Enzymatic Synthesis of Fatty Acid Isoamyl Monoesters from Soybean Oil Deodorizer Distillate: A Renewable and Ecofriendly Base Stock for Lubricant Industries
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization of Soybean Oil Deodorizer Distillate (SODD)
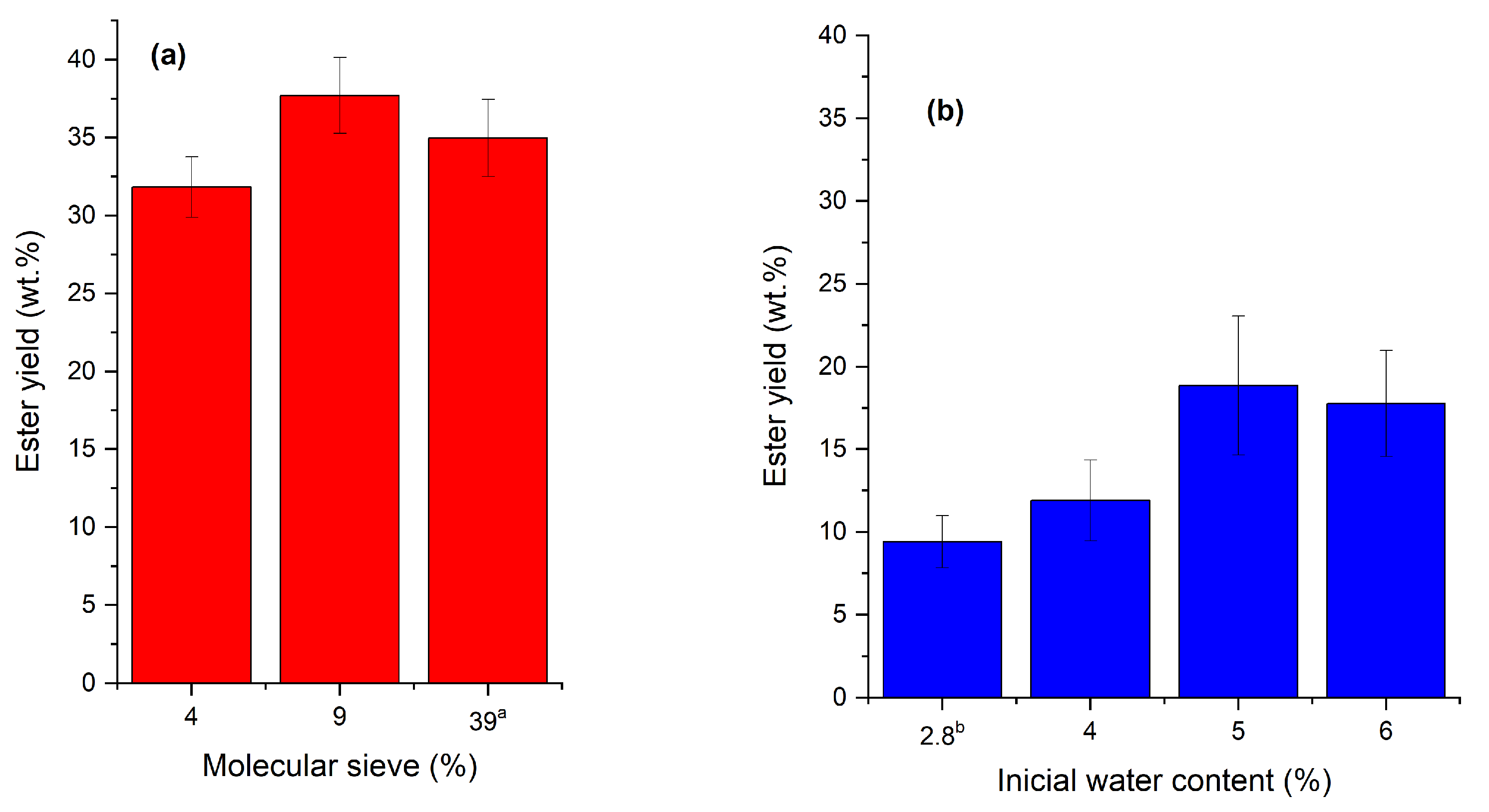
2.2. Rotational Central Composite Design (RCCD) and Analysis by Response Surface Methodology (RSM)
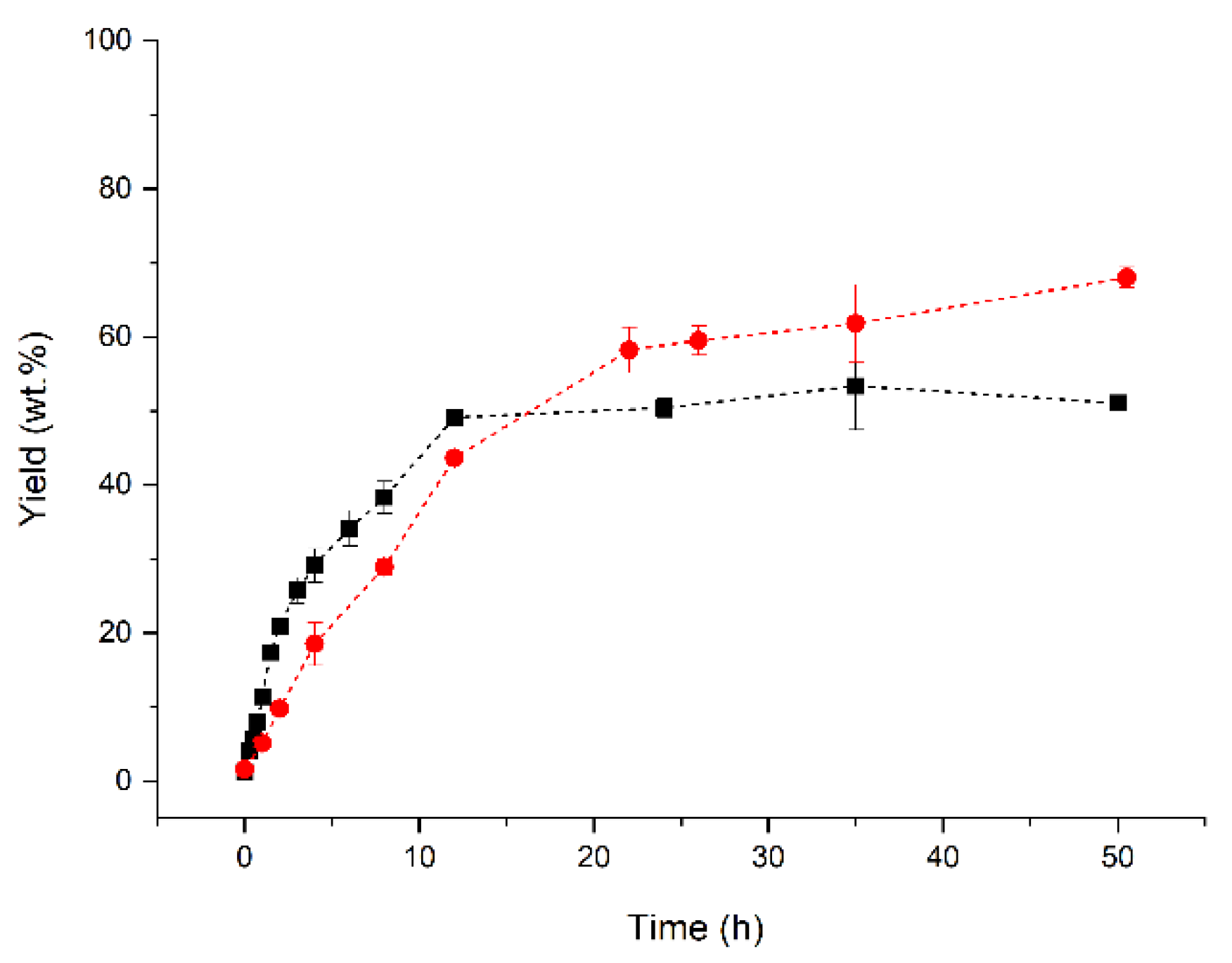
2.3. Reaction Course under Optimal Conditions
2.4. Chemical Composition and Some Physicochemical Properties of the Products
3. Materials and Methods
3.1. Materials
3.2. Rotational Central Composite Design (RCCD)
3.3. Reaction Courses
3.4. Chromatographic Analysis
3.4.1. Ester Analysis
3.4.2. Analysis of Acylglycerides and Glycerin
3.5. Chemical and Physicochemical Characterizations of the Reaction Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agric. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Afifah, A.N.; Syahrullail, S.; Wan Azlee, N.I.; Rohah, A.M. Synthesis and tribological studies of epoxidized palm stearin methyl ester as a green lubricant. J. Clean. Prod. 2021, 280, 124320. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A review on the science and technology of natural and synthetic biolubricants. J. Bio Tribo-Corrosion 2017, 3, 11. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Improved thermo-oxidative stability of structurally modified waste cooking oil methyl esters for bio-lubricant application. J. Clean. Prod. 2016, 112, 4515–4524. [Google Scholar] [CrossRef]
- Angulo, B.; Fraile, J.M.; Gil, L.; Herrerías, C.I. Bio-lubricants production from fish oil residue by transesterification with trimethylolpropane. J. Clean. Prod. 2018, 202, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Prasannakumar, P.; Edla, S.; Thampi, A.D.; Arif, M.; Santhakumari, R. A comparative study on the lubricant properties of chemically modified Calophyllum inophyllum oils for bio-lubricant applications. J. Clean. Prod. 2022, 339, 130733. [Google Scholar] [CrossRef]
- ExxonMobil Synthetic Lubricant Base Stocks Formulations Guide. Available online: https://www.exxonmobilchemical.com/-/media/project/wep/exxonmobil-chemicals/chemicals/low-viscosity-polyalphaolefins/synthetic_lubricant_base_stocks_formulations_guide_en_2017pdf.pdf (accessed on 8 February 2022).
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Chemical transformations of renewable lubricant feedstocks. In Biolubricants: Science and Technology; Bart, J.C.J., Gucciardi, E., Cavallaro, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 249–350. [Google Scholar]
- Bolina, I.C.A.; Gomes, R.A.B.; Mendes, A.A. Biolubricant production from several oleaginous feedstocks using lipases as catalysts: Current scenario and future perspectives. BioEnergy Res. 2021, 14, 1039–1057. [Google Scholar] [CrossRef]
- Devi, B.L.A.P.; Reddy, T.V.K.; Yusoff, M.F.M. Ionic liquids in the production of biodiesel and other oleochemicals. In Ionic Lquids in Lipid Processing and Analysis: Opportunities and Challenges; Xu, X., Guo, Z., Cheong, L.-Z., Eds.; Academic Press & AOCS Press: London, UK, 2016; p. 486. ISBN 978-1-63067-047-4. [Google Scholar]
- Boyde, S.; Randles, S.J. Esters. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; Rudnick, L.R., Ed.; CRC Press: New York, NY, USA, 2013; p. 1018. [Google Scholar]
- Salih, N. A review on new trends, challenges and prospects of ecofriendly friendly green food-grade biolubricants. Biointerface Res. Appl. Chem. 2021, 12, 1185–1207. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Renewable lubricants. In Biolubricants: Science and Technology; Bart, J.C.J., Gucciardi, E., Cavallaro, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–9. [Google Scholar]
- BASF. Esters–Base Stocks Selection Guide for Lubricants and Metalworking Fluids. Available online: https://www.btc-europe.com/fileadmin/user_upload/Downloads/Pdf_s/Industries/Brochure_Selection-Guide-Base-Stocks-Esters.pdf (accessed on 15 April 2021).
- Luzuriaga, S. Biolubricants Market during COVID-19. Available online: https://www.stle.org/files/TLTArchives/2020/09_September/Market_Trends.aspx (accessed on 19 February 2022).
- Markets and Markets. Lubricants Market by Base Oil (Mineral Oil, Synthetic Oil, Bio-Based Oil), Product Type (Engine Oil, Hydraulic Fluid, Metalworking Fluid), Application (Transportation and Industrial Lubricants), Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/lubricants-market-182046896.html?gclid=EAIaIQobChMIyqOy3LP27gIVBA-RCh0iYQCKEAAYASAAEgIj6_D_BwE (accessed on 19 February 2022).
- Tsagaraki, E.; Karachaliou, E.; Delioglanis, I.; Kouzi, E. Bio-Based Products and Applications Potential (3.0); Zenodo: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
- Byerlee, D.; Falcon, W.P.; Naylor, R.L. Soybean production and supply chains in the tropics. In The Tropical Oil Crop Revolution: Food, Feed, Fuel, and Forests; Byerlee, D., Falcon, W.P., Naylor, R.L., Eds.; Oxford University Press: New York, NY, USA, 2017; p. 305. [Google Scholar]
- Avagyan, A.B.; Singh, B. Biodiesel from plant oil and waste cooking oil. In Biodiesel: Feedstocks, Technologies, Economics and Barriers; Avagyan, A.B., Singh, B., Eds.; Springer: Singapore, 2019; p. 137. ISBN 978-981-13-5745-9. [Google Scholar]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. The transition from reliance on fossil resources to biomass valorisation. In Biolubricants: Science and Technology; Bart, J.C.J., Gucciardi, E., Cavallaro, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 74–120. [Google Scholar]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Greyt, W. De Deodorization. Available online: https://lipidlibrary.aocs.org/edible-oil-processing/deodorization (accessed on 17 August 2020).
- Gunawan, S.; Kasim, N.; Ju, Y. Separation and purification of squalene from soybean oil deodorizer distillate. Sep. Purif. Technol. 2008, 60, 128–135. [Google Scholar] [CrossRef]
- Sherazi, T.H.S.; Mahesar, A.S. Vegetable oil deodorizer distillate: A rich source of the natural bioactive components. J. Oleo Sci. 2016, 65, 957–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, A.C.; Cansian, A.B.M.; Guimarães, J.R.; Vieira, A.M.S.; Fernandez-Lafuente, R.; Tardioli, P.W. Performance of liquid Eversa on fatty acid ethyl esters production by simultaneous esterification/transesterification of low-to-high acidity feedstocks. Catalysts 2021, 11, 1486. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Souza, S.L.; Langone, M.A.P. Study of immobilized lipase Lipozyme RM IM in esterification reactions for biodiesel synthesis. Quim. Nova 2013, 36, 646–650. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Wei, W.; Wang, S.; Li, X.; Zhang, Y.; Wang, Z. Immobilization of Lipozyme TL 100L for methyl esterification of soybean oil deodorizer distillate. 3 Biotech 2020, 10, 51. [Google Scholar] [CrossRef]
- Du, W.; Wang, L.; Liu, D. Improved methanol tolerance during Novozym435-mediated methanolysis of SODD for biodiesel production. Green Chem. 2007, 9, 173–176. [Google Scholar] [CrossRef]
- Panpipat, W.; Xu, X.; Guo, Z. Towards a commercially potential process: Enzymatic recovery of phytosterols from plant oil deodoriser distillates mixture. Process Biochem. 2012, 47, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.S.; Aguieiras, E.C.G.; da Silva, M.A.P.; Langone, M.A.P. Biodiesel synthesis via esterification of feedstock with high content of free fatty acids. Appl. Biochem. Biotechnol. 2009, 154, 74–88. [Google Scholar] [CrossRef]
- Visioli, L.J.; de Castilhos, F.; Cardozo-Filho, L.; de Mello, B.T.F.; da Silva, C. Production of esters from soybean oil deodorizer distillate in pressurized ethanol. Fuel Process. Technol. 2016, 149, 326–331. [Google Scholar] [CrossRef]
- Yin, X.; You, Q.; Ma, H.; Dai, C.; Zhang, H.; Li, K.; Li, Y. Biodiesel production from soybean oil deodorizer distillate enhanced by counter-current pulsed ultrasound. Ultrason. Sonochem. 2015, 23, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Duan, X.; You, Q.; Dai, C.; Tan, Z.; Zhu, X. Biodiesel production from soybean oil deodorizer distillate usingcalcined duck eggshell as catalyst. Energy Convers. Manag. 2016, 112, 199–207. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, X.; Wan, M.; Duan, X.; You, Q.; Zhang, J.; Li, S. Intensification of biodiesel production using dual-frequency counter-current pulsed ultrasound. Ultrason. Sonochem. 2017, 37, 136–143. [Google Scholar] [CrossRef]
- Yun, L.; Ling, W.; Yunjun, Y. Cogeneration of biodiesel and tocopherols by combining pretreatment with supercritical carbon dioxide extraction from soybean oil deodorizer distillate. Chem. Technol. Fuels Oils 2010, 46, 79–86. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Fernandez-Lafuente, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic synthesis of biolubricants from by-product of soybean oil processing catalyzed by different biocatalysts of Candida rugosa lipase. Catal. Today 2021, 362, 122–129. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the fusel oil distillation process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351. [Google Scholar] [CrossRef]
- Incauca, S.A.S. Fusel Oil. Available online: http://www.incauca.com/en/producto/fusel-oil/ (accessed on 22 August 2018).
- Pérez, E.R.; Cardoso, D.R.; Franco, D.W. Análise dos álcoois, ésteres e compostos carbonílicos em amostras de óleo fúsel. Quim. Nova 2001, 24, 10–12. [Google Scholar] [CrossRef] [Green Version]
- Cerón, A.A.; Vilas Boas, R.N.; Biaggio, F.C.; de Castro, H.F. Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: Batch and continuous processes. Biomass Bioenergy 2018, 119, 166–172. [Google Scholar] [CrossRef]
- Dörmő, N.; Bélafi-Bakó, K.; Bartha, L.; Ehrenstein, U.; Gubicza, L. Manufacture of an environmental-safe biolubricant from fusel oil by enzymatic esterification in solvent-free system. Biochem. Eng. J. 2004, 21, 229–234. [Google Scholar] [CrossRef]
- Falkeborg, M.; Berton-Carabin, C.C.; Cheong, L.-Z. Ionic liquids in the synthesis of antioxidant targeted compounds. In Ionic Liquids in Lipid Processing and Analysis: Opportunities and Challenges; Xu, X., Guo, Z., Cheong, L.-Z., Eds.; AOCS Press: London, UK, 2016; p. 486. [Google Scholar]
- Raof, N.A.; Yunus, R.; Rashid, U.; Azis, N.; Yaakub, Z. Effect of molecular structure on oxidative degradation of ester based transformer oil. Tribol. Int. 2019, 140, 105852. [Google Scholar] [CrossRef]
- Konwar, B.K.; Sagar, K. Introduction. In Lipase: An industrial Enzyme through Metagenomics; Konwar, B.K., Sagar, K., Eds.; Apple Academic Press: Oakville, ON, Canada, 2018; p. 231. [Google Scholar]
- Parkin, K.L. Enzymes. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; p. 1125. ISBN 9781482208146. [Google Scholar]
- Paul, C.E.; Fernández, V.G. Biocatalysis and biotransformation in ionic liquids. In Ionic Liquids in Lipid Pocessing and Analysis; Xu, X., Guo, Z., Cheong, L.-Z., Eds.; Academic Press & AOCS Press: London, UK, 2016; p. 486. ISBN 978-1-63067-047-4. [Google Scholar]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Da Silva, J.A.C.; Freire, D.M.G. Produção de biolubrificantes catalisada por lipases: Fundamentos e aplicações. In Biotecnologia Aplicada à Agro & Indústria—Volume 4; Resende, R.R., Ed.; Edgard Blücher LTDA: São Paulo, Brazil, 2016; p. 1070. ISBN 978-85-212-1115-0. [Google Scholar]
- Miranda, L.P.; Guimarães, J.R.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Composites of crosslinked aggregates of Eversa® transform and magnetic nanoparticles. Performance in the ethanolysis of soybean oil. Catalysts 2020, 10, 817. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via alcoholysis of sunflower oil by Eversa lipases immobilized on hydrophobic supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized biocatalysts of Eversa® Transform 2.0 and lipase from Thermomyces lanuginosus: Comparison of some properties and performance in biodiesel production. Catalysts 2020, 10, 738. [Google Scholar] [CrossRef]
- Mibielli, G.M.; Fagundes, A.P.; Bender, J.P.; Oliveira, J.V. Lab and pilot plant FAME production through enzyme-catalyzed reaction of low-cost feedstocks. Bioresour. Technol. Rep. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- Remonatto, D.; Santin, C.M.T.; de Oliveira, D.; Di Luccio, M.; de Oliveira, J.V. FAME production from waste oils through commercial soluble lipase Eversa® catalysis. Ind. Biotechnol. 2016, 12, 254–262. [Google Scholar] [CrossRef]
- Novozymes A/S. The Novozymes Enzymatic Biodiesel Handbook. Available online: https://www.novozymes.com/en/advance-your-business/food-and-beverage/vegetable-oils-processing/biodiesel (accessed on 11 February 2021).
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization of Eversa lipase on octyl agarose beads and preliminary characterization of stability and activity features. Catalysts 2018, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Marty, A.; Dossat, V.; Condoret, J.-S. Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: Influence of the production of hydrophilic compounds. Biotechnol. Bioeng. 1997, 56, 232–237. [Google Scholar] [CrossRef]
- Alves, J.S.; Garcia-Galan, C.; Schein, M.F.; Silva, A.M.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paludo, N.; Alves, J.S.; Altmann, C.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem. 2015, 22, 89–94. [Google Scholar] [CrossRef]
- Martins, A.B.; Schein, M.F.; Friedrich, J.L.R.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Ultrasound-assisted butyl acetate synthesis catalyzed by Novozym 435: Enhanced activity and operational stability. Ultrason. Sonochem. 2013, 20, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.-S.; Monsan, P.; Marty, A. Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzyme Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J. Mol. Catal. B Enzym. 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Vescovi, V.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Immobilized lipases on functionalized silica particles as potential biocatalysts for the synthesis of fructose oleate in an organic solvent/water system. Molecules 2017, 22, 212. [Google Scholar] [CrossRef]
- Vescovi, V.; Kopp, W.; Guisán, J.M.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino-glutaraldehyde spacer arms. Process Biochem. 2016, 51, 2055–2066. [Google Scholar] [CrossRef]
- Lima, L.N.; Oliveira, G.C.; Rojas, M.J.; Castro, H.F.; Da Rós, P.C.M.; Mendes, A.A.; Giordano, R.L.C.; Tardioli, P.W. Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. J. Ind. Microbiol. Biotechnol. 2015, 42, 523–535. [Google Scholar] [CrossRef]
- Martins, A.B.; Friedrich, J.L.R.; Cavalheiro, J.C.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Du, W.; Liu, D.; Li, L.; Dai, N. Lipase-catalyzed biodiesel production from soybean oil deodorizer distillate with absorbent present in tert-butanol system. J. Mol. Catal. B Enzym. 2006, 43, 29–32. [Google Scholar] [CrossRef]
- Colombié, S.; Tweddell, R.J.; Condoret, J.-S.; Marty, A. Water activity control: A way to improve the efficiency of continuous lipase esterification. Biotechnol. Bioeng. 1998, 60, 362–368. [Google Scholar] [CrossRef]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- Yahya, A.R.; Anderson, W.A.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzyme Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Nott, K.; Brognaux, A.; Richard, G.; Laurent, P.; Favrelle, A.; Jérôme, C.; Blecker, C.; Wathelet, J.-P.; Paquot, M.; Deleu, M. (Trans)esterification of mannose catalyzed by lipase B from Candida antarctica in an improved reaction medium using co-solvents and molecular sieve. Prep. Biochem. Biotechnol. 2012, 42, 348–363. [Google Scholar] [CrossRef]
- Omar, I.C.; Nishio, N.; Nagai, S. The role of water on the equilibrium of esterification by immobilized lipase packed-bed column reactor. Biotechnol. Lett. 1988, 10, 799–804. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experiments: Design, Innovation, and Discovery, 2nd ed.; Balding, D.J., Boomfield, P., Cressie, N.A.C., Fisher, N.L., Johntsone, L.M., Kadane, J.B., Ryan, L.M., Scott, D.W., Smith, A.F.M., Teugels, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 13 978-0471-718713-0. [Google Scholar]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in enzymatic biodiesel production and commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.S.A.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A review from thermodynamic and kinetic perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Walde, P.; Luisi, P.L. Dependence of lipase activity on water content and enzyme concentration in reverse micelles. Biocatalysis 1990, 4, 153–161. [Google Scholar] [CrossRef]
- Petro-Canada Lubricants Inc. Petro-Canada Lubricants Handbook 2017; Petro-Canada Lubricants Inc.: Mississauga, ON, Canada, 2017; pp. 1–228. [Google Scholar]
- Shell. Marine Lubricants for Marine Applications; Shell: London, UK, 2019; pp. 1–8. [Google Scholar]
- Perez, J.M.; Rudnick, L.R.; Erhan, S.Z. Natural oils as lubricants. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 1018. [Google Scholar]
- Sathwik Chatra, K.R.; Jayadas, N.H.; Kailas, S.V. Natural oil-based lubricants. In Green Tribology—Biomimetics, Energy Conservation and Sustainability; Nosonovsky, M., Bhushan, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; p. 647. [Google Scholar]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. (Eds.) Renewable feedstocks for lubricant production. In Biolubricants: Science and Technology; Woodhead Publishing: Cambridge, UK, 2013; p. 944. [Google Scholar]
- Kenbeck, D.; Bunemann, T.F. Organic friction modifiers. In Lubricant Additives: Chemistry and Applications; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 709. [Google Scholar]
- Luther, R. Bio-based and biodegradable base oils. In Encyclopedia of Lubricants and Lubrication—Volume 1; Mang, T., Ed.; Springer: Weinheim, Germany, 2014; p. 2413. [Google Scholar]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Duvekot, C. Determination of Total FAME and Linolenic Acid Methyl Esters in Biodiesel According to EN-14103. Available online: https://www.agilent.com/cs/library/applications/5990-8983EN.pdf (accessed on 18 June 2020).
- McCurry, J.D.; Wang, C.-X. Analysis of Glycerin and Glycerides in Biodiesel (B100) Using ASTM D6584 and EN14105. Available online: https://www.agilent.com/cs/library/applications/5989-7269CHCN.pdf (accessed on 18 June 2020).
- AOCS Official Method Ca 2e-84. Moisture Karl Fischer reagent. In Official Methods and Recommended Pratices of the AOCS; Firestone, D., Ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2004. [Google Scholar]
- AOCS Official Method Cd 3d-63. Acid value. In Official Methods and Recommended Practices of the AOCS; Firestone, D., Ed.; AOCS Press: Champaign, IL, USA, 2004. [Google Scholar]
- AOCS Official Method Cd 3-25. Saponification value. In Official Methods and Recommended Practices of the AOCS; Firestone, D., Ed.; AOCS Press: Champaign, IL, USA, 2004. [Google Scholar]

| Parameter | Value |
|---|---|
| Saponification value (mgKOH/g) Saponification value (wt.%) | 186.75 ± 4.25 93.84 ± 2.13 |
| Acid value (mgKOH/g) Acid value (wt.%) | 36.85 ± 0.07 18.52 ± 0.03 |
| Moisture (wt.%) | 0.18 |
| Relative density | 0.921 |
| Viscosity at 40 °C (mm2/s) | 33.5 |
| Viscosity at 100 °C (mm2/s) | 7.3 |
| Viscosity index | 191.6 |
| Pour point (°C) | −3.0 |
| Flash point (°C) | 210 |
| Oxidation stability (min) | 43 |
| Corrosiveness to copper | 1A (a) |
| Free glycerol (wt.%) | 0.003 |
| Monoglycerides (wt.%) | 1.38 |
| Diglycerides (wt.%) | 4.39 |
| Triglycerides (wt.%) | 69.5 |
| Run | SODDSap: Isoamyl Alcohol Molar Ratio (Risoamyl, ) | Temperature (°C) (T, ) | Enzyme Mass (wt.%) (menz, ) | Reaction Yield (wt.%) () |
|---|---|---|---|---|
| 1 | 1:1 (−1) | 30.0 (−1) | 1.0 (−1) | 14.63 |
| 2 | 1:1 (−1) | 30.0 (−1) | 10.0 (+1) | 20.00 |
| 3 | 1:1 (−1) | 60.0 (+1) | 1.0 (−1) | 8.71 |
| 4 | 1:1 (−1) | 60.0 (+1) | 10.0 (+1) | 14.81 |
| 5 | 1:3 (+1) | 30.0 (−1) | 1.0 (−1) | 11.79 |
| 6 | 1:3 (+1) | 30.0 (−1) | 10.0 (+1) | 33.76 |
| 7 | 1:3 (+1) | 60.0 (+1) | 1.0 (−1) | 4.56 |
| 8 | 1:3 (+1) | 60.0 (+1) | 10.0 (+1) | 47.96 |
| 9 | 1:2 (0) | 45.0 (0) | 5.5 (0) | 39.79 |
| 10 | 1:2 (0) | 45.0 (0) | 5.5 (0) | 41.45 |
| 11 | 1:2 (0) | 45.0 (0) | 5.5 (0) | 43.29 |
| 12 | 1:2 (0) | 45.0 (0) | 5.5 (0) | 42.83 |
| 13 | 1:2 (0) | 45.0 (0) | 5.5 (0) | 48.62 |
| 14 | 1:0.318 (−1.68) | 45.0 (0) | 5.5 (0) | 29.09 |
| 15 | 1:2 (0) | 19.8 (−1.68) | 5.5 (0) | 38.74 |
| 16 | 1:2 (0) | 45.0 (0) | 0 (−1.22) | 1.22 |
| 17 | 1:3.682 (+1.68) | 4)5.0 (0) | 5.5 (0) | 33.33 |
| 18 | 1:2 (0) | 70.2 (+1.68) | 5.5 (0) | 35.57 |
| 19 | 1:2 (0) | 45.0 (0) | 13.1 (+1.68) | 46.92 |
| Source of Variation | SS a | DF b | MS c | Fcalculated | Ftabulated |
|---|---|---|---|---|---|
| Regression | 4129.56 | 5 | 825.91 | 31.09 | 3.02 |
| Residue | 345.37 | 13 | 26.57 | ||
| Lack of fit | 86.04 | 3 | 28.68 | 1.11 | 3.71 |
| Pure error | 259.32 | 10 | 25.93 | ||
| Total | 4474.92 | 18 | 248.61 |
| Parameters | SODD | Product 1 a | Product 2 b | Unity | Standard |
|---|---|---|---|---|---|
| FAIE content | 0 | 43.7 | 55.3 | wt.% | |
| Viscosity at 40 °C | 33.5 | 20.5 | 13.5 | cSt | ASTM D445 |
| Viscosity at 100 °C | 7.3 | 4.9 | 3.6 | cSt | ASTM D445 |
| Viscosity index | 191.6 | 175.0 | 163.8 | - | ASTM 2270 |
| Relative density | 0.921 | 0.911 | 0.899 | - | ASTM D1298 |
| Pour point | −3.0 | −6.0 | −9.0 | °C | ASTM D97 |
| Flash point | 210 | 178 | 104 | °C | ASTM D93 |
| Oxidative stability | 43 | 37 | 53 | min | ASTM D2272 |
| Corrosiveness to copper c | 1A | - | 1B | - | ASTM D130 |
| Saponification value | 186.75 ± 4.25 | 153.3 ± 11.4 | 152.2 ± 0.46 | mgKOH/g | AOCS Cd 3-25 |
| Acid values | 36.85 ± 0.07 | 9.78 ± 0.04 | 15.45 ± 0.08 | mgKOH/g | AOCS Cd 3d-63 |
| Free glycerol | 0.003 | 0.25 | 0.50 | wt.% | ASTM D6584 |
| Monoglycerides | 1.38 | 4.36 | 7.96 | wt.% | ASTM D6584 |
| Diglycerides | 4.39 | 9.04 | 8.38 | wt.% | ASTM D6584 |
| Triglycerides | 69.5 | 3.05 | 1.40 | wt.% | ASTM D6584 |
| Factors | −1.68 | −1 | 0 | +1 | +1.68 |
|---|---|---|---|---|---|
| SODDSap: isoamyl alcohol molar Ratio (Risoamyl) ( ) | 1:0.318 | 1:1 | 1:2 | 1:3 | 1:3.682 |
| Temperature (°C) (T) () | 19.8 | 30 | 45 | 60 | 70.2 |
| Enzyme mass/SODD mass, wt.% (menz) () | 0 | 1 | 5.5 | 10 | 13.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo-Silva, R.; Vieira, A.C.; de Campos Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Enzymatic Synthesis of Fatty Acid Isoamyl Monoesters from Soybean Oil Deodorizer Distillate: A Renewable and Ecofriendly Base Stock for Lubricant Industries. Molecules 2022, 27, 2692. https://doi.org/10.3390/molecules27092692
de Araujo-Silva R, Vieira AC, de Campos Giordano R, Fernandez-Lafuente R, Tardioli PW. Enzymatic Synthesis of Fatty Acid Isoamyl Monoesters from Soybean Oil Deodorizer Distillate: A Renewable and Ecofriendly Base Stock for Lubricant Industries. Molecules. 2022; 27(9):2692. https://doi.org/10.3390/molecules27092692
Chicago/Turabian Stylede Araujo-Silva, Rafael, Ana Carolina Vieira, Roberto de Campos Giordano, Roberto Fernandez-Lafuente, and Paulo Waldir Tardioli. 2022. "Enzymatic Synthesis of Fatty Acid Isoamyl Monoesters from Soybean Oil Deodorizer Distillate: A Renewable and Ecofriendly Base Stock for Lubricant Industries" Molecules 27, no. 9: 2692. https://doi.org/10.3390/molecules27092692
APA Stylede Araujo-Silva, R., Vieira, A. C., de Campos Giordano, R., Fernandez-Lafuente, R., & Tardioli, P. W. (2022). Enzymatic Synthesis of Fatty Acid Isoamyl Monoesters from Soybean Oil Deodorizer Distillate: A Renewable and Ecofriendly Base Stock for Lubricant Industries. Molecules, 27(9), 2692. https://doi.org/10.3390/molecules27092692









