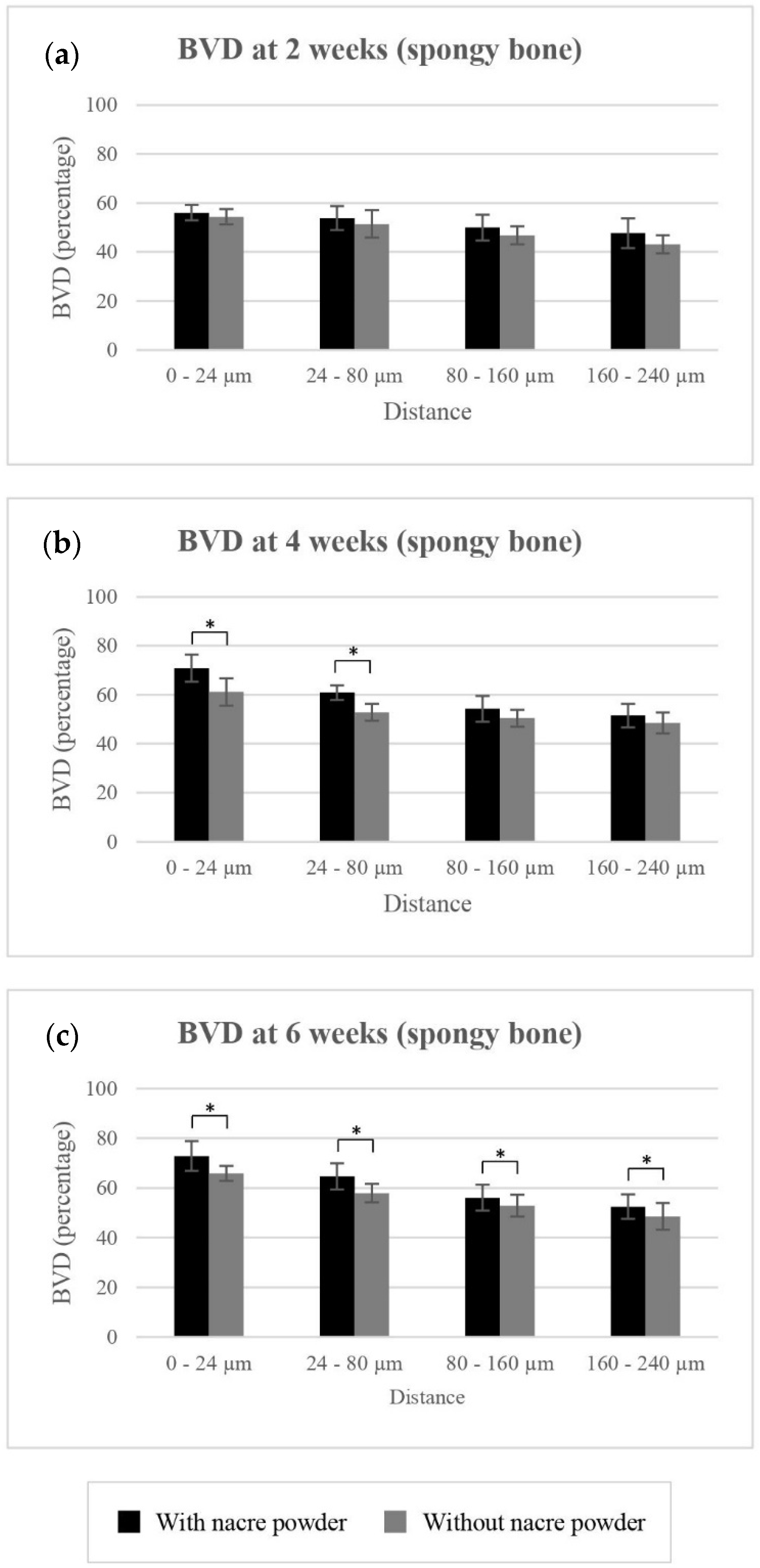
2.2.2. Bone Volume Density (BVD)
BVD of Cortical Bone
BVD of Spongy Bone
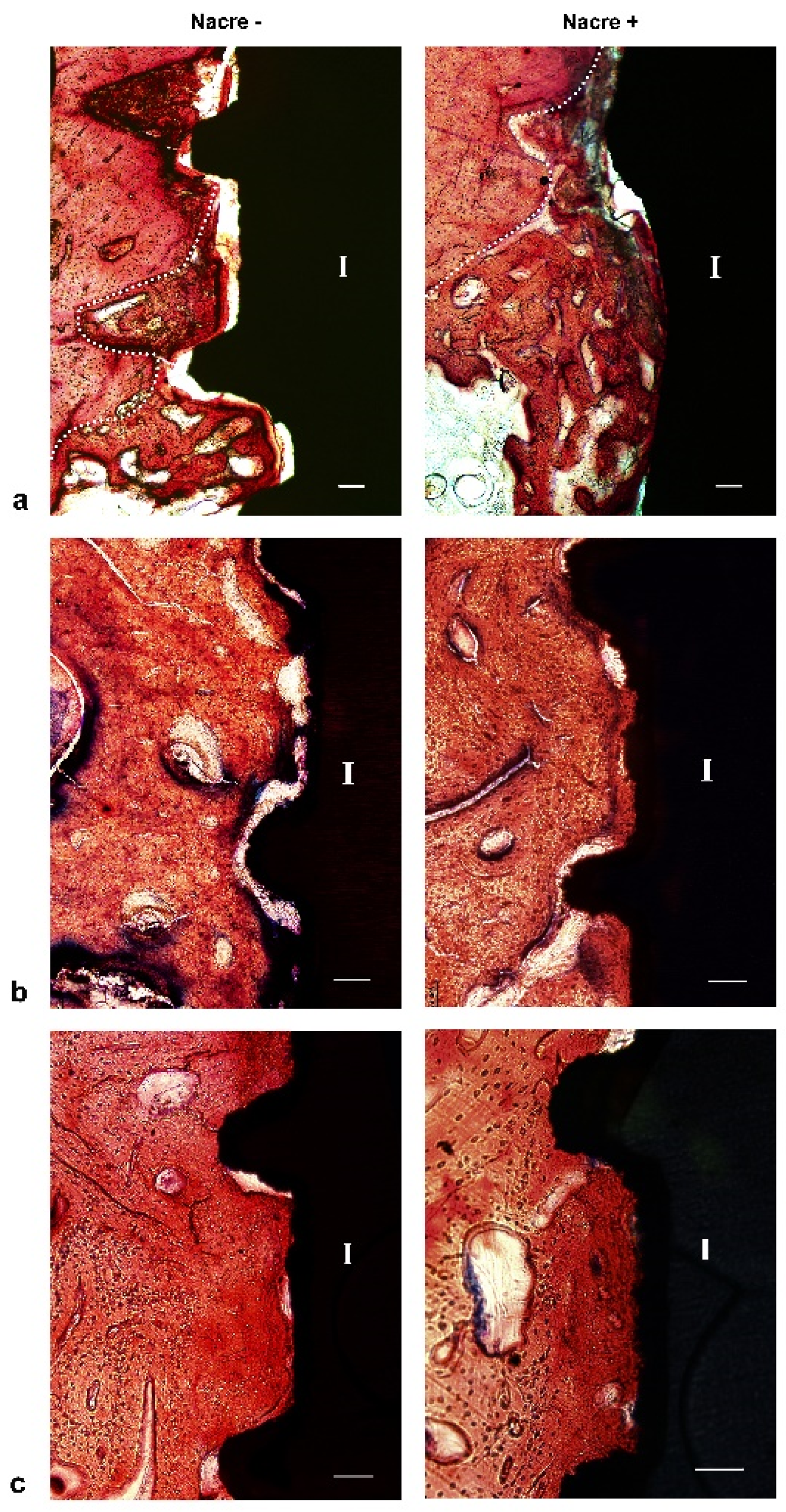
2.3. Histological Study
3. Discussion
4. Materials and Methods
4.1. Implanted Materials
4.1.1. Nacre Powder
4.1.2. Dental Implants
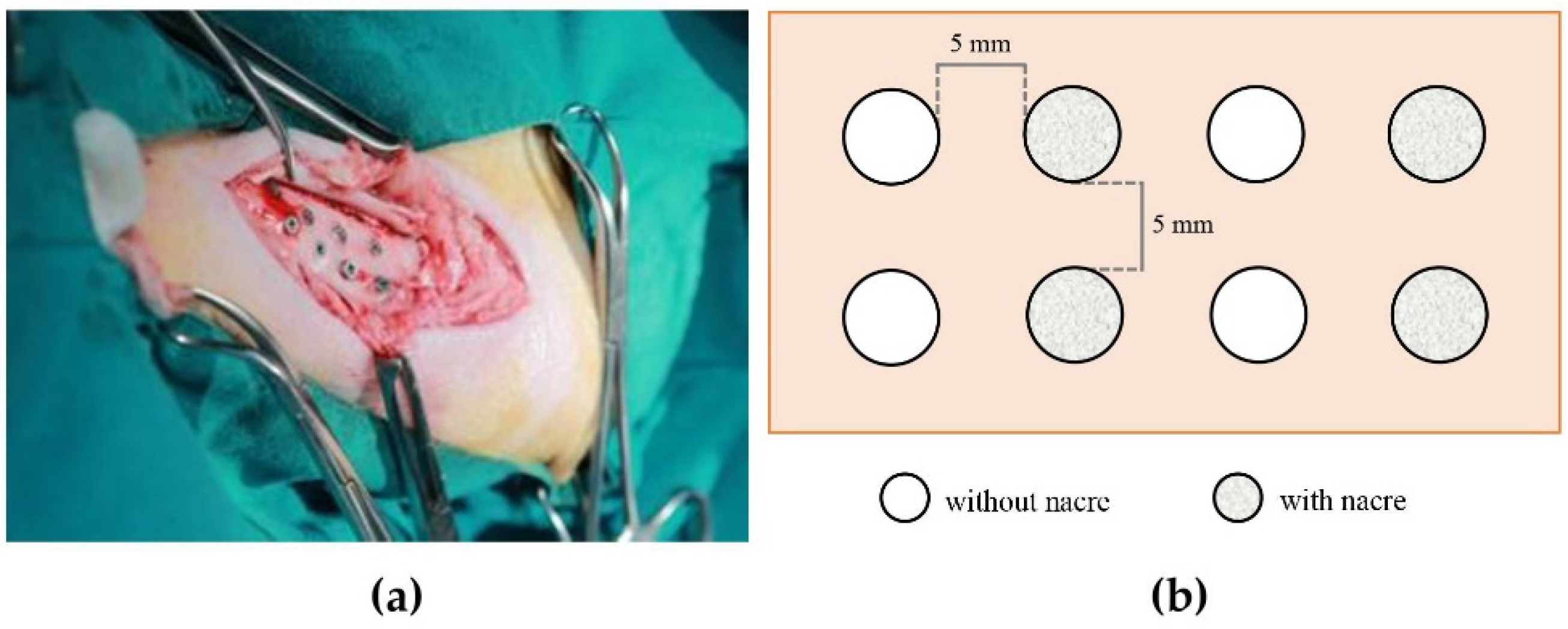
4.2. Animal Implantation
4.3. Micro-CT Study
4.4. Histological Study
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, C.M. Surface modifications of dental implants. Aust. Dent. J. 2008, 53 (Suppl. 1), S26–S33. [Google Scholar] [CrossRef]
- Yeo, I.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Wennerberg, A.; Hallgren, C.; Johansson, C.; Danelli, S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin. Oral Implant. Res. 1998, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Khang, W.; Feldman, S.; Hawley, C.E.; Gunsolley, J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J. Periodontol. 2001, 72, 1384–1390. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Hallgren, C.; Widmark, G.; Sennerby, L.; Wennerberg, A. Histologic evaluation of the bone integration of TiO(2) blasted and turned titanium microimplants in humans. Clin. Oral Implant. Res. 2001, 12, 128–134. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. The combination of micron and nanotopography by H(2)SO(4)/H(2)O(2) treatment and its effects on osteoblast-specific gene expression of hMSCs. J. Biomed. Mater. Res. A 2010, 94, 169–179. [Google Scholar] [CrossRef]
- Lamers, E.; Walboomers, X.F.; Domanski, M.; te Riet, J.; van Delft, F.C.; Luttge, R.; Winnubst, L.A.; Gardeniers, H.J.; Jansen, J.A. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 2010, 31, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Sewing, A.; Aref, A.; Roessler, S. Functionalization of dental implant surfaces using adhesion molecules. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chemistry 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design strategies and applications of nacre-based biomaterials. Acta Biomater. 2017, 54, 21–34. [Google Scholar] [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.S.; Piet, M.H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a natural, multi-use, and timely biomaterial for bone graft substitution. J. Biomed. Mater. Res. A 2017, 105, 662–671. [Google Scholar] [CrossRef]
- Lopez, E.; Vidal, B.; Berland, S.; Camprasse, S.; Camprasse, G.; Silve, C. Demonstration of the capacity of nacre to induce bone formation by human osteoblasts maintained in vitro. Tissue Cell 1992, 24, 667–679. [Google Scholar] [CrossRef]
- Asvanund, P.; Chunhabundit, P.; Suddhasthira, T. Potential induction of bone regeneration by nacre: An in vitro study. Implant Dent. 2011, 20, 32–39. [Google Scholar] [CrossRef]
- Green, D.W.; Kwon, H.J.; Jung, H.S. Osteogenic potency of nacre on human mesenchymal stem cells. Mol. Cells 2015, 38, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Westbroek, P.; Marin, F. A marriage of bone and nacre. Nature 1998, 392, 861–862. [Google Scholar] [CrossRef]
- Liao, H.; Brandsten, C.; Lundmark, C.; Wurtz, T.; Li, J. Responses of bone to titania-hydroxyapatite composite and nacreous implants: A preliminary comparison by in situ hybridization. J. Mater. Sci. Mater. Med. 1997, 8, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Mutvei, H.; Sjöström, M.; Hammarström, L.; Li, J. Tissue responses to natural aragonite (Margaritifera shell) implants in vivo. Biomaterials 2000, 21, 457–468. [Google Scholar] [CrossRef]
- Lamghari, M.; Almeida, M.J.; Berland, S.; Huet, H.; Laurent, A.; Milet, C.; Lopez, E. Stimulation of bone marrow cells and bone formation by nacre: In vivo and in vitro studies. Bone 1999, 25, 91S–94S. [Google Scholar] [CrossRef]
- Duplat, D.; Chabadel, A.; Gallet, M.; Berland, S.; Bédouet, L.; Rousseau, M.; Kamel, S.; Milet, C.; Jurdic, P.; Brazier, M.; et al. The in vitro osteoclastic degradation of nacre. Biomaterials 2007, 28, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Atlan, G.; Delattre, O.; Berland, S.; LeFaou, A.; Nabias, G.; Cot, D.; Lopez, E. Interface between bone and nacre implants in sheep. Biomaterials 1999, 20, 1017–1022. [Google Scholar] [CrossRef]
- Liao, H.; Mutvei, H.; Hammarström, L.; Wurtz, T.; Li, J. Tissue responses to nacreous implants in rat femur: An in situ hybridization and histochemical study. Biomaterials 2002, 23, 2693–2701. [Google Scholar] [CrossRef]
- Asvanund, P.; Chunhabundit, P. Alveolar bone regeneration by implantation of nacre and B-tricalcium phosphate in guinea pig. Implant Dent. 2012, 21, 248–253. [Google Scholar] [CrossRef]
- Laiblin, C.; Jaeschke, G. Klinisch-chemische Untersuchungen des Knochen- und Muskelstoffwechsels unter Belastung beim Göttinger Miniaturschwein--eine experimentelle Studie [Clinical chemistry examinations of bone and muscle metabolism under stress in the Göttingen miniature pig--an experimental study]. Berl. Münchener Tierärztliche Wochenschr. 1979, 92, 124–128. [Google Scholar]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Butz, F.; Ogawa, T.; Chang, T.L.; Nishimura, I. Three-dimensional bone-implant integration profiling using micro-computed tomography. Int. J. Oral Maxillofac. Implant. 2006, 1, 687–695. [Google Scholar]
- Marco, F.; Milena, F.; Gianluca, G.; Vittoria, O. Peri-implant osteogenesis in health and osteoporosis. Micron 2005, 36, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Franchi, M.; Orsini, E.; Trire, A.; Quaranta, M.; Martini, D.; Piccari, G.G.; Ruggeri, A.; Ottani, V. Osteogenesis and morphology of the peri-implant bone facing dental implants. Sci. World J. 2004, 4, 1083–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laothumthut, T.; Jantarat, J.; Paemanee, A.; Roytrakul, S.; Chunhabundit, P. Shotgun proteomics analysis of proliferating STRO-1-positive human dental pulp cell after exposure to nacreous water-soluble matrix. Clin. Oral Investig. 2015, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, W.; Fan, H.; Xu, P. Water-soluble nano-pearl powder promotes MC3T3-E1 cell differentiation by enhancing autophagy via the MEK/ERK signaling pathway. Mol. Med. Rep. 2018, 18, 993–1000. [Google Scholar] [CrossRef]


| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Without Nacre Powder (Controls) | With Nacre Powder | ||||||
| Time Points | 2 Weeks | 4 Weeks | 6 Weeks | 2 Weeks | 4 Weeks | 6 Weeks | |
| BIC (%) | |||||||
| 63.20 ± 2.20 | 70.67 ± 3.23 | 71.99 ± 3.24 | 68.99 ± 4.13 | 78.47 ± 5.15 | 81.19 ± 5.96 | ||
| BVD (%): cortical bone | |||||||
| 0 to 24 µm | 70.52 ± 2.91 | 71.45 ± 3.42 | 76.62 ± 5.97 | 80.61 ± 4.78 | 85.62 ± 2.18 | 89.79 ± 3.94 | |
| 24 to 80 µm | 69.09 ± 3.24 | 73.88 ± 3.77 | 77.62 ± 6.71 | 81.27 ± 3.75 | 86.68 ± 2.63 | 91.21 ± 3.39 | |
| 80 to 160 µm | 69.56 ± 5.64 | 74.01 ± 2.60 | 78.92 ± 4.95 | 82.49 ± 4.75 | 87.78 ± 1.97 | 92.14 ± 3.92 | |
| 160 to 240 µm | 70.22 ± 4.22 | 75.05 ± 4.19 | 79.71 ± 6.85 | 83.96 ± 4.36 | 88.99 ± 2.41 | 93.01 ± 3.77 | |
| BVD (%): spongy bone | |||||||
| 0 to 24 µm | 54.36 ± 3.14 | 61.12 ± 5.54 | 65.84 ± 3.02 | 56.02 ± 3.10 | 70.81 ± 5.49 | 72.83 ± 6.01 | |
| 24 to 80 µm | 51.46 ± 5.52 | 52.79 ± 3.45 | 57.89 ± 3.72 | 53.85 ± 4.84 | 60.86 ± 2.98 | 64.64 ± 5.33 | |
| 80 to 160 µm | 46.80 ± 3.72 | 50.45 ± 3.43 | 52.87 ± 4.40 | 49.95 ± 5.27 | 54.23 ± 5.30 | 56.05 ± 5.18 | |
| 160 to 240 µm | 43.15 ± 3.71 | 48.50 ± 4.24 | 48.54 ± 5.30 | 47.65 ± 6.03 | 51.47 ± 4.86 | 52.42 ± 4.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leelatian, L.; Chunhabundit, P.; Charoonrut, P.; Asvanund, P. Induction of Osseointegration by Nacre in Pigs. Molecules 2022, 27, 2653. https://doi.org/10.3390/molecules27092653
Leelatian L, Chunhabundit P, Charoonrut P, Asvanund P. Induction of Osseointegration by Nacre in Pigs. Molecules. 2022; 27(9):2653. https://doi.org/10.3390/molecules27092653
Chicago/Turabian StyleLeelatian, Leena, Panjit Chunhabundit, Phingphol Charoonrut, and Pattapon Asvanund. 2022. "Induction of Osseointegration by Nacre in Pigs" Molecules 27, no. 9: 2653. https://doi.org/10.3390/molecules27092653









