Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Screening of Antifungal Activity
2.3. Antifungal Activity Affected by Concentration (EC50 Value)
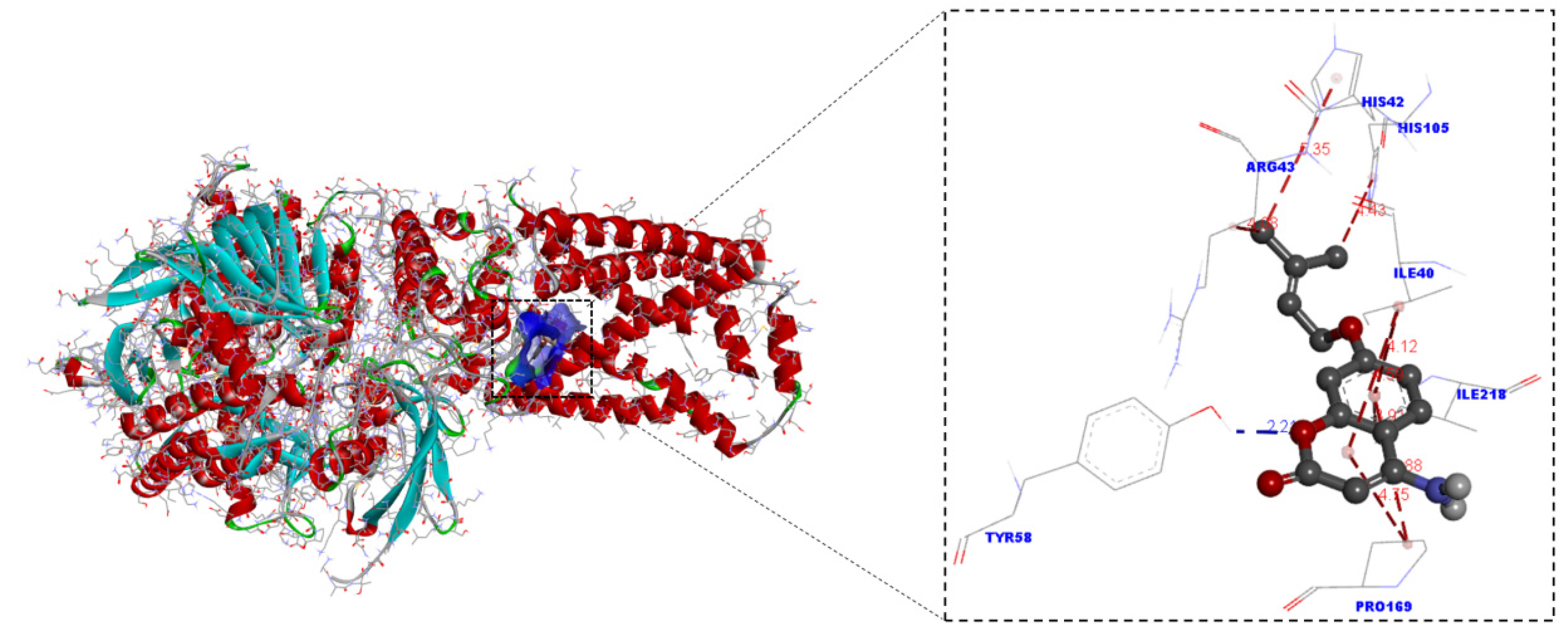
2.4. Molecular Docking
3. Conclusions
4. Experimental Section
4.1. Chemicals and Instruments
4.2. Synthesis and Characterization
4.2.1. General Procedure of Synthesis
4.2.2. Characterization
4.3. Procedure of Antifungal Activity
4.4. Docking Studies and Results Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mckay, A.H.; Hagerty, G.C.; Follas, G.B.; Moore, M.S.; Christie, M.S.; Beresford, R.M. Succinate dehydrogenase inhibitor (SDHI) fungicide resistance prevention strategy. N. Z. Plant Prot. 2011, 64, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Veloukas, T.; Markoglou, A.N.; Karaoglanidis, G.S. Differential effect of Sdh B gene mutations on the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellman, J.K.; Tourneau, D.L.; Stiers, D.L. Effects of some carboxamide fungicides on growth and sclerotium formation of Sclerotium rolfsii. Trans. Br. Mycol. Soc. 1983, 80, 170–172. [Google Scholar] [CrossRef]
- Yang, J.H.; Brannen, P.M.; Schnabel, G. Resistance in Alternaria alternata to SDHI fungicides causes rare disease outbreak in peach orchards. Plant Dis. 2015, 99, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, G.; Wallnfer, P.R.; Plapp, R. Degradation of linuron and some other herbicides and fungicides by a linuron-inducible enzyme obtained from Bacillus sphaericus. Appl. Microbiol. 1971, 22, 284–288. [Google Scholar] [CrossRef]
- Xu, G.F.; Song, B.A.; Pinaki, S.B.; Yang, S.; Zhang, P.W.; Jin, L.H.; Xue, W.; Hu, D.Y.; Lu, P. Synthesis and antifungal activity of novel s-substituted 6-fluoro-4-alkyl (aryl) thioquinazoline derivatives. Bioorg. Med. Chem. 2007, 15, 3768–3774. [Google Scholar] [CrossRef]
- Tarun, K.C.; Prem, D.J. Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food. Chem. 2006, 54, 2129–2133. [Google Scholar]
- Wu, Z.; Zhou, X.; Ye, Y.; Wang, P.; Yang, S. Design, synthesis and insecticidal activities of novel 1-substituted-5-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Chin. Chem. Lett. 2017, 28, 121–125. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, G.; Zhao, X.; Wu, J.; Wu, S. Design, synthesis and antifungal evaluation of N-substituted-1-(3-chloropyridin-2-yl)-N-(pyridin-4-yl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxamide Derivatives. J. Heterocycl. Chem. 2019, 56, 234–238. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, G.; Duan, W.; Zhang, Q.; Huang, Y.; Lei, F. Design, synthesis, and antifungal activity of novel longifolene-derived diacylhydrazine compounds. ACS Omega. 2021, 6, 9104–9111. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, B.; Hao, Z.; Chen, L.; Cao, L.; Guo, X.; Zhang, N.; Yang, D.; Tang, L.; Fan, Z. Design, synthesis and biological evaluation of pyrazole-aromatic containing carboxamides as potent SDH inhibitors. Eur. J. Med. Chem. 2021, 214, 113230. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, L.M.; de Marchi, A.A.; Kanashiro, A.; Lopes, N.P.; da Silva, C.H.T.P.; Pupo, M.T.; Lucisano-Valim, Y.M. Inhibition of horseradish peroxidase catalytic activity by new 3-phenylcoumarin derivatives: Synthesis and structure–activity relationships. Bioorg. Med. Chem. 2007, 15, 516–1524. [Google Scholar] [CrossRef] [PubMed]
- Traykova, M.; Kostova, I. Coumarin derivatives and oxidative stress. Int. J. Pharm. 2005, 1, 29–32. [Google Scholar]
- Belluti, F.; Fontana, G.; Bo, L.D.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Matos, M.J.; Gaspar, A.; Kachler, S.; Klotz, K.N.; Borges, F.; Santana, L.; Uriarte, E. Targeting adenosine receptors with coumarins: Synthesis and binding activities of amide and carbamate derivatives. J. Pharm. Pharmacol. 2013, 65, 30–34. [Google Scholar] [CrossRef]
- Lan, J.S.; Ding, Y.; Liu, Y.; Kang, P.; Hou, J.W.; Zhang, X.Y.; Xie, S.S.; Zhang, T. Design, synthesis and biological evaluation of novel coumarin-N-benzyl pyridinium hybrids as multi-target agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 48–59. [Google Scholar] [CrossRef]
- Xie, S.; Wang, X.; Jiang, N.; Yu, W.; Wang, K.D.G.; Lan, J.; Li, Z.; Kong, L. Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase B inhibition properties against Alzheimer’s disease. Eur. J. Med. Chem. 2015, 95, 153–165. [Google Scholar] [CrossRef]
- Roussaki, M.; Kontogiorgies, C.A.; Hadjipavlou-Litina, D.; Hamilaki, S.; Detsi, A. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3889–3892. [Google Scholar] [CrossRef]
- Ostrov, D.A.; Prada, J.A.H.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Anti-Microb Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Vina, D.; Picciau, C.; Orallo, F.; Santana, L.; Uriarte, E. Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5053–5055. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Coumarins as inhibitors of HIV reverse transcriptase. Curr. HIV Res. 2006, 4, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Shi, Y.; Yang, M.; Sun, Z.; Liu, X.; Li, B.; Sun, N. Design, synthesis, DFT study and antifungal activity of pyrazolecarboxamide derivatives. Molecules. 2016, 21, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Li, X.; Yan, Z.; Guo, H.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure–activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef]
- Pan, L.; Lei, D.; Jin, L.; He, Y.; Yang, Q. Promising fungicides from allelochemicals: Synthesis of umbelliferone derivatives and their structure–activity relationships. Molecules. 2018, 23, 3002. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.; World Intellectual Property Organization. Antiviral Compounds and Antihypertensive Compounds. International publication number: WO 92/04327, 19 March 1992. [Google Scholar]
- Afonso, A. Ester and alkoxy substituted benzopyransHypotensive and antiviral agents. U.S. Patent 5412104, Schering Corp: Kenilworth, NJ, USA, 1995. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger, L.; DeLano, W. PyMOL, version 2.3; Schrödinger Inc: New York, NY, USA, 2019. [Google Scholar]

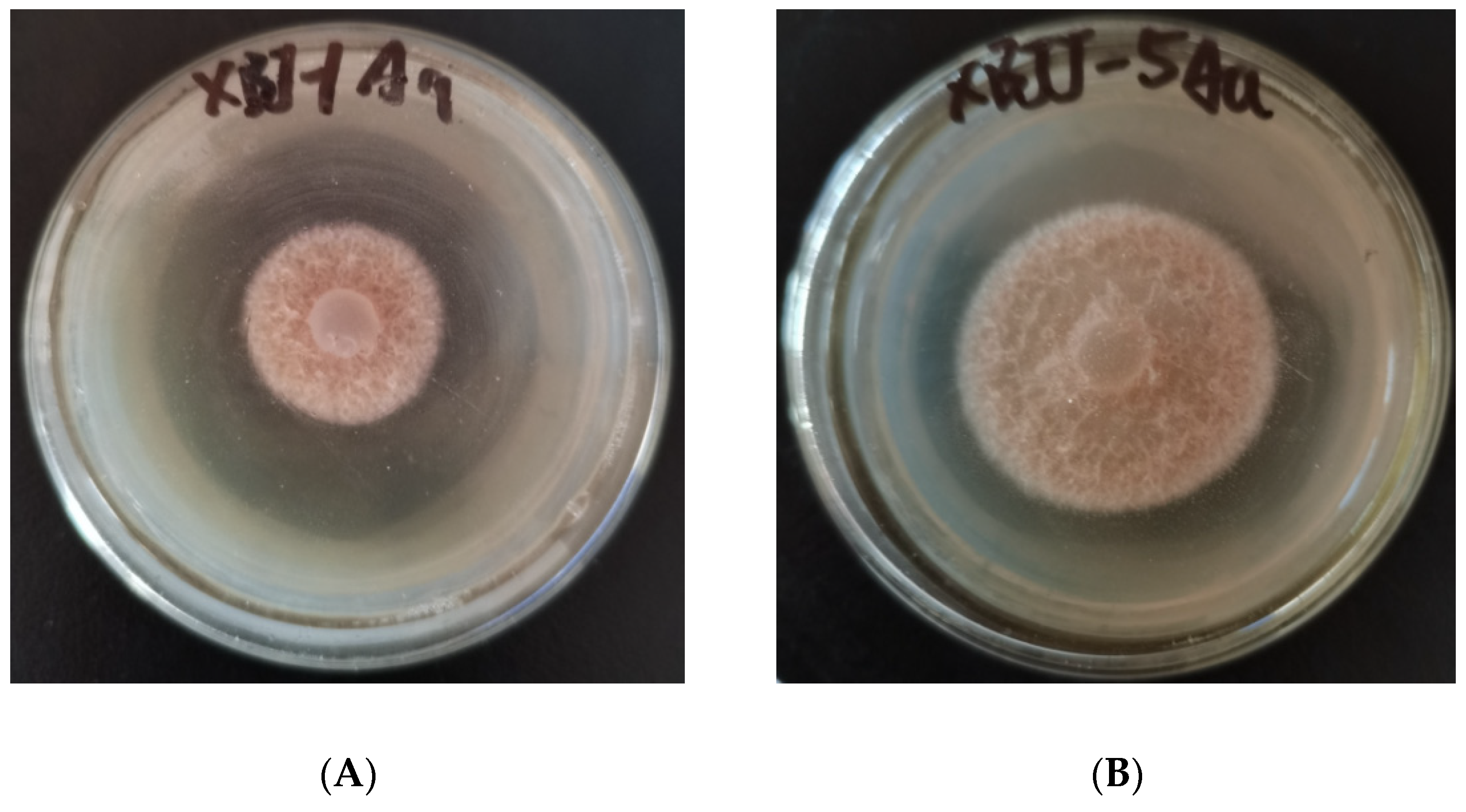
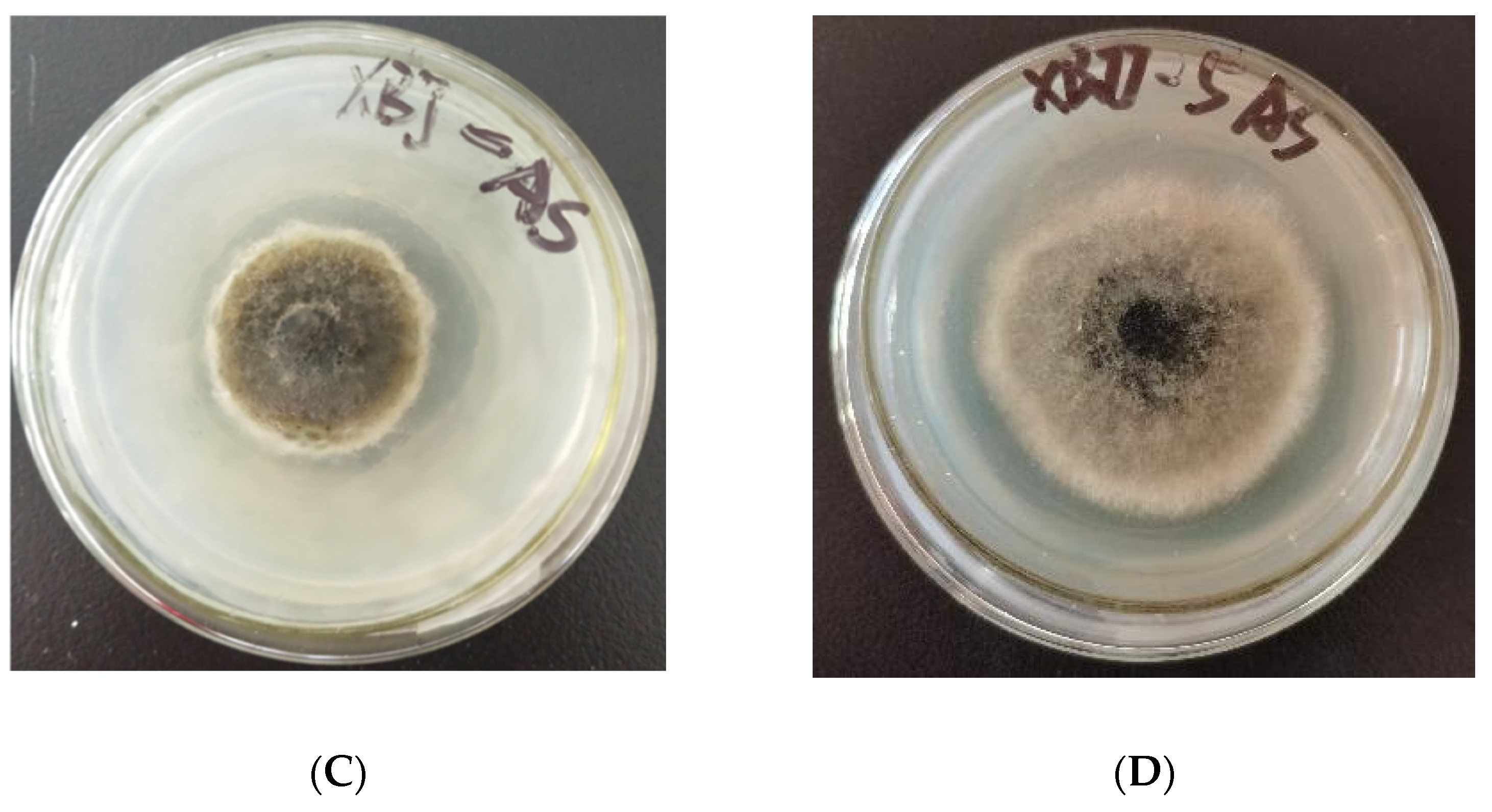
| Compd. | The Inhibition Rate (96 h) (%; Mean ± SD; N = 3) | |||
|---|---|---|---|---|
| B. Cinerea | A. Salani | F. Oxysporum | A. Alternata | |
| 2 | - | 1.70 ± 2.4 | - | 3.10 ± 2.6 |
| 3a | 18.40 ± 2.2 | 33.10 ± 1.2 | 15.40 ± 2.5 | 49.60 ± 1.0 |
| 3b | 5.50 ± 3.4 | 19.40 ± 0.9 | 8.20 ± 1.8 | 17.60 ± 2.5 |
| 3c | 0.00 ± 1.9 | 20.00 ± 1.0 | - | 31.10 ± 2.5 |
| 3d | 23.90 ± 2.2 | 13.70 ± 1.2 | 10.60 ± 0.6 | 24.40 ± 1.8 |
| 3e | 10.00 ± 3.4 | 12.62 ± 1.6 | 10.86 ± 3.8 | 22.64 ± 2.9 |
| 3f | 20.65 ± 3.1 | 37.22 ± 2.3 | 16.10 ± 1.6 | 23.90 ± 1.9 |
| 3g | 5.81 ± 1.0 | 4.21 ± 3.1 | 7.12 ± 2.4 | 2.52 ± 4.0 |
| 3h | 0.06 ± 12.6 | 0.21 ± 5.7 | - | 0.02 ± 6.6 |
| 3i | 14.84 ± 0.4 | 19.09 ± 2.0 | 13.86 ± 0.8 | 25.79 ± 4.2 |
| 3j | 0.00 ± 1.7 | 3.56 ± 1.6 | - | 0.63 ± 3.1 |
| 3k | 7.10 ± 1.6 | 6.15 ± 0.6 | 10.11 ± 3.8 | 6.29 ± 1.3 |
| 3l | 6.45 ± 2.6 | - | 2.62 ± 2.7 | 8.81 ± 0.6 |
| 3m | 9.68 ± 1.5 | 22.98 ± 1.0 | - | 8.18 ± 3.1 |
| 3n | 44.80 ± 0.6 | 76.00 ± 2.5 | 40.20 ± 2.0 | 73.70 ± 3.4 |
| 3o | 38.68 ± 0.1 | 28.70 ± 0.1 | 27.14 ± 0.1 | 23.68 ± 0.1 |
| Carbendazim | 91.88 ± 0.0 | 15.16 ± 0.2 | 86.81 ± 0.0 | 14.67 ± 0.2 |
| Chlorothalonil | 78.36 ± 0.0 | 58.25 ± 0.1 | 66.85 ± 0.0 | 56.69 ± 0.1 |
| Compd. | The Inhibition Rate (96 h) (%; Mean ± SD; N = 3) | |||
|---|---|---|---|---|
| B. Cinerea | A. Salani | F. Oxysporum | A. Alternata | |
| 2′ | 2.00 ± 3.8 | - | 12.80 ± 3.4 | 22.88 ± 3.3 |
| 4a | 7.20 ± 2.4 | 4.00 ± 0.9 | 2.42 ± 0.7 | 19.75 ± 3.8 |
| 4b | 7.74 ± 2.8 | - | - | 1.80 ± 8.3 |
| 4c | - | 15.69 ± 1.2 | 5.19 ± 1.7 | 27.90 ± 3.0 |
| 4d | 2.62 ± 9.2 | 21.51 ± 3.2 | 6.71 ± 4.2 | 16.37 ± 3.0 |
| 4e | 38.34 ± 4.3 | 45.00 ± 4.9 | 38.00 ± 0.7 | 49.28 ± 4.3 |
| 4f | 16.70 ± 1.8 | 27.60 ± 2.5 | 15.11 ± 5.1 | 0.95 ± 1.9 |
| 4g | 22.70 ± 2.8 | 40.59 ± 2.7 | 28.33 ± 8.0 | 44.06 ± 8.3 |
| 4h | - | 4.00 ± 1.0 | 13.49 ± 1.9 | 4.08 ± 2.2 |
| 4i | 32.00 ± 0.7 | 35.31 ± 3.9 | 5.47 ± 1.6 | 10.48 ± 1.5 |
| 4j | 14.40 ± 2.8 | - | 5.88 ± 1.4 | 12.85 ± 1.8 |
| 4k | 1.60 ± 1.7 | 3.38 ± 0.9 | 5.19 ± 1.4 | 12.85 ± 1.7 |
| 4l | 6.19 ± 4.3 | 6.49 ± 4.9 | 2.86 ± 0.7 | 17.12 ± 4.3 |
| 4m | 7.38 ± 1.8 | 38.43 ± 2.4 | 21.21 ± 12.1 | 21.56 ± 2.6 |
| 4n | 16.00 ± 1.6 | 45.85 ± 0.9 | 21.11 ± 1.8 | 44.20 ± 2.1 |
| 4o | 8.04 ± 0.2 | 27.29 ± 0.0 | 20.71 ± 0.0 | 30.22 ± 0.0 |
| Carbendazim | 91.88 ± 0.0 | 15.16 ± 0.2 | 86.81 ± 0.0 | 14.67 ± 0.2 |
| Chlorothalonil | 78.36 ± 0.0 | 58.25 ± 0.1 | 66.85 ± 0.0 | 56.69 ± 0.1 |
| Compd. | EC50 (μg/mL) | |
|---|---|---|
| A. Alternata | A. Salani | |
| 3n | 107.4 | 96.7 |
| 3e | >500 | >500 |
| 4n | 492.4 | 474 |
| 4e | 144.5 | 92.1 |
| 3g | >500 | >500 |
| 4g | 436.9 | >500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yu, J.; Jin, L.; Pan, L. Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives. Molecules 2022, 27, 2738. https://doi.org/10.3390/molecules27092738
Xu L, Yu J, Jin L, Pan L. Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives. Molecules. 2022; 27(9):2738. https://doi.org/10.3390/molecules27092738
Chicago/Turabian StyleXu, Lu, Jinmeng Yu, Lu Jin, and Le Pan. 2022. "Design, Synthesis, and Antifungal Activity of 4-Amino Coumarin Based Derivatives" Molecules 27, no. 9: 2738. https://doi.org/10.3390/molecules27092738







