The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties
Abstract
:1. Introduction
2. Results
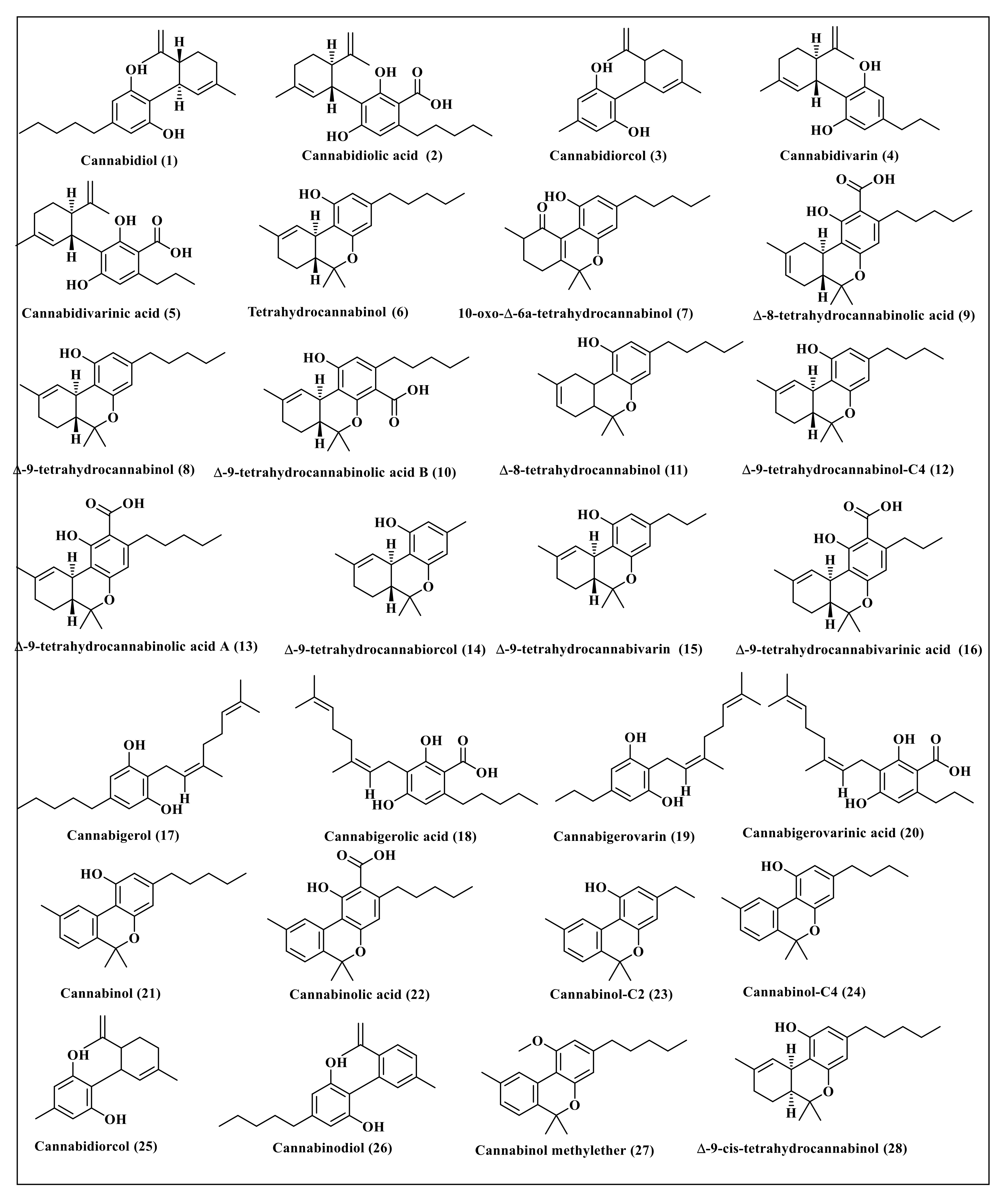
2.1. Selection of Secondary Metabolites
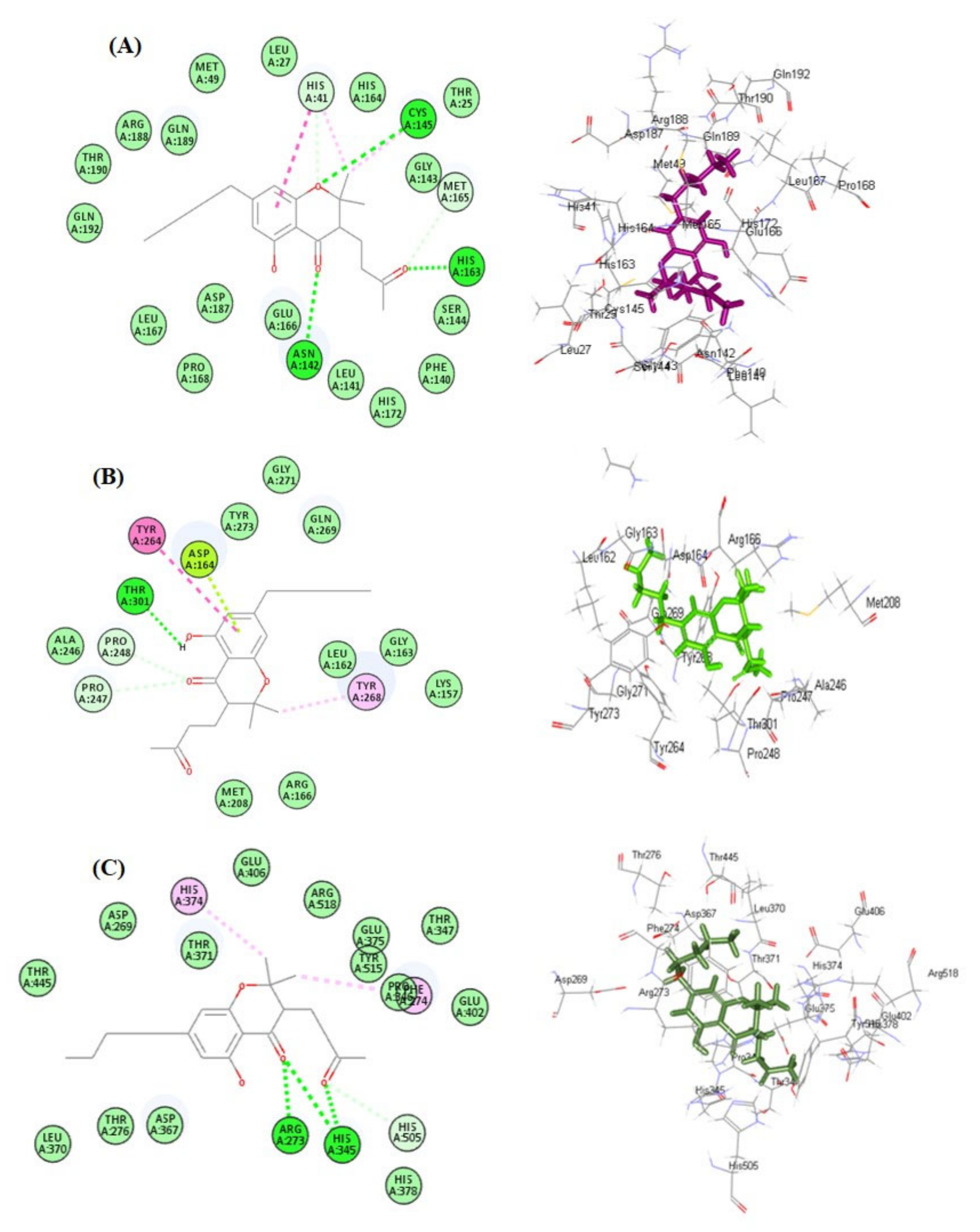
2.2. Molecular Docking Studies
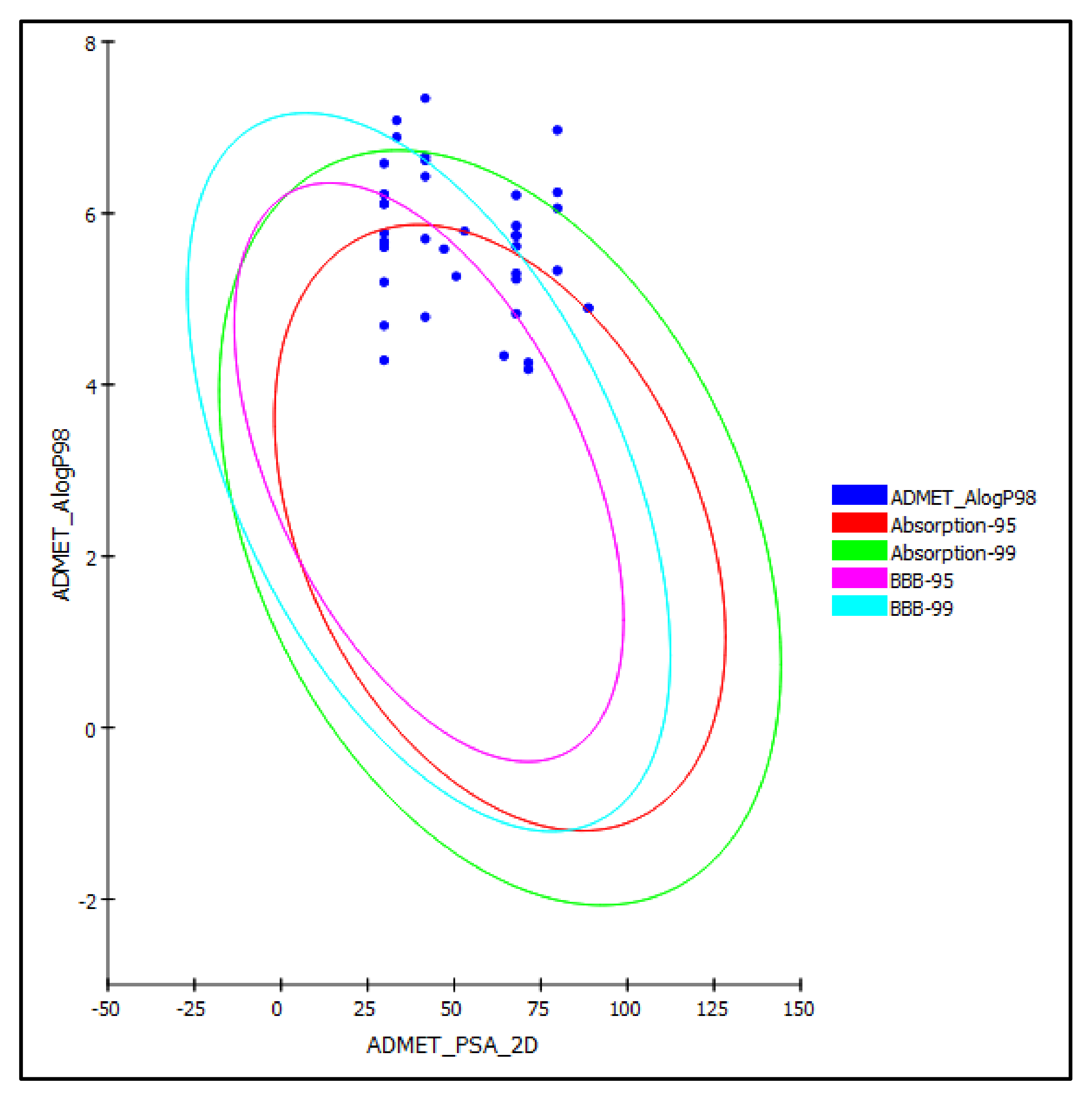
2.3. ADME/TOPAKT Evaluation
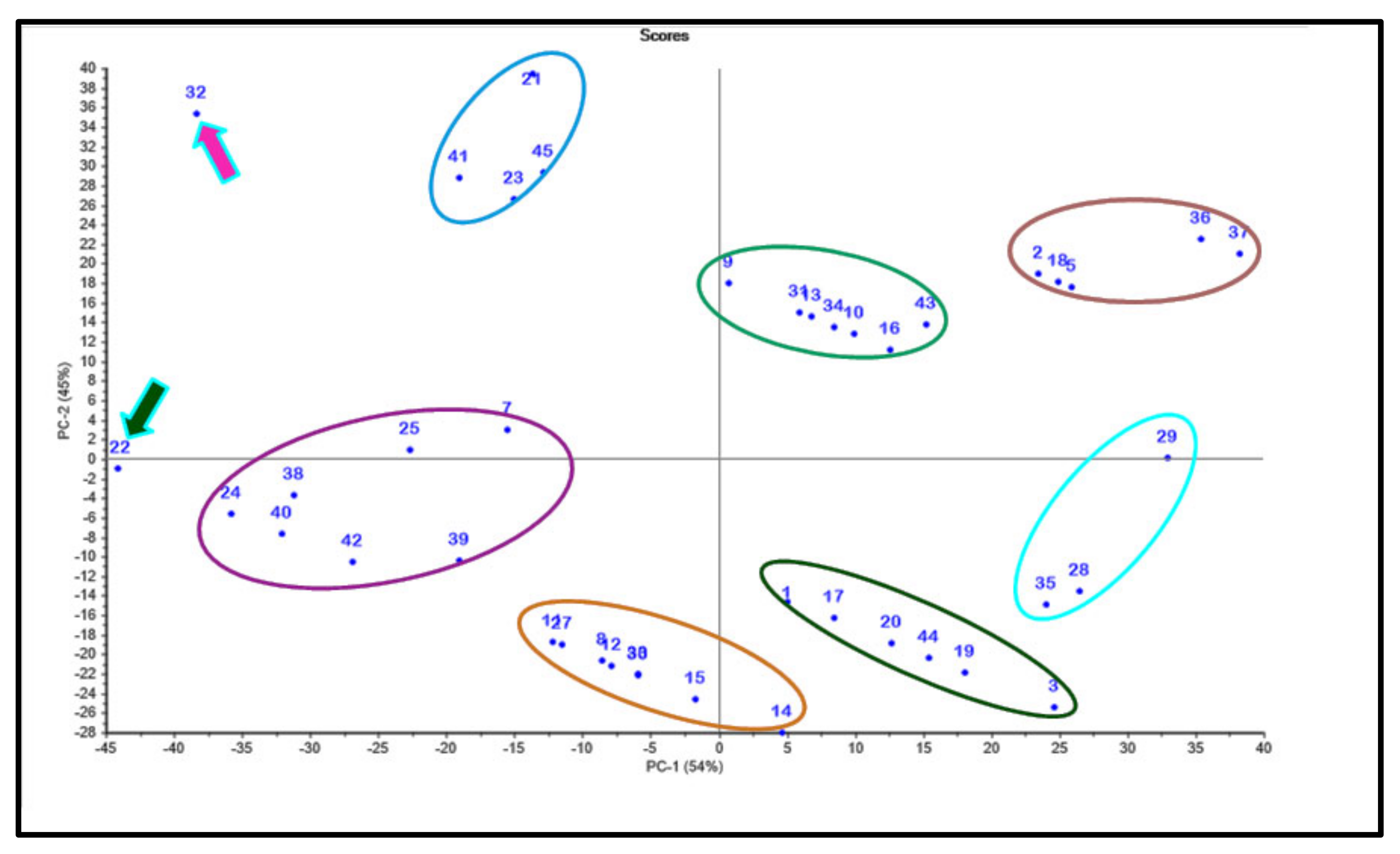
2.4. Chemometric Analysis
3. Discussion
4. Materials and Methods
4.1. Selection of Compounds Used in this Study
4.2. Molecular Docking Studies
4.3. ADME/TOPKAT Evaluation
4.4. Chemometric Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Verma, R.; Hoda, F.; Arshad, M.; Iqubal, A.; Siddiqui, A.N.; Khan, M.A.; Haque, S.E.; Akhtar, M.; Najmi, A.K. Cannabis, a Miracle Drug with Polyvalent Therapeutic Utility: Preclinical and Clinical-Based Evidence. Med. Cannabis Cannabinoids 2021, 4, 43–60. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Puntel, L.A.; Sawyer, J.E.; Barker, D.W.; Dietzel, R.; Poffenbarger, H.; Castellano, M.J.; Moore, K.J.; Thorburn, P.; Archontoulis, S.V. Modeling long-term corn yield response to nitrogen rate and crop rotation. Front. Plant Sci. 2016, 7, 1630. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. In Marijuana and the Cannabinoids; Humana Press, Inc.: Totowa, NJ, USA, 2007; pp. 17–49. [Google Scholar]
- Glass, M.; Dragunow, M.; Faull, R. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABAA receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 2000, 97, 505–519. [Google Scholar] [CrossRef]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Almanza, C.; Pakdel, A.; Lee, J.; Limbad, C. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Canc. Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; Pereira da Silva, V.M.; Cantao Freitas, L.; Gomes Silva, S.; Nevez Cruz, J.; de Aguiar Andrade, E.H. Extraction yield, chemical composition, preliminary toxicity of Bignonia nocturna (bignoniaceae) essential oil and in silico evaluation of the interaction. Chem. Biodivers. 2021, 18, e2000982. [Google Scholar] [CrossRef]
- Costa, E.; Silva, R.; Espejo-Román, J.; Neto, M.d.A.; Cruz, J.; Leite, F.; Silva, C.; Pinheiro, J.; Macêdo, W.; Santos, C. Chemometric methods in antimalarial drug design from 1, 2, 4, 5-tetraoxanes analogues. SAR QSAR Environ. Res. 2020, 31, 677–695. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Molecular targets for antiviral agents. J. Pharmacol. Exp.Ther. 2001, 297, 1–10. [Google Scholar] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Khafaji, K.; Al-Duhaidahawi, D.; Taskin Tok, T. Using integrated computational approaches to identify safe and rapid treatment for SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 39, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Park, J.G.; Cho, K.-H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Hok, L.; Rimac, H.; Mavri, J.; Vianello, R. COVID-19 infection and neurodegeneration: Computational evidence for interactions between the SARS-CoV-2 spike protein and monoamine oxidase enzymes. Comput. Struct. Biotechnol. J. 2022, 20, 1254–1263. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, M.; Bhargava, S. Potential use of turmeric in COVID-19. Clin. Exp. Dermatol. 2020, 45, 902–903. [Google Scholar] [CrossRef]
- Rollinger, J.; Langer, T.; Stuppner, H. Strategies for efficient lead structure discovery from natural products. Curr. Med. Chem. 2006, 13, 1491–1507. [Google Scholar] [CrossRef]
- Vora, J.; Patel, S.; Sinha, S.; Sharma, S.; Srivastava, A.; Chhabria, M.; Shrivastava, N. Molecular docking, QSAR and ADMET based mining of natural compounds against prime targets of HIV. J. Biomol. Struct. Dyn. 2019, 37, 131–146. [Google Scholar] [CrossRef]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; El-Beshbishy, H.A.; Singab, A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018, 114, 302–310. [Google Scholar] [CrossRef]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal metabolic variation in the essential oil composition of its leaf and verification of its anti-Ageing potential via in vitro assays and molecular modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Labib, R.M.; Ebada, S.S.; Youssef, F.S.; Ashour, M.L.; Ross, S.A. Ursolic acid, a natural pentacylcic triterpene from Ochrosia elliptica and its role in the management of certain neglected tropical diseases. Pharmacogn. Mag. 2016, 12, 319–325. [Google Scholar] [PubMed]
- The RCSB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 18 March 2022).
- Mollica, A.; Zengin, G.; Durdagi, S.; Ekhteiari Salmas, R.; Macedonio, G.; Stefanucci, A.; Dimmito, M.P.; Novellino, E. Combinatorial peptide library screening for discovery of diverse α-glucosidase inhibitors using molecular dynamics simulations and binary QSAR models. J. Biomol. Struct. Dyn. 2019, 37, 726–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamadalieva, N.Z.; Youssef, F.S.; Hussain, H.; Zengin, G.; Mollica, A.; Al Musayeib, N.M.; Ashour, M.L.; Westermann, B.; Wessjohann, L.A. Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules 2021, 26, 6331. [Google Scholar] [CrossRef]
- Bouzabata, A.; Montoro, P.; Gil, K.A.; Piacente, S.; Youssef, F.S.; Al Musayeib, N.M.; Cordell, G.A.; Ashour, M.L.; Tuberoso, C.I.G. HR-LC-ESI-Orbitrap-MS-based metabolic profiling coupled with chemometrics for the discrimination of different Echinops spinosus organs and evaluation of their antioxidant activity. Antioxidants 2022, 11, 453. [Google Scholar] [CrossRef]

| Compound N° | SARS-CoV-2 MPro | SARS-CoV-2 PLpro | ACE2 |
|---|---|---|---|
| 1 | 5.87 | 8.82 | −5.63 |
| 2 | 3.80 | 8.91 | −3.20 |
| 3 | 16.57 | 18.76 | 12.42 |
| 4 | FD | FD | Fd |
| 5 | 5.43 | 11.35 | −0.54 |
| 6 | FD | FD | FD |
| 7 | −9.86 | −10.30 | −21.88 |
| 8 | −3.02 | 2.48 | −7.84 |
| 9 | −6.26 | −5.98 | −12.97 |
| 10 | −2.26 | −0.42 | −11.52 |
| 11 | −4.93 | −1.53 | −11.36 |
| 12 | −3.56 | 4.79 | −5.66 |
| 13 | −2.94 | −2.22 | −9.13 |
| 14 | 5.89 | 9.91 | 3.45 |
| 15 | −0.36 | 8.00 | −2.83 |
| 16 | −2.26 | 4.29 | −3.51 |
| 17 | 3.61 | 10.54 | −1.86 |
| 18 | 3.87 | 6.00 | −6.51 |
| 19 | 12.51 | 16.34 | 3.87 |
| 20 | 6.11 | 14.17 | −2.43 |
| 21 | −21.60 | −18.60 | −30.36 |
| 22 | −23.24 | −22.81 | −31.34 |
| 23 | −19.40 | −15.13 | −25.34 |
| 24 | −21.40 | −16.33 | −27.52 |
| 25 | −16.24 | −11.56 | −27.85 |
| 26 | FD | FD | FD |
| 27 | −4.17 | −0.21 | −7.51 |
| 28 | 14.43 | 15.37 | 5.34 |
| 29 | 15.47 | 16.11 | 5.34 |
| 30 | −1.28 | 2.67 | −4.98 |
| 31 | −4.73 | −2.41 | −9.59 |
| 32 | −33.63 | −28.36 | −41.77 |
| 33 | 2.11 | −1.42 | −1.97 |
| 34 | −3.44 | −2.11 | −8.77 |
| 35 | 14.62 | 16.89 | 3.58 |
| 36 | 7.38 | 11.34 | 0.08 |
| 37 | 10.88 | 13.37 | −2.03 |
| 38 | −18.05 | −13.08 | −24.23 |
| 39 | −10.05 | −6.90 | −17.9 |
| 40 | −17.07 | −17.41 | −22.38 |
| 41 | −19.79 | −19.89 | −27.15 |
| 42 | −15.12 | −9.96 | −19.65 |
| 43 | 0.78 | 4.60 | −11.60 |
| 44 | 9.12 | 15.58 | −1.97 |
| 45 | −19.39 | −14.40 | −25.61 |
| SARS-CoV-2 MPro ligand (FHR/PRD_002347) | −4.58 | - | - |
| SARS-CoV-2 PLpro ligand (Y97) | - | −4.05 | - |
| ACE2 ligand (XX5) | - | - | −72.19 |
| Remdesivir | −35.56 | 2.28 | −44.62 |
| Compound | Absorption Level | Solubility Level | BBB Level | PPB Level | CPY2D6 | Hepato-Toxic | PSA-2D | Alog p98 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 0 | true | Inh | NT | 41.631 | 6.613 |
| 2 | 2 | 2 | 4 | true | NI | NT | 79.747 | 6.243 |
| 3 | 0 | 2 | 1 | true | NI | NT | 41.631 | 4.788 |
| 4 | - | - | - | - | - | - | - | - |
| 5 | 1 | 2 | 4 | true | NI | Tox | 79.747 | 5.331 |
| 6 | - | - | - | - | - | - | - | - |
| 7 | 0 | 1 | 0 | true | Inh | Tox | 47.046 | 5.581 |
| 8 | 1 | 1 | 0 | true | NI | Tox | 29.745 | 6.109 |
| 9 | 1 | 2 | 4 | true | NI | Tox | 67.861 | 5.739 |
| 10 | 1 | 2 | 4 | true | NI | Tox | 67.861 | 5.739 |
| 11 | 1 | 1 | 0 | true | NI | Tox | 29.745 | 6.109 |
| 12 | 0 | 1 | 0 | true | NI | Tox | 29.745 | 5.653 |
| 13 | 1 | 2 | 4 | true | NI | Tox | 67.861 | 5.739 |
| 14 | 0 | 2 | 0 | true | NI | Tox | 29.745 | 4.284 |
| 15 | 0 | 2 | 0 | true | NI | Tox | 29.745 | 5.197 |
| 16 | 0 | 2 | 1 | true | NI | Tox | 67.861 | 4.827 |
| 17 | 3 | 2 | 4 | true | Inh | NT | 41.631 | 7.34 |
| 18 | 2 | 2 | 4 | true | NI | NT | 79.747 | 6.969 |
| 19 | 1 | 2 | 0 | true | Inh | NT | 41.631 | 6.427 |
| 20 | 1 | 2 | 0 | true | Inh | NT | 41.631 | 6.427 |
| 21 | 2 | 2 | 4 | true | NI | NT | 79.747 | 6.057 |
| 22 | 1 | 1 | 0 | true | NI | Tox | 29.745 | 6.223 |
| 23 | 1 | 1 | 4 | true | NI | Tox | 67.861 | 5.853 |
| 24 | 0 | 1 | 0 | true | NI | Tox | 29.745 | 5.767 |
| 25 | 1 | 2 | 4 | true | NI | Tox | 41.631 | 6.659 |
| 26 | - | - | - | - | - | - | - | - |
| 27 | 1 | 3 | 0 | true | NI | true | 29.745 | 6.109 |
| 28 | 1 | 3 | 0 | true | NI | NT | 52.954 | 5.791 |
| 29 | 1 | 3 | 4 | false | NI | NT | 67.861 | 5.614 |
| 30 | 0 | 1 | 0 | true | NI | NT | 29.745 | 6.58 |
| 31 | 1 | 2 | 4 | true | NI | NT | 67.861 | 6.21 |
| 32 | 1 | 2 | 1 | true | NI | NT | 64.347 | 4.336 |
| 33 | 0 | 2 | 0 | true | NI | Tox | 29.745 | 5.668 |
| 34 | 0 | 2 | 1 | true | NI | NT | 67.861 | 5.297 |
| 35 | 0 | 2 | 1 | true | NI | NT | 50.561 | 5.264 |
| 36 | 0 | 2 | 4 | true | NI | Tox | 88.677 | 4.893 |
| 37 | 1 | 2 | 4 | true | NI | Tox | 88.677 | 4.893 |
| 38 | 3 | 1 | 4 | true | NI | Tox | 33.369 | 7.082 |
| 39 | 2 | 1 | 4 | true | NI | Tox | 33.369 | 6.886 |
| 40 | 0 | 1 | 0 | true | NI | Tox | 29.745 | 5.601 |
| 41 | 0 | 2 | 1 | true | NI | Tox | 67.861 | 5.23 |
| 42 | 0 | 2 | 0 | true | NI | Tox | 29.745 | 4.688 |
| 43 | 0 | 2 | 1 | true | NI | Tox | 71.376 | 4.256 |
| 44 | 0 | 2 | 0 | true | NI | Tox | 41.631 | 5.701 |
| 45 | 0 | 2 | 1 | true | NI | NT | 71.376 | 4.176 |
| Compound | Ames Prediction | Rat Oral LD50 | Rat Chronic LOAEL | Skin Irritancy | Ocular Irritancy | Rat Female FDA | Rat Male FDA |
|---|---|---|---|---|---|---|---|
| 1 | Non-Mutagen | 0.75 | 0.21 | Moderate | None | Non-Carcinogen | Non-Carcinogen |
| 2 | Non-Mutagen | 1.38 | 0.21 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 3 | Non-Mutagen | 0.59 | 0.11 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 4 | - | - | - | - | - | - | - |
| 5 | Non-Mutagen | 1.17 | 0.16 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 6 | - | - | - | - | - | - | - |
| 7 | Non-Mutagen | 1.52 | 0.09 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 8 | Non-Mutagen | 0.64 | 0.05 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 9 | Non-Mutagen | 0.96 | 0.03 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 10 | Non-Mutagen | 0.59 | 0.04 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 11 | Non-Mutagen | 0.61 | 0.04 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 12 | Non-Mutagen | 0.76 | 0.03 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 13 | Non-Mutagen | 1.01 | 0.04 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 14 | Non-Mutagen | 0.43 | 0.02 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 15 | Non-Mutagen | 0.46 | 0.02 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 16 | Non-Mutagen | 0.73 | 0.02 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 17 | Non-Mutagen | 2.26 | 0.30 | Moderate | None | Non-Carcinogen | Non-Carcinogen |
| 18 | Non-Mutagen | 4.14 | 0.29 | None | None | Non-Carcinogen | Non-Carcinogen |
| 19 | Non-Mutagen | Non-Carcinogen | Non-Carcinogen | ||||
| 20 | Non-Mutagen | 1.89 | 0.23 | Moderate | None | Non-Carcinogen | Non-Carcinogen |
| 21 | Non-Mutagen | 3.52 | 0.22 | None | None | Non-Carcinogen | Non-Carcinogen |
| 22 | Non-Mutagen | 2.31 | 0.23 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 23 | Non-Mutagen | 3.63 | 0.18 | None | Mild | Non-Carcinogen | Non-Carcinogen |
| 24 | Non-Mutagen | 2.57 | 0.11 | None | Mild | Non-Carcinogen | Non-Carcinogen |
| 25 | Non-Mutagen | 3.90 | 0.90 | None | Mild | Non-Carcinogen | Non-Carcinogen |
| 26 | - | - | - | - | - | - | - |
| 27 | Non-Mutagen | 0.65 | 0.05 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 28 | Non-Mutagen | 5.01 | 0.42 | Mild | None | Non-Carcinogen | Non-Carcinogen |
| 29 | Non-Mutagen | 5.32 | 0.13 | Mild | None | Non-Carcinogen | Non-Carcinogen |
| 30 | Non-Mutagen | 1.85 | 0.07 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 31 | Non-Mutagen | 2.94 | 0.06 | None | Mild | Non-Carcinogen | Non-Carcinogen |
| 32 | Non-Mutagen | 3.64 | 0.22 | None | Moderate | Non-Carcinogen | Non-Carcinogen |
| 33 | Non-Mutagen | 1.33 | 0.04 | Moderate | Severe | Single-Carcinogen | Non-Carcinogen |
| 34 | Non-Mutagen | 2.13 | 0.03 | None | Mild | Non-Carcinogen | Non-Carcinogen |
| 35 | Non-Mutagen | 1.85 | 0.04 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 36 | Non-Mutagen | 2.3 | 0.03 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 37 | Non-Mutagen | 1.34 | 0.03 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 38 | Non-Mutagen | 0.09 | 0.23 | Severe | Severe | Non-Carcinogen | Non-Carcinogen |
| 39 | Non-Mutagen | 0.34 | 0.24 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 40 | Non-Mutagen | 0.98 | 0.07 | Moderate | Severe | Non-Carcinogen | Non-Carcinogen |
| 41 | Non-Mutagen | 1.54 | 0.06 | None | Severe | Single-Carcinogen | Non-Carcinogen |
| 42 | Non-Mutagen | 0.71 | 0.04 | None | Severe | Single-Carcinogen | Non-Carcinogen |
| 43 | Non-Mutagen | 3.24 | 0.05 | None | Severe | Non-Carcinogen | Non-Carcinogen |
| 44 | Non-Mutagen | 0.63 | 0.16 | Moderate | None | Non-Carcinogen | Non-Carcinogen |
| 45 | Non-Mutagen | 1.15 | 0.09 | None | Severe | Non-Carcinogen | Non-Carcinogen |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altyar, A.E.; Youssef, F.S.; Kurdi, M.M.; Bifari, R.J.; Ashour, M.L. The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties. Molecules 2022, 27, 2797. https://doi.org/10.3390/molecules27092797
Altyar AE, Youssef FS, Kurdi MM, Bifari RJ, Ashour ML. The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties. Molecules. 2022; 27(9):2797. https://doi.org/10.3390/molecules27092797
Chicago/Turabian StyleAltyar, Ahmed E., Fadia S. Youssef, Maram M. Kurdi, Renad J. Bifari, and Mohamed L. Ashour. 2022. "The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties" Molecules 27, no. 9: 2797. https://doi.org/10.3390/molecules27092797
APA StyleAltyar, A. E., Youssef, F. S., Kurdi, M. M., Bifari, R. J., & Ashour, M. L. (2022). The Role of Cannabis sativa L. as a Source of Cannabinoids against Coronavirus 2 (SARS-CoV-2): An In Silico Study to Evaluate Their Activities and ADMET Properties. Molecules, 27(9), 2797. https://doi.org/10.3390/molecules27092797









