Nigella sativa L. and COVID-19: A Glance at The Anti-COVID-19 Chemical Constituents, Clinical Trials, Inventions, and Patent Literature
Abstract
:1. Introduction
2. Materials and Methods
3. N. sativa L.
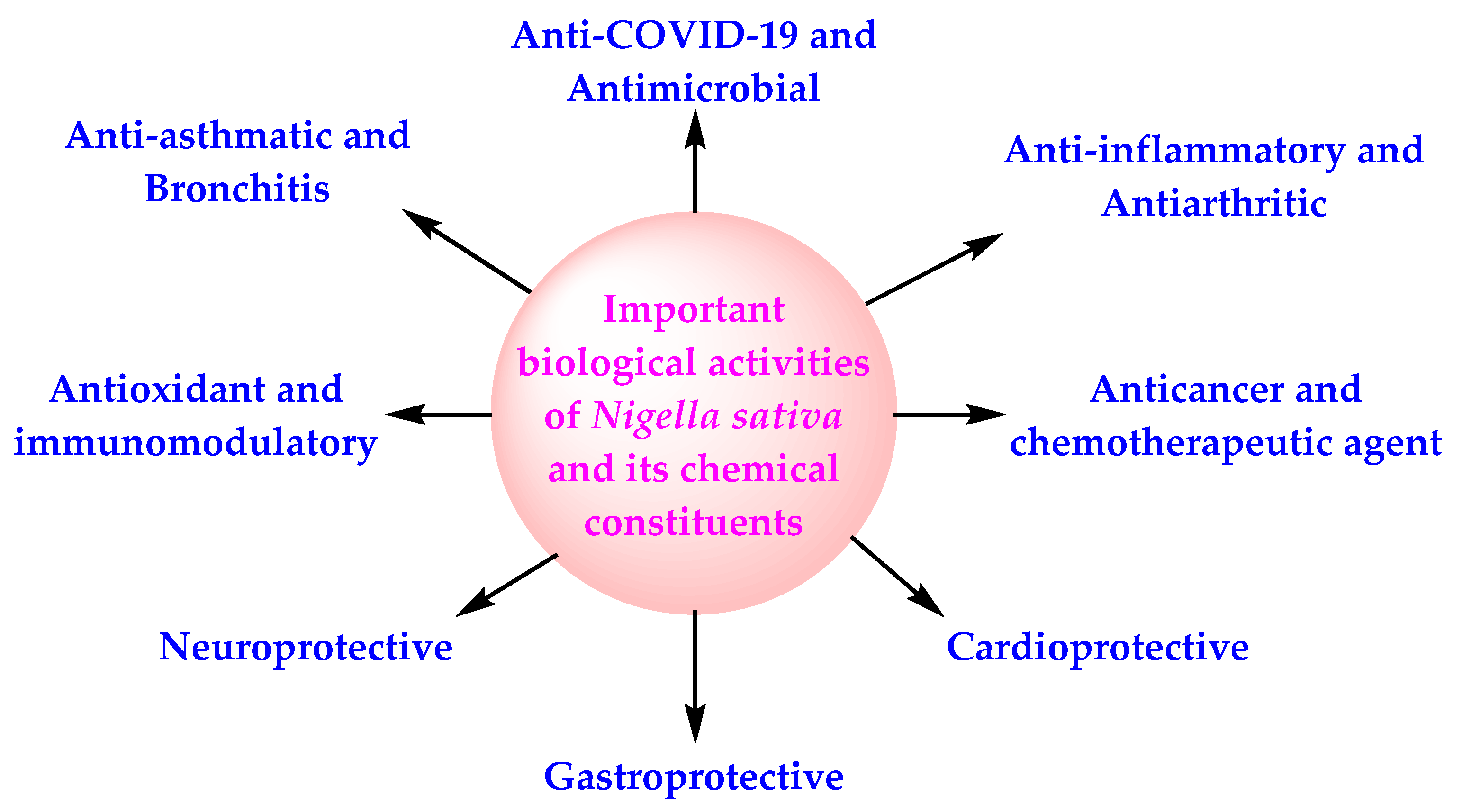
3.1. Introduction
3.2. Important Biologically Active Chemical Constituents of NS
3.3. Anti-COVID-19 Chemical Constituents of NS
3.4. Anti-COVID-19 Clinical Trials on NS and Its Chemical Constituents
4. Patent Summary
5. Conclusions
6. Discussion and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Worldometer on COVID-19 Cases. Available online: https://www.worldometers.info/coronavirus/ (accessed on 1 April 2022).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Emergency Use Authorization. Available online: https://www.fda.gov.ph/list-of-fda-issued-emergency-use-authorization/ (accessed on 31 March 2022).
- Alshrari, A.S.; Hudu, S.A.; Imran, M.; Asdaq, S.M.B.; Ali, A.M.; Rabbani, S.I. Innovations and development of COVID-19 vaccines: A patent review. J. Infect. Public Health 2022, 15, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Rajan, A.; Damodaran, A.; Kamath, S.R.; Nair, K.S.; Zachariah, S.M.; Sahu, R.K.; Fattepur, S.; Sreeharsha, N.; Nair, A.; et al. Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention. Antibiotics 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Asdaq, S.M.B.; Rabbani, S.I.; Alkahtani, M.; Aldohyan, M.M.; Alabdulsalam, A.M.; Alshammari, M.S.; Alajlan, S.A.; Binrokan, A.; Mohzari, Y.; Alrashed, A.; et al. A Patent Review on the Therapeutic Application of Monoclonal Antibodies in COVID-19. Int. J. Mol. Sci. 2021, 22, 11953. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.K.; Grover, P.; Asdaq, S.M.B.; Mehta, L.; Tomar, R.; Imran, M.; Pathak, A.; Jangra, A.; Sahoo, J.; Alamri, A.S.; et al. Potential role of nicotinamide analogues against SARS-COV-2 target proteins. Saudi J. Biol. Sci. 2021, 28, 7567–7574. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Ikbal, A.M.A.; Sahu, R.K.; Bhattacharjee, B.; Paul, T.; Deka, B.; Fattepur, S.; Widyowati, R.; Vijaya, J.; Al Mohaini, M.; et al. Nanotechnology integration for SARS-CoV-2 diagnosis and treatment: An approach to preventing pandemic. Nanomaterials 2021, 11, 1841. [Google Scholar] [CrossRef]
- Asdaq, S.M.B.; Alshrari, A.S.; Imran, M.; Sreeharsha, N.; Sultana, R. Knowledge, attitude and practices of healthcare professionals of Riyadh, Saudi Arabia towards COVID-19: A cross-sectional study. Saudi J. Biol. Sci. 2021, 28, 5275–5282. [Google Scholar]
- Asdaq, S.M.B.; Rabbani, S.I.; Imran, M.; Alanazi, A.A.; Alnusir, G.Y.; Al-Shammari, A.A.; Alsubaie, F.H.; Alsalman, A.J. A review on potential antimutagenic plants of Saudi Arabia. Appl. Sci. 2021, 11, 8494. [Google Scholar] [CrossRef]
- Villena-Tejada, M.; Vera-Ferchau, I.; Cardona-Rivero, A.; Zamalloa-Cornejo, R.; Quispe-Florez, M.; Frisancho-Triveño, Z.; Abarca-Meléndez, R.C.; Alvarez-Sucari, S.G.; Mejia, C.R.; Yañez, J.A. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: A cross-sectional survey. PLoS ONE 2021, 16, e0257165. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Intan, D.; Hananto, J.E.; Harapan, H.; Kurniawan, A. Vitamin D supplementation and COVID-19 outcomes: A 614 systematic review, meta-analysis and meta-regression. Rev. Med. Virol. 2021, 32, e2269. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Tripathi, J.; Manik, S.; Umar, L.; Rabia, J. Preliminary phytochemical studies of the miracle herb of the century, Nigella sativa L. (Black seed). Indo Am. J. Pharm. Res. 2013, 3, 3000–3007. [Google Scholar]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A promising natural remedy for wide range of illnesses. Evid. Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naz, H. Nigella sativa: The miraculous herb. Pak. J. Biochem. Mol. Biol. 2011, 44, 44–48. [Google Scholar]
- Ijaz, H.; Tulain, U.R.; Qureshi, J.; Danish, Z.; Musayab, S.; Akhtar, M.F.; Saleem, A.; Khan, K.A.; Zaman, M.; Waheed, I.; et al. Nigella sativa (Prophetic Medicine): A review. Pak. J. Pharm. Sci. 2017, 30, 229–234. [Google Scholar]
- Ismail, M.Y.; Yaheya, M. Therapeutic role of prophetic medicine Habbat El Baraka (Nigella sativa L.)—A review. World Appl. Sci. J. 2009, 7, 1203–1208. [Google Scholar]
- Yarnell, E.; Abascal, K. Nigella sativa: Holy herb of the middle East. Altern. Complement. Ther. 2011, 17, 99–105. [Google Scholar] [CrossRef]
- El-Tahir, K.E.; Bakeet, D.M. The black seed Nigella sativa Linnaeus-A mine for multi cures: A plea for urgent clinical evaluation of its volatile oil. J. Taibah. Univ. Med. Sci. 2006, 1, 1–19. [Google Scholar]
- Sudhir, S.P.; Deshmukh, V.O.; Verma, H.N. Nigella sativa seed, a novel beauty care ingredient: A review. Int. J. Pharm. Sci. Res. 2016, 7, 3185. [Google Scholar]
- Dajani, E.Z.; Shahwan, T.G.; Dajani, N.E. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): A complementary drug with historical and clinical significance. J. Physiol. Pharmacol. 2016, 67, 801–817. [Google Scholar]
- Areefa, A.; Mohd, A.; Shah, C.S. A review on Nigella sativa (Kalonji) seeds: A universal healer. Cellmed 2020, 10, 11.1–11.14. [Google Scholar]
- Begum, S.; Mannan, A. A review on Nigella sativa: A Marvel herb. J. Drug Deliv. Therap. 2020, 10, 213–219. [Google Scholar] [CrossRef]
- Thakur, S.; Kaurav, H.; Chaudhary, G. Nigella sativa (Kalonji): A black seed of miracle. Int. J. Res. Rev. 2021, 8, 342–357. [Google Scholar] [CrossRef]
- Momin, M.; Momin, S.; Kurhade, S.; Butte, K. Nigella sativa: Blessed seed. Int. J. Res. Phytochem. Pharmacol. 2013, 3, 78–84. [Google Scholar]
- Ara, I.; Maqbool, M.; Fekadu, G.; Hajam, T.A.; Dar, M.A. Pharmaceutical significance of Nigella Sativa L., a Wonder herb. J. Appl. Pharm. Sci. Res. 2020, 3, 4–13. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Imran, M.; Ul-Haq, I.; Živković, J.; Abu-Reidah, I.M.; Sen, S.; Taheri, Y.; Acharya, K.; Azadi, H.; et al. Nigella plants-Traditional uses, bioactive phytoconstituents, preclinical and clinical studies. Front. Pharmacol. 2021, 12, 625386. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Damirchi, S.A.; Geoffrey, P.; Savage, G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019, 35, 342–362. [Google Scholar] [CrossRef]
- Farooq, J.; Sultana, R.; Taj, T.; Asdaq, S.M.B.; Alsalman, A.J.; Mohaini, M.A.; Al Hawaj, M.A.; Kamal, M.; Alghamdi, S.; Imran, M.; et al. Insights into the protective effects of thymoquinone against toxicities induced by chemotherapeutic agents. Molecules 2021, 27, 226. [Google Scholar] [CrossRef]
- Khan, M.A.; Afzal, M. Chemical composition of Nigella sativa Linn: Part 2 Recent advances. Inflammopharmacology 2016, 24, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ali, M.; Albratty, M.M.; Najmi, A.Y.; Azeem, U.; Khan, S.A.; Rather, M.A. Nigella sativa: From chemistry to medicine. In Black Seeds (Nigella sativa); Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–62. [Google Scholar]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Balbaa, M.; El-Zeftawy, M.; Abdulmalek, S.A.; Shahin, Y.R. Health-promoting activities of Nigella sativa fixed oil. In Black Cumin (Nigella sativa) Seeds: Chemistry, Technology, Functionality, and Application; Springer: Cham, Switzerland, 2021; pp. 361–379. [Google Scholar]
- Gheita, T.A.; Kenawy, S.A. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: A placebo controlled study. Phytother. Res. 2012, 26, 1246–1248. [Google Scholar] [CrossRef]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef]
- Kalamegam, G.; Alfakeeh, S.M.; Bahmaid, A.O.; AlHuwait, E.A.; Gari, M.A.; Abbas, M.M.; Ahmed, F.; Abu-Elmagd, M.; Pushparaj, P.N. In vitro Evaluation of the anti-inflammatory effects of thymoquinone in osteoarthritis and in silico analysis of inter-related pathways in age-related degenerative diseases. Front. Cell Dev. Biol. 2020, 8, 646. [Google Scholar] [CrossRef]
- Boskabady, M.; Khazdair, M.R.; Bargi, R.; Saadat, S.; Memarzia, A.; Roshan, N.M.; Hosseini, M.; Askari, V.R.; Boskabady, M.H. Thymoquinone ameliorates lung inflammation and pathological changes observed in lipopolysaccharide-induced lung injury. Evid. Based Complement. Altern. Med. 2021, 2021, 6681729. [Google Scholar] [CrossRef]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.F.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Immunomodulatory and anti-inflammatory effects of thymoquinone. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 52–60. [Google Scholar] [CrossRef]
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783. [Google Scholar] [CrossRef]
- AbuKhader, M.M.; Khan, S.A. Thymoquinone and nanoparticles: A promising approach for the clinical trials. J. Bionanosci. 2017, 11, 258–265. [Google Scholar] [CrossRef]
- Staniek, K.; Gille, L. Is thymoquinone an antioxidant? BMC Pharmacol. 2010, 10, A9. [Google Scholar] [CrossRef] [Green Version]
- Wienkötter, N.; Höpner, D.; Schütte, U.; Bauer, K.; Begrow, F.; El-Dakhakhny, M.; Verspohl, E.J. The effect of nigellone and thymoquinone on inhibiting trachea contraction and mucociliary clearance. Planta Med. 2008, 74, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Stefanowicz-Hajduk, J.; Ochocka, J.R. Alpha-Hederin, the active saponin of Nigella sativa, as an anticancer agent inducing apoptosis in the SKOV-3 cell line. Molecules 2019, 24, 2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepdiremen, A.; Mshvildadze, V.; Süleyman, H.; Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: Alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine 2005, 12, 440–444. [Google Scholar] [CrossRef]
- Saadat, S.; Mohammadi, M.; Fallahi, M.; Keyhanmanesh, R.; Aslani, M.R. The protective effect of α-hederin, the active constituent of Nigella sativa, on tracheal responsiveness and lung inflammation in ovalbumin-sensitized guinea pigs. J. Physiol. Sci. 2015, 65, 285–292. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Ghafari, S.; Sadeghi, M. Possible therapeutic effects of Nigella sativa and its thymoquinone on COVID-19. Pharm. Biol. 2021, 59, 696–703. [Google Scholar] [CrossRef]
- Khan, S.A.; Al-Balushi, K. Combating COVID-19: The role of drug repurposing and medicinal plants. J. Infect. Public Health 2021, 14, 495–503. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Badary, O.A.; Hamza, M.S.; Tikamdas, R. Thymoquinone: A Promising natural compound with potential benefits for COVID-19 prevention and cure. Drug Des. Dev. Ther. 2021, 15, 1819–1833. [Google Scholar] [CrossRef]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef]
- Banerjee, A.; Kanwar, M.; Mohapatra, P.K.D.; Saso, L.; Nicoletti, M.; Maiti, S. Nigellidine (Nigella sativa, black-cumin seed) docking to SARS CoV-2 nsp3 and host inflammatory proteins may inhibit viral replication/transcription and FAS-TNF death signal via TNFR 1/2 blocking. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duru, C.E.; Duru, I.A.; Adegboyega, A.E. In silico identification of compounds from Nigella sativa seed oil as potential inhibitors of SARS-CoV-2 targets. Bull. Natl. Res. Cent. 2021, 45, 57. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Abbasi, H.W.; Shahid, S.; Gul, S.; Abbasi, S.W. Molecular docking, simulation and MM-PBSA studies of Nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2021, 39, 4225–4233. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Firoz, A.; Alaidarous, M.; Alshehri, B.; Bin Dukhyil, A.A.; Banawas, S.; Alsagaby, S.A.; Alturaiki, W.; Bhat, G.A.; Kashoo, F.; et al. Identification of SARS-CoV-2 RNA-dependent RNA polymerase inhibitors from the major phytochemicals of Nigella sativa: An in silico approach. Saudi J. Biol. Sci. 2022, 29, 394–401. [Google Scholar] [CrossRef]
- Rizvi, S.M.D.; Hussain, T.; Moin, A.; Dixit, S.R.; Mandal, S.P.; Adnan, M.; Jamal, Q.M.S.; Sharma, D.C.; Alanazi, A.S.; Unissa, R. Identifying the most potent dual-targeting compound(s) against 3CLprotease and NSP15exonuclease of SARS-CoV-2 from Nigella sativa: Virtual screening via physicochemical properties, docking and dynamic simulation analysis. Processes 2021, 9, 1814. [Google Scholar] [CrossRef]
- Baig, A.; Srinivasan, H. SARS-CoV-2 Inhibitors from Nigella sativa. Appl. Biochem. Biotechnol. 2022, 194, 1051–1092. [Google Scholar] [CrossRef]
- Maiti, S.; Banerjee, A.; Nazmeen, A.; Kanwar, M.; Das, S. Active-site Molecular docking of Nigellidine with nucleocapsid- NSP2-MPro of COVID-19 and to human IL1R-IL6R and strong antioxidant role of Nigella-sativa in experimental rats. J. Drug Target. 2022, 30, 511–521. [Google Scholar] [CrossRef]
- Khan, S.L.; Siddiqui, F.A.; Jain, S.P.; Sonwane, G.M. Discovery of potential inhibitors of SARS-CoV-2 (COVID-19) main protease (Mpro) from Nigella sativa (Black seed) by molecular docking study. Coronaviruses 2021, 2, 384–402. [Google Scholar] [CrossRef]
- Mani, R.J.; Sehgal, N.; Dogra, N.; Saxena, S.; Katare, D.P. Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: In-silico study. J. Biomol. Struct. Dyn. 2022, 40, 2417–2429. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Mazumder, A.; Rana, A.K.; Srivastava, Y. Inhibitory potential of dietary phytocompounds of Nigella sativa against key targets of novel coronavirus (COVID-19). Indian J. Pharm. Educ. Res. 2021, 55, 190–197. [Google Scholar] [CrossRef]
- Esharkawy, E.R.; Almalki, F.; Hadda, T.B. In vitro potential antiviral SARS-CoV-19- activity of natural product thymohydroquinone and dithymoquinone from Nigella sativa. Bioorg. Chem. 2022, 102, 105587. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, B.; Xiao, Z.; Zhou, M.; Ge, L.; Jia, F.; Liu, Y.; Jin, H.; Zhu, X.; Gao, J.; et al. Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infect. Dis. Ther. 2021, 10, 483–494. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/home (accessed on 1 April 2022).
- Imran, M.; Alshrari, S.A.; Thabet, H.K.; Abida Bakht, A.M. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opin. Ther. Patents 2021, 31, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alshrari, S.A.; Tauseef, M.; Khan, S.A.; Hudu, S.A.; Abid. Mucormycosis medications: A patent review. Expert Opin. Ther. Patents 2021, 31, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Asdaq, S.M.B.; Khan, S.A.; Unnikrishnan, M.D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals 2021, 14, 710. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Alshammari, M.K.; Alreshidi, M.A.; Alreshidi, A.A.; Alghonaim, R.S.; Alanazi, F.A.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Discovery, development, inventions, and patent trends on Mobocertinib succinate: The first-in-class oral treatment for NSCLC with EGFR Exon 20 insertions. Biomedicines 2021, 9, 1938. [Google Scholar] [CrossRef]
- Ichim, T.E.; Dixon, T.G.; Veltmeyer, J. Nutraceuticals for Suppressing Indolamine 2,3 Deoxygenase. U.S. Patent Number US11229674B1, 25 January 2022. [Google Scholar]
- Ichim, T.E.; Dixon, T.G. Nutraceuticals for the Prevention, Inhibition, and Treatment of SARS-Cov-2 and Associated COVID-19. U.S. Patent Application Publication Number US20210338763A1, 4 November 2021. [Google Scholar]
- Haddadi, M.; Taghdisi, H.P.M. Herbal Medicine for the Treatment of COVID-19. PCT International Patent Application Publication Number WO2022009236A1, 13 January 2022. [Google Scholar]
- Al Jasim, N.A.N.; Ahmed, N.Z. Immunomodulatory Composition to Treat and/or Prevent COVID-19 Illness. U.S. Patent Application Publication Number US20220000958A1, 6 January 2022. [Google Scholar]
- Omar, A. Herbal Anti-Viral Drug Containing Black Seed Oil for Treating Coronavirus Disease (COVID-19). Turkish Patent Application Publication Number TR2020004046A2, 21 April 2020. [Google Scholar]
- Muhammad, T. Compositions and Prevention and Intervention Methods for COVID-19 with Divine Ayats’ Fitra30 COVID-19 Protocol. PCT International Patent Application Publication Number WO202120, 14 October 2021. [Google Scholar]
- Ichim, T.E.; Veltmeyer, J.; Dixon, T.G. Additive and/or Synergistic Combinations of Metformin with Nutraceuticals for the Prevention, Inhibition and Treatment of Sars-Cov-2 and Associated COVID-19. U.S. Patent Application Publication Number US2022023237A1, 27 January 2022. [Google Scholar]
- Ichim, T.E.; Dixon, T.G.; Veltmeyer, J. Neuroprotection and Neuroregeneration by Pterostilbene and Compositions Thereof. U.S. Patent Application Publication Number US2022031793A1, 3 February 2022. [Google Scholar]
- Ichim, T.E.; Ramos, F.; Veltmeyer, J.; Dixon, T.G. Prevention of Neuroinflammation Associated Memory Loss Using Nutraceutical Compositions. U.S. Patent Application Publication Number US2022040248A1, 10 February 2022. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating and Preventing a Coronavirus Infection. PCT International Patent Application Publication Number WO2021186453A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating and Preventing Non-Malignant Respiratory Disease. PCT International Patent Application Publication Number WO2021186454A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating or Preventing Inflammatory Diseases Including Diabetes Mellitus 10 Type I and Type II and Thyroid Diseases. PCT International Patent Application Publication Number WO2021186455A1, 23 September 2021. [Google Scholar]
- Alkalay, R. Compositions and Methods for Treating Solid and Soft Tumors and Proliferative Diseases. PCT International Patent Application Publication Number WO2021186456A1, 23 September 2021. [Google Scholar]
- Popov, T.; Josling, P.D. Compositions and Applications Thereof. PCT International Patent Application Publication Number WO2021160982A1, 19 August 2021. [Google Scholar]
- Hoag, G.E.; Salerno, J. Method for Treating Viral and Bacterial Infection through Inhalation Therapy. PCT International Patent Application Publication Number WO2021216749A1, 28 October 2021. [Google Scholar]
- Mehmood, A.; Khan, S.; Khan, S.; Ahmed, S.; Ali, A.; Xue, M.; Ali, L.; Hamza, M.; Munir, A.; Ur Rehman, S.; et al. In silico analysis of quranic and prophetic medicinals plants for the treatment of infectious viral diseases including corona virus. Saudi J. Biol. Sci. 2021, 28, 3137–3151. [Google Scholar] [CrossRef]
- Holgersen, E.M.; Gandhi, S.; Zhou, Y.; Kim, J.; Vaz, B.; Bogojeski, J.; Bugno, M.; Shalev, Z.; Cheung-Ong, K.; Gonçalves, J.; et al. Transcriptome-Wide off-target effects of steric-blocking oligonucleotides. Nucleic Acid Ther. 2021, 31, 392–403. [Google Scholar] [CrossRef]
- From the American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Zarei, A.; Soleymani, S.; Jamalimoghadamsiahkali, S.; Asadi, A.; Shati, M.; Jafari, M.; Rezadoost, H.; Kordafshar, G.; Naghizadeh, A.; et al. Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother. Res. 2021, 35, 6295–6309. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.; Li, R.; Mehmood, K.; Waqas, M.; Li, K.; Li, J. Potential influence of Nigella sativa (Black cumin) in reinforcing immune system: A hope to decelerate the COVID-19 pandemic. Phytomedicine 2021, 85, 153277. [Google Scholar] [CrossRef] [PubMed]
- Kadil, Y.; Mouhcine, M.; Filali, H. In Silico Investigation of the SARS CoV2 protease with thymoquinone, the major constituent of Nigella Sativa. Curr. Drug Discov. Technol. 2021, 18, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Younus, H. Potential implications of black seed and its principal constituent thymoquinone in the treatment of COVID-19 patients. Curr. Pharm. Biotechnol. 2021, 22, 1315–1324. [Google Scholar] [CrossRef]
- Awad, E.M.; Binder, B.R. In vitro induction of endothelial cell fibrinolytic alterations by Nigella Sativa. Phytomedicine 2005, 12, 194–202. [Google Scholar] [CrossRef]
- Imran, M.; Alshrari, A.S.; Asdaq, S.M.B.; Abida. Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J. Infect. Public Health 2021, 14, 1075–1086. [Google Scholar] [CrossRef]
- Imran, M.; Kumar, A.M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq, A.A.; Al-Shammeri, A.M.; et al. Discovery, development, and patent trends on molnupiravir: A prospective oral treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J. Pharmacopunct. 2017, 20, 179–193. [Google Scholar]
- Webber, P.M. A guide to drug discovery. Protecting your inventions: The patent system. Nat. Rev. Drug Discov. 2003, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Jalal, Z.; Bakour, M.; Lyoussi, B. Medicinal Plants and zinc: Impact on COVID-19 pandemic. Sci. World J. 2021, 2021, 9632034. [Google Scholar] [CrossRef] [PubMed]


| NS Constituent Identified as Anti-COVID-19 | Type of Study | Finding of the Study |
|---|---|---|
| Caryophyllene oxide, β-bisabolene | In silico | Molecular docking studies (PDB IDs: 6YHU, 6W4B, 6VXS, 6LU7, 7BTF 6LZG) revealed caryophyllene oxide to possess the highest binding affinity towards 3CLpro, NSP3, NSP9, and RdRp molecular targets in COVID-19. ACE-2 binding affinity of β-bisabolene and remdesivir was almost similar [56] |
| Dithymoquinone (DTQ) | In silico | The binding affinity (PDB ID: 6VW1) of DTQ on SARS-CoV-2-ACE-2 was better than chloroquine. It was found to be stable at the docked site in molecular dynamics simulation studies [57] |
| α-Hederin | In silico | α-Hederin was found to be a better inhibitor of RdRp (PDB ID: 6M71) than DTQ, nigellicine, and nigellidine [58] |
| DTQ | In silico | DTQ was found to be active against 3CLpro (PDB ID: 6LU7) and Nsp15 (PDB ID: 6VWW) targets [59] |
| α -Hederin, rutin, and nigellamine A2 | In silico | α-Hederin, rutin, and nigellamine A2 were identified as potential inhibitors of SARS-CoV-2 proteins (PDB IDs: 6W9C, 6Y2E, 6M71, 6ZSL, 6W4B, 6VWW, 6M17, and 6VYO) related to RdRp, protease, and helicase [60] |
| Nigellidine | In silico and in vivo | Nigellidine showed a good affinity toward COVID-19 Nsp2 and IL1R proteins (PDB IDs: 6LU7, 6VSB, 1ITB, and 1P9M). Nigellidine in vivo study in rats showed antioxidant, hepato-protective, and anti-inflammatory activities [61] |
| α-Hederin | In silico | NS chemical constituents such as α-hederin, stigmasterol glucoside, nigellidine-4-O-sulfite, nigellidine, sterol-3-β-D-glucoside, DTQ, β-sitosterol were identified as potential inhibitors of main protease (Mpro) (PDB IDs: 6LU7). Nigllimine, nigellimine N-oxide, carvacrol, TQ, THQ, thymol, anthole, etc., showed weaker binding affinity than remdesivir, lopinavir, and nelfinavir. α-Hederin was identified as the most promising anti-COVID agent [62] |
| α-Hederin, THQ, and TQ | In silico | In molecular docking studies, α-hederin, THQ, and TQ were found to be efficiently binding to ACE-2 (PDB ID: 1R4L) of SARS-CoV-2 [63] |
| Nigellone | In silico | Nigellone (DTQ) upon molecular docking studies with four COVID-19 protein targets (spike glycoprotein, 3CLpro/Mpro (PDB ID: 6LU7), human ACE-2) was observed to bind more strongly than carvacrol, nigellicine, nigellidine, TQ, THQ, and thymol. Its binding affinity on other viral proteins (PDB IDs: 6LU7, 6VSB, and 6VX) was better than remdesivir and hydroxychloroquine [64]. This study also advocated further in vitro experiments to establish Nigellone as an anti-COVID-19 lead compound |
| Nigellidine | In silico | Nigellidine was exposed to prevent SARS-CoV-2 NSP3 replication/transcription. It also blocked the pro-inflammatory cytokines TNF R1 and TNF R2 and Fas-induced apoptotic death [55] |
| DTQ and THQ | In vitro | Cytotoxicity of DTQ and THQ was tested in VERO-E6 cells by MTT assay. HTQ presented anti-SARS-CoV-2 action at non-cytotoxic nanomolar concentration (IC50 = 23.15 ng/mL) while DTQ showed an IC50 of 275.2 ng/mL [65] |
| TQ | In Silico and in vitro | TQ is bound strongly to ACE-2 of SARS-CoV-2 (PDB ID: 6VW1). In vitro results showed it to inhibit SARS-CoV-2 pseudo particles infecting HEK293-ACE2 cells with IC50 of 4.999 μM and CC50 of 35.100 μM; SI = 7.02) [66] |
| Summary of the Title (Intervention) | Primary Purpose (Phase; Number of Enrollments; Status; Results) | NCT Number (Allocation; Intervention Model; Completion Date) | Sponsor (Location of the Clinical Trial) | Primary Outcomes/Conclusion |
|---|---|---|---|---|
| NS in COVID-19 (Oral soft gel capsule containing 500 mg NSO two times a day for 10 days) | Treatment (2; 183; Completed; Available) | NCT04401202 (Randomized; Parallel Assignment; 31 December 2020) | King Abdulaziz University (Saudi Arabi) | The dietary supplement helped the faster recovery of COVID-19 patients |
| Safety and efficacy of NSO against COVID-19 (Six 500 mg capsules of NSO per day for 14 days) | Treatment (2; 60; Recruiting; Not available) | NCT04914377 (Randomized; Parallel Assignment; November 2021) | Novatek Pharmaceuticals (United States) | The reduction in the COVID-19 signs and symptoms |
| Effectiveness of NSO to treat COVID-19 (One capsule of NS every 2 h for the first 3 days followed by one capsule three times a day for 12 days. The dose of NSO is not mentioned) | Treatment (1; 500; Completed; Not available) | NCT04914767 (Randomized; Parallel Assignment; 31 December 2021) | Sahloul University Hospital (Tunisia) | Rate of death, readmission, and oxygen supplementation among high-risk COVID-19 patients |
| Honey and NS seeds for COVID-19 treatment (Honey 1 g/kg daily + NS seed capsule, 80 mg/kg daily for 14 days) | Treatment (3; 313; Completed; Not available) | NCT04347382 (Randomized; Parallel Assignment; 30 August 2020) | Sohaib Ashraf and Sheikh Zayed Federal Postgraduate Medical Institute (Pakistan) | Days needed to obtain a negative COVID-19 PCR of a COVID-19 positive patient |
| Efficacy of NS versus vitamin D3 against COVID-19 (NS capsule, 900 mg two times a day for 14 days) | Treatment (Not Applicable; 100; Recruiting; Not available) | NCT04981743 (Randomized; Parallel Assignment; 30 December 2021) | Ain Shams University (Egypt) | The safety and efficacy of NS versus vitamin D3 against COVID-19 will be evaluated and recorded utilizing COVID-19 signs and symptoms (fever, runny nose, fatigue, cough, sore throat, and headache) |
| Impact of the composition of NSO and Omega 3 on the immunity of COVID-19 patient (1g Omega 3 and 1g NSO containing 3% TQ for 14 days) | Treatment (2 & 3; Recruiting; Not available) | NCT04553705 (Randomized; Sequential Assignment; 4 December 2020) | Beni-Suef University, Maternity and Children Hospital (Makkah), and University of Arizona (Saudi Arabia) | The recovery rate from COVID-19 positive to COVID-19 negative |
| NS for the prevention of influenza syndrome (One capsule of NS per day for 21 days followed by weekly follow-up for COVID-19 checking. The dose is not mentioned) | Prevention (Not applicable; 500; Completed; Not available) | NCT04989101 (Randomized; Parallel Assignment; 31 August 2021) | Sahloul University Hospital (Tunisia) | SARS-CoV-19 infection |
| Honey and NS seeds for COVID-19 prophylaxis (Honey 0.5 g/kg daily + NS seeds 40 mg/kg daily for 14 days) | Prevention (2 & 3; 1000; Recruiting; Not available) | NCT04767087 (Randomized; Parallel Assignment; 15 April 2022) | Sohaib Ashraf and Sheikh Zayed Federal Postgraduate Medical Institute (Pakistan) | The combination of honey and NS improved the symptoms, viral clearance, and mortality among COVID-19 patients |
| Patent/Patent Application Number (Applicant/Assignee; Publication Date; Priority Country) | Status (Family Members; International Patent Classification) | Summary of the Claimed Invention |
|---|---|---|
| US11229674B1 (Therapeutic Solutions International; 25 January 2022; United States) | Patented case (None; A61K36/31, A61K36/45, A61K36/71, A61K36/82, A61P29/00) | A quadramune composition comprising 100–200 ug of green tea extract (epigallocatechin-3-gallate), 100–200 ug of NS extract (TQ), 100–200 ug of broccoli extract (sulforaphane), and 50-100 ug of blueberry extract (pterostilbene) to treat COVID-19 patient. This composition is said to possess anti-inflammatory activity and improves immunity by inhibiting the expression of indoleamine 2,3-dioxygenase. However, no clinical or in vitro analysis data have been provided in support of the claimed method of treatment [72]. |
| US20210338763A1 (Therapeutic Solutions International; 4 November 2021; United States) | Under examination (None; A61K31/09, A61K31/122, A61K31/26, A61K31/353, A61K36/31, A61K36/45, A61K36/71, A61K36/82) | It claims a nutraceutical composition similar to US11229674B1 [72] comprising NS, green tea, blueberry, and broccoli for treating or preventing complications linked with the infection of SARS-CoV-2. The composition is claimed to reduce the expression of inflammatory markers in the human body. No example has been provided in the specification to support the claimed invention, but inventors tried to justify their claims based on prior studies [73]. |
| WO2022009236A1 (Mozhdeh Haddadi and Mahdyar Taghdisi Hadi Pour; 13 January 2022; Iran) | No national phase entry (A61K36/00) | It claims four types of compositions of NS (capsule/tablet) for the treatment of COVID-19 (10 days course) containing different dry and powdered herbs. First composition for patients < 3 years comprised of NS and Terminalia chebula (TC). Second composition for patients of 3–7 years comprised of NS, Apple seed, and TC. Third composition for patients 7–15 years comprised of NS, Peganum harmala (PH), and TC. Fourth composition for patients >15 years NS, PH, Apple seed, and TC. This document does not deliver any experimental proof (in vitro, in vivo, or clinical) for the claimed treatment [74]. |
| US20220000958A1 (Covimmune Pharma; 6 January 2022; United States) | Under examination (A61K36/71, A61P31/14) | A biologically active immunostimulant extract obtained by the extraction of NS plant, NS seed, or its oil with aqueous ethanoic acid (vinegar) for the treatment of COVID-19. The patent application provides a pictorial mechanism of action of the NS extract to treat COVID-19. However, no anti-COVID-19 activity data (in vitro, in vivo, and clinical) of the extract have been exemplified [75]. |
| TR2020004046A2 (Alravvi, Omar, Turk; 21 April 2020; Turkey) | Granted patent (Not available online) | An antiviral herbal composition comprising NSO (60–80%), olive oil (10–20%), and clove oil (1–5%) for treating COVID-19. The complete document was not available for analyzing the examples [76]. |
| WO2021205196A1 (Muhammad Taliah; 14 October 2021; International Bureau of The World Intellectual Property Organization) | No national phase entry (WO2021205196A4; A61K36/19, A61K36/38, A61K36/48, A61K36/70, A61P31/14) | A composition comprising NS seed (anti-SARS-CoV-2) and Saussurea lappa root (anti-SARS-CoV-2) for treating/preventing COVID-19. The optional components of the composition include, Astragalus membranaceus root (immunomodulator/reduces viral load), Paeonia lactiflora root (anti-inflammatory/immunomodulator), Radix bupleuri root (anti-inflammatory), Nelumbo nucifera seed (antioxidant), Angelica archangelica root (antioxidant), Citrus sinensis peel (antioxidant), Rosa canina fruit (antioxidant), Vaccinium angustifolium fruit (antioxidant), Polygonum cuspidatum root (antioxidant/antiviral), Ocimum sanctum leaf (antiviral), Andrographis paniculate aerial parts (antiviral), Artemisia vulgaris leaf (autophagy inducer), Zingiber officinale root (autophagy inducer), Glycyrrhiza glabra root (antimutagenic), and Panax ginseng root (reduces lethargy and fatigue associated with COVID-19). This patent application provides a protocol for the clinical study of the claimed composition but is silent about its data [77]. |
| US2022023237A1 (Therapeutic Solutions International; 27 January 2022; United States) | Under examination (None; A23L33/105, A61K31/155, A61K36/31, A61K36/45, A61K36/82) | A synergistic composition to treat/prevent COVID-19 comprising metformin, Green Tea extract (epigallocatechin-3-gallate), blueberry extract (pterostilebene), NS extract (TQ), and broccoli extract (sulforaphane). The in vivo activity data demonstrated that the claimed composition enhanced type 2 monocytes, IL-10 (anti-inflammatory protein), and HGF-1 (regenerative protein). It also reduced lung injury, and IL-17 (inflammatory protein). However, no specific data have been provided against SARS-CoV-2 or COVID-19 treatment [78]. |
| US2022031793A1 (Therapeutic Solutions International; 3 February 2022; United States) | Under examination (None; A61K31/09, A61K31/122, A61K31/26, A61K31/353, A61K36/31, A61K36/45, A61K36/71, A61K36/82) | A method of protecting against neurological damage due to COVID-19 utilizing a composition comprising green tea extract (epigallocatechin-3-gallate), blueberry extract (pterostilbene), NS extract (TQ), and broccoli extract (sulforaphane) [79]. |
| US2022040248A1 (Therapeutic Solutions International; 10 February 2022; United States) | Under examination (None; A61K31/09, A61K31/122, A61K31/26, A61K31/353, A61K36/31, A61K36/45, A61K36/71, A61K36/82, A61P25/28) | A method of treating inflammation associated with neurological damage due to COVID-19 utilizing a composition comprising green tea extract (epigallocatechin-3-gallate), blueberry extract (pterostilbene), NS extract (TQ), and broccoli extract (sulforaphane) [80]. |
| WO2021186453A1 (Alkalay Rachel; 23 September 2021; United States) | No national phase entry (WO2021186454A1, WO2021186455A1, WO2021186456A1; A61K31/05, A61K36/25, A61K36/258, A61K36/324, A61K36/424, A61K36/53, A61K36/537, A61P11/00, A61P31/14) | A method of preventing or treating SARS-CoV-2 infection using a composition containing oregano oil (1), thyme oil (2), NSO (3), sumac oil (4), sesame oil (5), olibanum oil (6). Different combinations of these oils were made as combination A (1+2+3), combination B (1+2+3+4), combination C (1+2+3+4+5), and combination D (1+2+3+4+5+6). The in vitro analysis of these combinations demonstrated that these combinations digested the S-1 and S-2 subunits of the spike protein of SARS-CoV-2 and attenuated it. The clinical trial in a patient showed that the combination-A relieved sore throat and cough in 24 and 40 h, respectively [81]. |
| WO2021186454A1 (Alkalay Rachel; 23 September 2021; United States) | No national phase entry (WO2021186453A1, WO2021186455A1, WO2021186456A1; A61K31/05, A61K36/25, A61K36/258, A61K36/324, A61K36/424, A61K36/53, A61K36/537, A61P11/00, A61P31/14) | This is a family member of WO2021186453A1 [81] with similar data. It claims a method of lowering the infectivity of a non-malignant respiratory disease virus using a combination of NS along with other herbs as mentioned in WO2021186453A1 [81,82]. |
| WO2021186455A1 (Alkalay Rachel; 23 September 2021; United State) | No national phase entry (WO2021186453A1, WO2021186454A1, WO2021186456A1; A61K31/05, A61K36/25, A61K36/258, A61K36/324, A61K36/424, A61K36/53, A61K36/537, A61P31/12, C07K14/47) | This is a family member of WO2021186453A1 [81] with similar data. It claims an anti-inflammatory composition of NS along with other herbs as mentioned in WO2021186453A1 [81,83]. |
| WO2021186456A1 (Alkalay Rachel; 23 September 2021; United States) | No national phase entry (WO2021186453A1, WO2021186454A1, WO2021186455A1; A61K31/045, A61K31/05, A61K31/121, A61K31/198, A61K31/352, A61K36/258, A61K36/424, A61K38/48, A61P35/00) | This is a family member of WO2021186453A1 [81] with similar data. It claims the antiviral composition of NS along with other herbs as mentioned in WO2021186453A1 [81]. However, it is silent about the antiviral activity against SARS-CoV-2 [81,84]. |
| WO2021160982A1 (Nasaleze Patents Limited; 19 August 2021; United Kingdom) | No national phase entry (None; A61K31/685, A61K36/8962, A61K47/38, A61K47/46, A61K9/00, A61K9/14, A61P31/12) | A homogenized powdered composition consisting of hydroxypropyl methylcellulose particles, at least one signaling agent (menthol, strawberry, mint, spearmint, peppermint, eucalyptus, lavender, and citrus), and optionally one or more biologically active agents like NS. This document does not furnish any rationale for using NS in the description part [85]. |
| WO2021216749A1 (Hoag George Edward and Salerno John; 28 October 2021; United States) | No national phase entry (None; A61K31/015, A61K31/045, A61K31/12, A61K31/35, A61K9/00, A61P31/04, A61P31/12) | A liquid pharmaceutical composition for inhalation to prevent/treat infectious diseases (COVID-19) containing a plant extract comprising one or more Transient Receptor Potential Cation Channel, Subfamily A, member 1 (TRPA1) antagonist (1,8-cineole), one or more plant extract antibacterial compound (b-caryophyllene), one or more plant extract antiviral compounds (TQ), and one or more plant extract antioxidants (berberine). This patent application does not exemplify the anti-SARS-CoV-2 activity data of the claimed composition [86]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Khan, S.A.; Abida; Alshammari, M.K.; Alkhaldi, S.M.; Alshammari, F.N.; Kamal, M.; Alam, O.; Asdaq, S.M.B.; Alzahrani, A.K.; et al. Nigella sativa L. and COVID-19: A Glance at The Anti-COVID-19 Chemical Constituents, Clinical Trials, Inventions, and Patent Literature. Molecules 2022, 27, 2750. https://doi.org/10.3390/molecules27092750
Imran M, Khan SA, Abida, Alshammari MK, Alkhaldi SM, Alshammari FN, Kamal M, Alam O, Asdaq SMB, Alzahrani AK, et al. Nigella sativa L. and COVID-19: A Glance at The Anti-COVID-19 Chemical Constituents, Clinical Trials, Inventions, and Patent Literature. Molecules. 2022; 27(9):2750. https://doi.org/10.3390/molecules27092750
Chicago/Turabian StyleImran, Mohd, Shah Alam Khan, Abida, Mohammed Kanan Alshammari, Saif M. Alkhaldi, Fayez Nafea Alshammari, Mehnaz Kamal, Ozair Alam, Syed Mohammed Basheeruddin Asdaq, A. Khuzaim Alzahrani, and et al. 2022. "Nigella sativa L. and COVID-19: A Glance at The Anti-COVID-19 Chemical Constituents, Clinical Trials, Inventions, and Patent Literature" Molecules 27, no. 9: 2750. https://doi.org/10.3390/molecules27092750
APA StyleImran, M., Khan, S. A., Abida, Alshammari, M. K., Alkhaldi, S. M., Alshammari, F. N., Kamal, M., Alam, O., Asdaq, S. M. B., Alzahrani, A. K., & Jomah, S. (2022). Nigella sativa L. and COVID-19: A Glance at The Anti-COVID-19 Chemical Constituents, Clinical Trials, Inventions, and Patent Literature. Molecules, 27(9), 2750. https://doi.org/10.3390/molecules27092750











