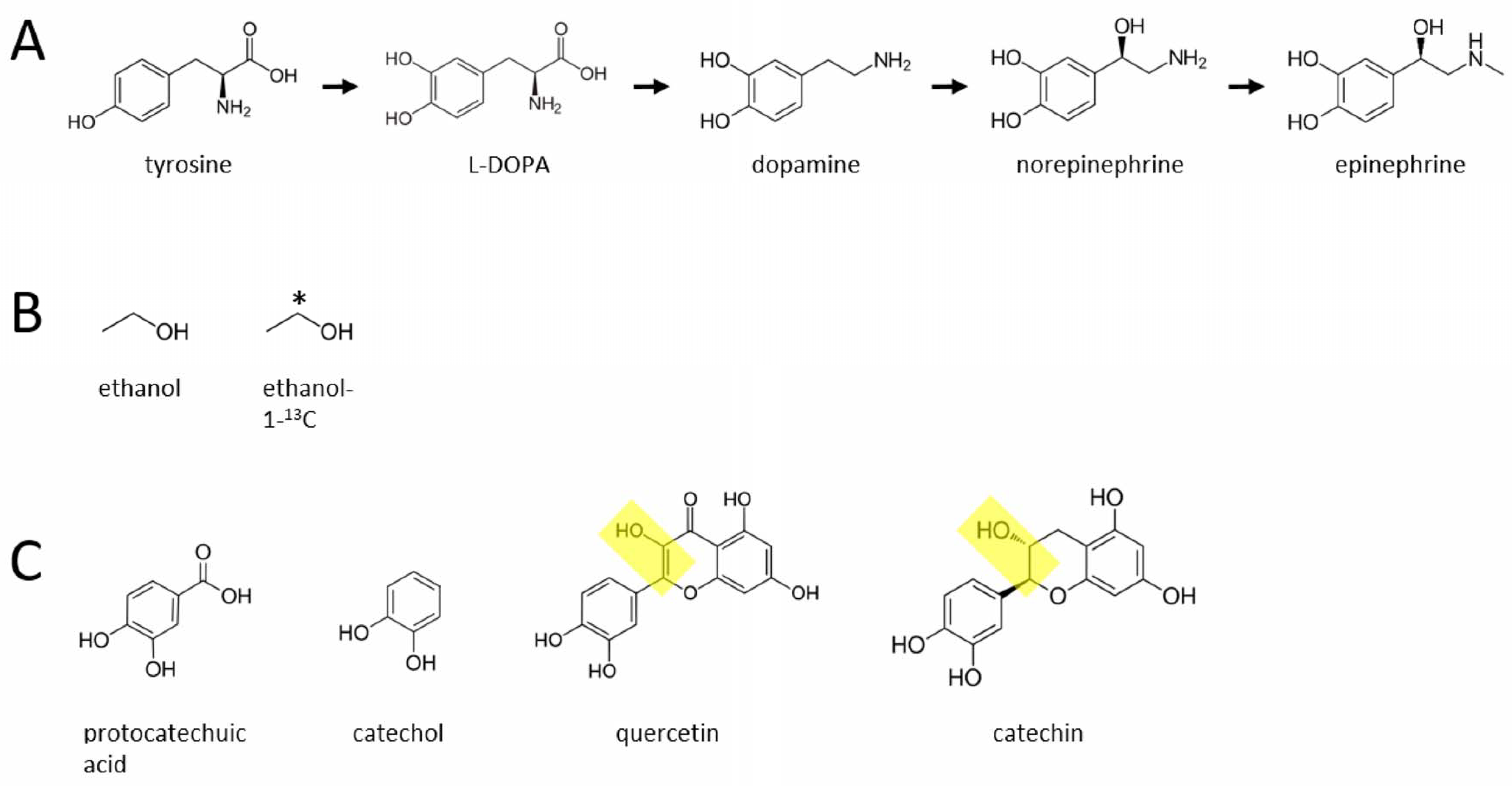
Is There a Novel Biosynthetic Pathway in Mice That Converts Alcohol to Dopamine, Norepinephrine and Epinephrine?
Abstract
:Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Al Ansari, M.; Dawson, A.; Conigrave, K. Alcohol: From Mesopotamia to Modern Iraq. J. Ethn. Subst. Abuse 2021, 20, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Gervilla, E.; Quigg, Z.; Duch, M.; Juan, M.; Guimarães, C. Adolescents’ alcohol use in Botellon and attitudes towards alcohol use and prevention policies. Int. J. Environ. Res. Public Health 2020, 17, 3885. [Google Scholar] [CrossRef] [PubMed]
- Sudhinaraset, M.; Wigglesworth, C.; Takeuchi, D.T. Social and cultural contexts of alcohol use: Influences in a social–ecological framework. Alcohol Res. Curr. Rev. 2016, 38, 35–45. [Google Scholar]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef] [PubMed]
- Axley, P.D.; Richardson, C.T.; Singal, A.K. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin. Liver Dis. 2019, 23, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Leslie, S.W.; Gonzales, R.A. The effects of chronic ethanol exposure on N-methyl-d-aspartate-stimulated overflow of [3H]catecholamines from rat brain. Brain Res. 1991, 547, 289–294. [Google Scholar] [CrossRef]
- Jadzic, D.; Bassareo, V.; Carta, A.R.; Carboni, E. Nicotine, cocaine, amphetamine, morphine, and ethanol increase norepinephrine output in the bed nucleus of stria terminalis of freely moving rats. Addict. Biol. 2019, 26, e12864. [Google Scholar] [CrossRef]
- You, C.; Vandegrift, B.; Brodie, M.S. Ethanol actions on the ventral tegmental area: Novel potential targets on reward pathway neurons. Psychopharmacology 2018, 235, 1711–1726. [Google Scholar] [CrossRef] [Green Version]
- Weinshenker, D.; Schroeder, J.P. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology 2007, 32, 1433–1451. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, P.J. Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: Alcohol, nicotine, marijuana, heroin, cocaine, and caffeine. Subst. Abus. Res. Treat. 2013, 7, 171–183. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3113–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.H.; Fang, Y.Y.; Xu, S.B.; Li, J.; Luo, X.; Pan, D.J.; Wang, M.H.; Wang, W. Alcohol, coffee and tea intake and the risk of cognitive deficits: A dose-response meta-analysis. Epidemiol. Psychiatr. Sci. 2021, 30, E13. [Google Scholar]
- Baum-Baicker, C. The psychological benefits of moderate alcohol consumption: A review of the literature. Drug Alcohol Depend. 1985, 15, 305–322. [Google Scholar] [CrossRef]
- Baum-Baicker, C. The health benefits of moderate alcohol consumption: A review of the literature. Drug Alcohol Depend. 1985, 15, 207–227. [Google Scholar] [CrossRef]
- Brown, F.C.; Zawad, J.O.H.N.; Harralson, J.D. Interactions of pyrazole and ethanol on norepinephrine metabolism in rat brain. J. Pharmacol. Exp. Ther. 1978, 206, 75–80. [Google Scholar] [PubMed]
- Patel, V.A.; Pohorecky, L.A. Acute and chronic ethanol treatment on beta-endorphin and catecholamine levels. Alcohol 1989, 6, 59–63. [Google Scholar] [CrossRef]
- Jaime, S.; Vena, A.A.; Gonzales, R.A. Intravenous Ethanol Administration and Operant Self-Administration Alter Extracellular Norepinephrine Concentration in the Mesocorticolimbic Systems of Male Long Evans Rats. Alcohol. Clin. Exp. Res. 2020, 44, 1529–1539. [Google Scholar] [CrossRef]
- Liljequist, S.; Engel, J. The effect of chronic ethanol administration on central neurotransmitter mechanisms. Med. Biol. 1979, 57, 199–210. [Google Scholar]
- Getachew, B.; Hauser, S.R.; Taylor, R.E.; Tizabi, Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol. Biochem. Behav. 2010, 96, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Ciccocioppo, R.; Panocka, I.; Froldi, R.; Colombo, G.; Gessa, G.L.; Massi, M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology 1999, 144, 151–157. [Google Scholar] [CrossRef]
- Murphy, J.M.; McBride, W.J.; Lumeng, L.; Li, T.K. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol. Biochem. Behav. 1982, 16, 145–149. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Koob, G.F. Effects of β-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology 2010, 212, 431–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.M.; Rasmussen, D.D.; Raskind, M.A.; Koob, G.F. Alpha1-Noradrenergic Receptor Antagonism Blocks Dependence-Induced Increases in Responding for Ethanol. Alcohol 2008, 42, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haass-Koffler, C.L.; Goodyear, K.; Zywiak, W.H.; Magill, M.; Eltinge, S.E.; Wallace, P.M.; Long, V.M.; Jayaram-Lindström, N.; Swift, R.M.; Kenna, G.A.; et al. Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend. 2017, 177, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J. Neurodrinking: Is alcohol a substrate in a novel, endogenous synthetic pathway for norepinephrine? Med. Hypotheses 2012, 78, 760–762. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J. Neurodining: Common dietary factors may be substrates in novel biosynthetic pathways for monoaminergic neurotransmitters. Med. Hypotheses 2020, 138, 109618. [Google Scholar] [CrossRef] [PubMed]
- Andreou, D.; Söderman, E.; Axelsson, T.; Sedvall, G.C.; Terenius, L.; Agartz, I.; Jönsson, E.G. Polymorphisms in genes implicated in dopamine, serotonin and noradrenalin metabolism suggest association with cerebrospinal fluid monoamine metabolite concentrations in psychosis. Behav. Brain Funct. 2014, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Blaschko, H. The specific action of l-dopa decarboxylase. J. Physiol. 1942, 101, 337–349. [Google Scholar] [CrossRef]
- Bulbring, E. The methylation of noradrenaline by minced suprarenal tissue. Br. J. Pharmacol. Chemother. 1949, 4, 234–244. [Google Scholar] [CrossRef]
- Holtz, P. Dopadecarboxylase. Naturwissenschaften 1939, 27, 724–725. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Keller, R.W.; Zigmond, M.J. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience 1988, 27, 897–904. [Google Scholar] [CrossRef]
- Cizza, G.; Pacak, K.; Kvetnansky, R.; Palkovits, M.; Goldstein, D.S.; Brady, L.S.; Fukuhara, K.; Bergamini, E.; Kopin, I.J.; Blackman, M.R.; et al. Decreased stress responsivity of central and peripheral catecholaminergic systems in aged 344/N Fischer rats. J. Clin. Investig. 1995, 95, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.A.; Kalén, P.; Mandel, R.J.; Björklund, A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: A microdialysis study in the rat. Brain Res. 1992, 581, 217–228. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzgerald, P.J. Is There a Novel Biosynthetic Pathway in Mice That Converts Alcohol to Dopamine, Norepinephrine and Epinephrine? Molecules 2022, 27, 2726. https://doi.org/10.3390/molecules27092726
Fitzgerald PJ. Is There a Novel Biosynthetic Pathway in Mice That Converts Alcohol to Dopamine, Norepinephrine and Epinephrine? Molecules. 2022; 27(9):2726. https://doi.org/10.3390/molecules27092726
Chicago/Turabian StyleFitzgerald, Paul J. 2022. "Is There a Novel Biosynthetic Pathway in Mice That Converts Alcohol to Dopamine, Norepinephrine and Epinephrine?" Molecules 27, no. 9: 2726. https://doi.org/10.3390/molecules27092726





