Green Synthesized ZnO Nanoparticles as Biodiesel Blends and Their Effect on the Performance and Emission of Greenhouse Gases
Abstract
:1. Introduction
2. Results and Discussion
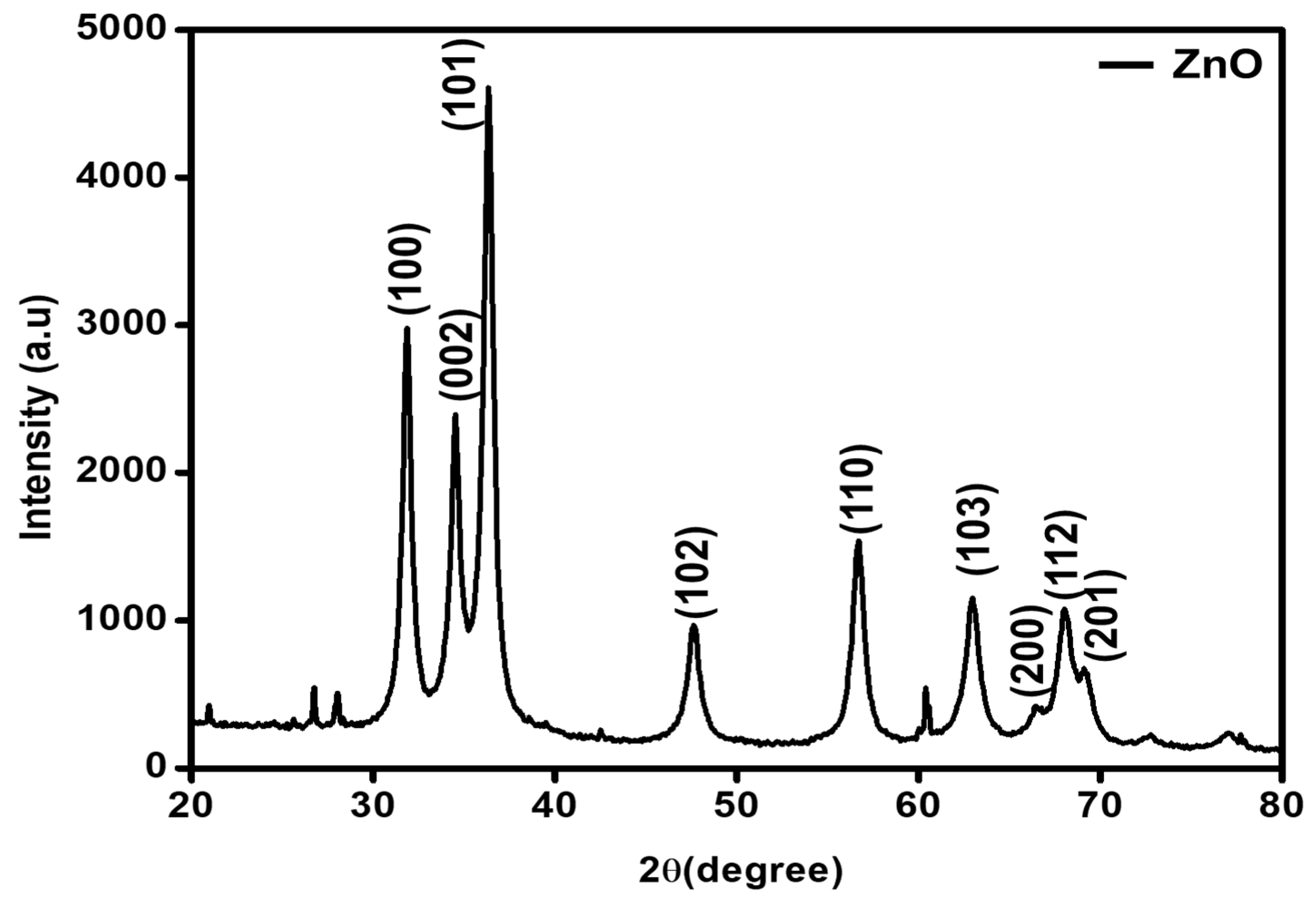
2.1. Characterization of the ZnO-GS Nanoparticles
2.2. Physical Properties of D-COME
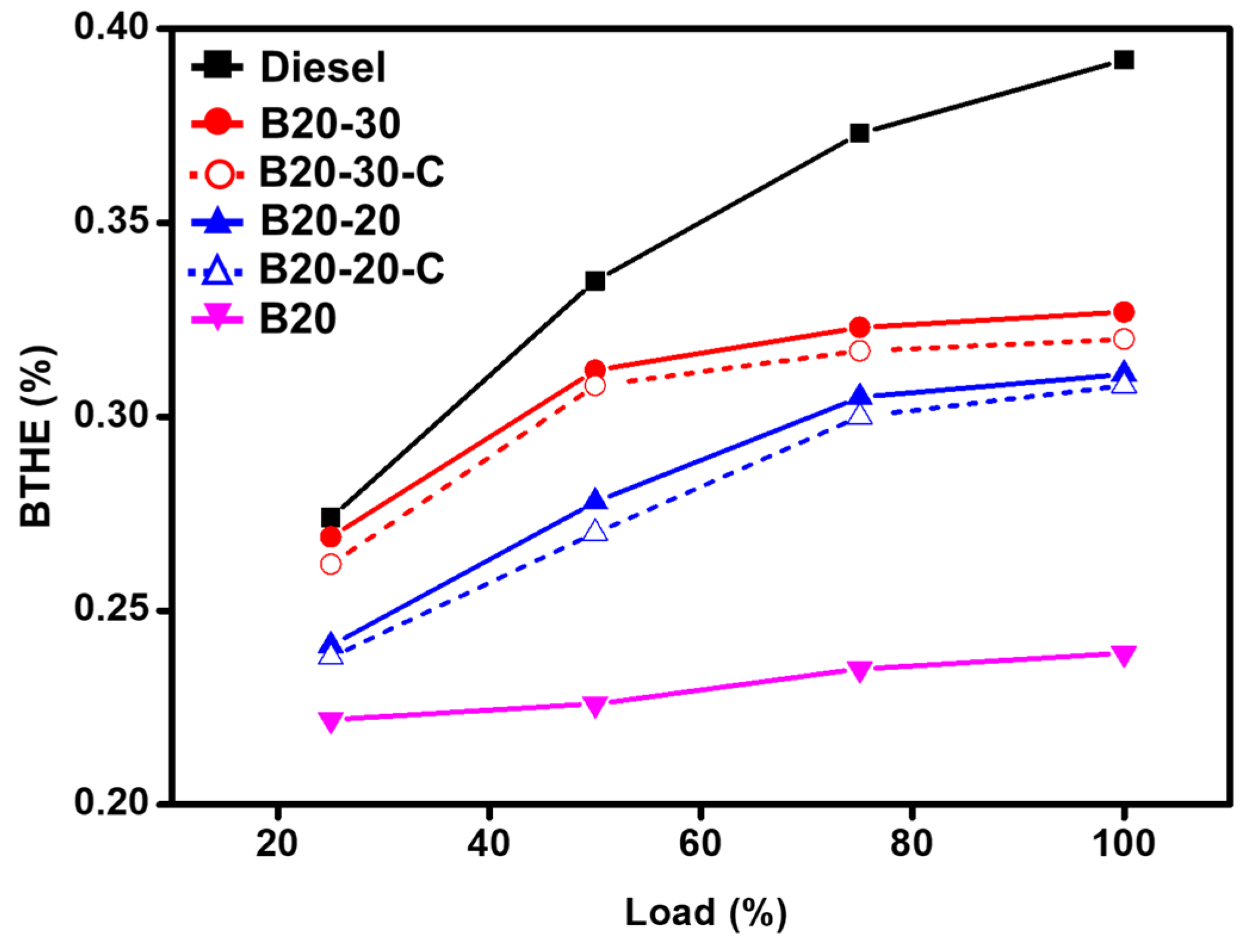
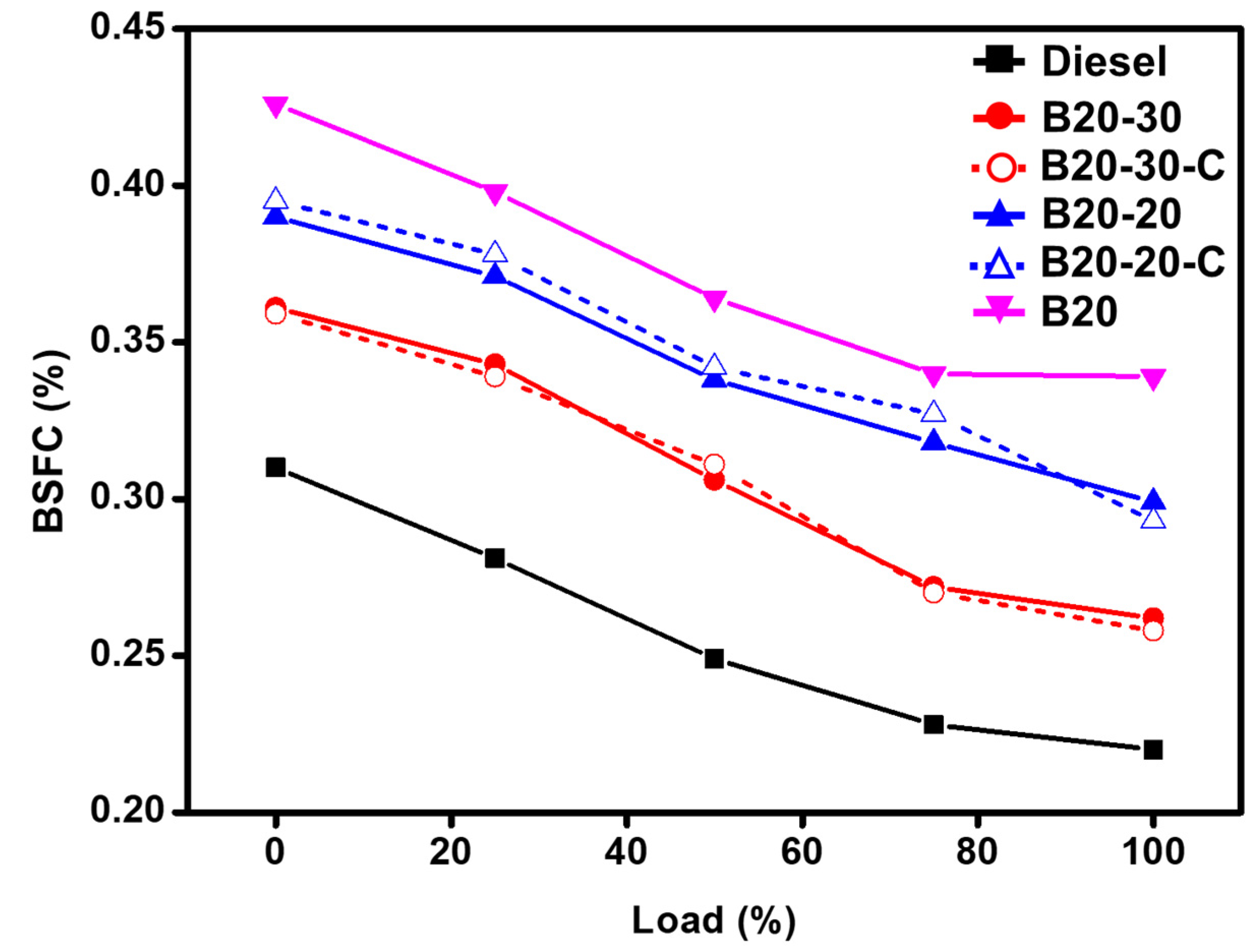
Brake Thermal Efficiency (BTHE) and Brake Specific Fuel Consumption (BSFC) Analysis
2.3. Carbon Monoxide (CO) Emission
2.4. Carbon Dioxide (CO2) Emission
2.5. Nitrogen Oxide (NOx) Emission
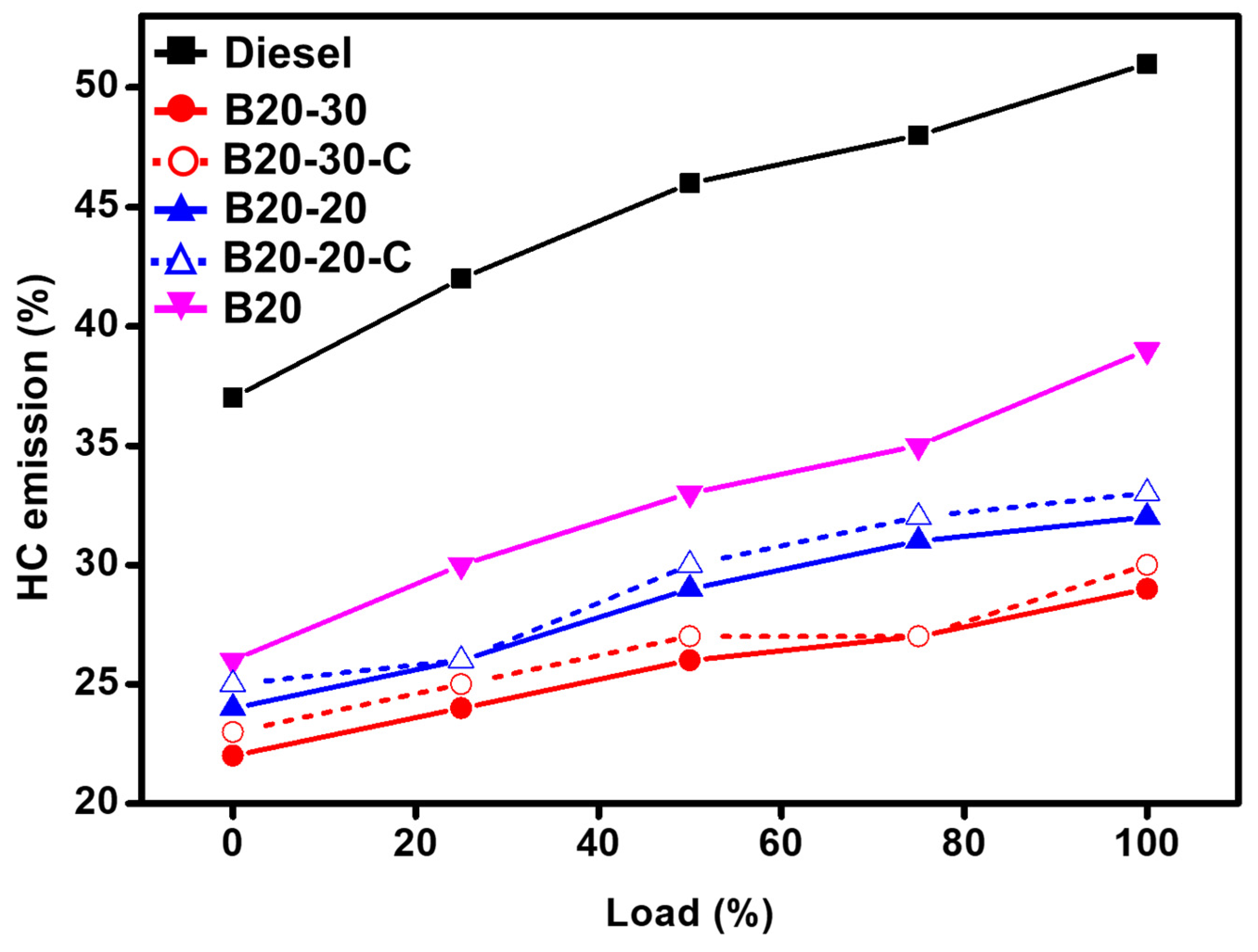
2.6. Hydrocarbon (HC) Emission
3. Materials and Methods
3.1. Materials
3.2. Green Synthesis of ZnO Nanocatalyst (ZnO-GS)
3.3. Synthesis of Discarded Cooking Oil Methyl Ester (D-COME)
3.4. Blending of D-COME
3.5. Characterization of ZnO-GS Nanocatalyst
3.6. Experimental Setup and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Othman, M.F.; Adam, A.; Najafi, G.; Mamat, R. Green fuel as alternative fuel for diesel engine: A review. Renew. Sust. Energy Rev. 2017, 80, 694–709. [Google Scholar] [CrossRef]
- Ali, B.; Yusup, S.; Quitain, A.T.; Alnarabiji, M.S.; Kamil, R.N.M.; Kida, T. Synthesis of novel graphene oxide/bentonite bi-functional heterogeneous catalyst for one-pot esterification and transesterification reactions. Energy Convers. Manag. 2018, 171, 1801–1812. [Google Scholar] [CrossRef]
- Jamil, F.; Kumar, P.S.M.; Al-Haj, L.; Myint, M.T.Z.; Ala’a, H. Heterogeneous carbon-based catalyst modified by alkaline earth metal oxides for biodiesel production: Parametric and kinetic study. Energy Convers. Manag. X 2021, 10, 100047. [Google Scholar] [CrossRef]
- Jume, B.H.; Gabris, M.A.; Nodeh, H.R.; Rezania, S.; Cho, J. Biodiesel production from waste cooking oil using a novel heterogeneous catalyst based on graphene oxide doped metal oxide nanoparticles. Renew. Energy 2020, 162, 2182–2189. [Google Scholar] [CrossRef]
- Yoo, S.J.; Lee, H.-S.; Veriansyah, B.; Kim, J.; Kim, J.-D.; Lee, Y.-W. Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour. Technol. 2010, 101, 8686–8689. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Wang, A.; Quan, W.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for efficient biodiesel production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Borah, R.; Deka, D. Synthesis and application of Co doped ZnO as heterogeneous nanocatalyst for biodiesel production from non-edible oil. Renew. Energy 2019, 133, 512–519. [Google Scholar] [CrossRef]
- Naveed Ul Haq, A.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis approaches of zinc oxide nanoparticles: The dilemma of ecotoxicity. J. Nanomater. 2017, 2017, 8510342. [Google Scholar] [CrossRef]
- Quirino, M.R.; Oliveira, M.J.C.; Keyson, D.; Lucena, G.L.; Oliveira, J.B.L.; Gama, L. Synthesis of zinc oxide by microwave hydrothermal method for application to transesterification of soybean oil (biodiesel). Mater. Chem. Phys. 2017, 185, 24–30. [Google Scholar] [CrossRef]
- Ali, M.A.; Idris, M.R.; Quayum, M.E. Fabrication of ZnO nanoparticles by solution-combustion method for the photocatalytic degradation of organic dye. J. Nanostruct. Chem. 2013, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Desantes, J.; Molina, S.; Novella, R.; Lopez-Juarez, M. Comparative global warming impact and NOX emissions of conventional and hydrogen automotive propulsion systems. Energy Convers. Manag. 2020, 221, 113137. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.-N.; Kalam, M.; Badruddin, I.A.; Banapurmath, N.R.; Ali, M.A.B.; Kamangar, S.; Cho, H.M.; Akram, N. An investigation on the influence of aluminium oxide nano-additive and honge oil methyl ester on engine performance, combustion and emission characteristics. Renew. Energy 2020, 146, 2291–2307. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Esfandeh, S. Improving engine oil lubrication in light-duty vehicles by using of dispersing MWCNT and ZnO nanoparticles in 5W50 as viscosity index improvers (VII). Appl. Therm. Eng. 2018, 143, 493–506. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Mahalingam, A. Investigation on behavior of diesel engine performance, emission, and combustion characteristics using nano-additive in neat biodiesel. Heat Mass Transfer. 2019, 55, 1641–1650. [Google Scholar] [CrossRef]
- Prabu, A.; Anand, R. Emission control strategy by adding alumina and cerium oxide nano particle in biodiesel. J. Energy Inst. 2016, 89, 366–372. [Google Scholar] [CrossRef]
- Elumalai, P.; Balasubramanian, D.; Parthasarathy, M.; Pradeepkumar, A.; Iqbal, S.M.; Jayakar, J.; Nambiraj, M. An experimental study on harmful pollution reduction technique in low heat rejection engine fuelled with blends of pre-heated linseed oil and nano additive. J. Clean. Prod. 2021, 283, 124617. [Google Scholar] [CrossRef]
- Javed, S.; Murthy, Y.S.; Satyanarayana, M.; Reddy, R.R.; Rajagopal, K. Effect of a zinc oxide nanoparticle fuel additive on the emission reduction of a hydrogen dual-fuelled engine with jatropha methyl ester biodiesel blends. J. Clean. Prod. 2016, 137, 490–506. [Google Scholar] [CrossRef]
- Hasannuddin, A.; Yahya, W.; Sarah, S.; Ithnin, A.; Syahrullail, S.; Sidik, N.; Kassim, K.A.; Ahmad, Y.; Hirofumi, N.; Ahmad, M. Nano-additives incorporated water in diesel emulsion fuel: Fuel properties, performance and emission characteristics assessment. Energy Convers. Manag. 2018, 169, 291–314. [Google Scholar] [CrossRef]
- Kumar, T.D.; Hussain, S.S.; Ramesha, D. Effect of a zinc oxide nanoparticle fuel additive on the performance and emission characteristics of a CI engine fuelled with cotton seed biodiesel blends. Mater. Today Proc. 2020, 26, 2374–2378. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Mujtaba, M.; Safaei, M.R.; Afzal, A.; Ahmed, W.; Banapurmath, N.; Hossain, N.; Bashir, S.; Badruddin, I.A.; Goodarzi, M. Effect of Sr@ ZnO nanoparticles and Ricinus communis biodiesel-diesel fuel blends on modified CRDI diesel engine characteristics. Energy 2021, 215, 119094. [Google Scholar] [CrossRef]
- Zak, A.K.; Abrishami, M.E.; Majid, W.H.A.; Yousefi, R.; Hosseini, S.M. Effects of annealing temperature on some structural and optical properties of ZnO nanoparticles prepared by a modified sol–gel combustion method. Ceram. Int. 2011, 37, 393–398. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, J.; Parashar, V.; Singh, K.; Tiwari, R.; Srivastava, O.; Ramam, K.; Pandey, A.C. Investigations on structural, optical and second harmonic generation in solvothermally synthesized pure and Cr-doped ZnO nanoparticles. CrystEngComm 2012, 14, 1653–1658. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, J.; Zhu, Q.; Cui, Y.; Liu, L.; Li, B.; Zhou, X. Monodispersed hollow microsphere of ZnO mesoporous nanopieces: Preparation, growth mechanism and photocatalytic performance. Mater. Res. Bull. 2010, 45, 2024–2030. [Google Scholar] [CrossRef]
- Garba, J.; Samsuri, W.A.; Othman, R.; Hamdani, M.S.A. Evaluation of Adsorptive Characteristics of Cow Dung and Rice Husk Ash for Removal of Aqueous Glyphosate and Aminomethylphoshonic Acid. Sci. Rep. 2019, 9, 17689. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Abd Majid, W.H.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Al_Dawody, M.F.; Bhatti, S. Experimental and computational investigations for combustion, performance and emission parameters of a diesel engine fueled with soybean biodiesel-diesel blends. Energy Procedia 2014, 52, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- McCormick, R.L.; Williams, A.; Ireland, J.; Hayes, R. Effects of Biodiesel Blends on Vehicle Emissions: Fiscal Year 2006 Annual Operating Plan Milestone 10.4; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2006. [Google Scholar]










| S. No. | Property | Diesel [29] | D-COME |
|---|---|---|---|
| 1 | Calorific Value (kJ/kg) | 42,000 | 39,216 |
| 2 | Flash Point [°C] | 52–96 | 182 |
| 3 | Fire Point [°C] | 62–106 | 134 |
| 4 | Density at 15 °C [g/m3] | 824 | 925 |
| 5 | Viscosity [mm2/s] | 1.2–2 | 4.56 |
| 6 | Octane Number | 48 | 55 |
| Variation | Yield (%) | |
|---|---|---|
| Temperature (°C) Conditions: Catalyst ZnO-GS 175 mg; Methanol 150 mL; Time 90 min | 55 | 69.5 |
| 60 | 82.3 | |
| 65 | 97.2 (96.8) | |
| 70 | 96.6 | |
| Methanol (mL) Conditions: Catalyst ZnO-GS 175 mg; Temperature 65 °C; Time 90 min | 100 | 82.5 |
| 125 | 92.5 | |
| 150 | 97.2 | |
| 175 | 97.3 | |
| ZnO-GS (mg) Conditions: Methanol 150 mL; Temperature 65 °C; Time 90 min | 125 | 81.4 |
| 150 | 90.3 | |
| 175 | 97.2 | |
| 200 | 97.5 | |
| Parameters | Value |
|---|---|
| Bore (mm) | 80 |
| Stroke Length (mm) | 110 |
| Connecting Rod Length (mm) | 234 |
| Displacement Volume (cc) | 552 |
| Rated Speed (RPM) | 1500 |
| Max Power (HP) | 5 |
| Max Torque (Nm) | 24 |
| Compression Ratio | 16.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavalli, K.; Hebbar, G.S.; Shubha, J.P.; Adil, S.F.; Khan, M.; Hatshan, M.R.; Almutairi, A.M.; Shaik, B. Green Synthesized ZnO Nanoparticles as Biodiesel Blends and Their Effect on the Performance and Emission of Greenhouse Gases. Molecules 2022, 27, 2845. https://doi.org/10.3390/molecules27092845
Kavalli K, Hebbar GS, Shubha JP, Adil SF, Khan M, Hatshan MR, Almutairi AM, Shaik B. Green Synthesized ZnO Nanoparticles as Biodiesel Blends and Their Effect on the Performance and Emission of Greenhouse Gases. Molecules. 2022; 27(9):2845. https://doi.org/10.3390/molecules27092845
Chicago/Turabian StyleKavalli, Kiran, Gurumoorthy S. Hebbar, Jayachamarajapura Pranesh Shubha, Syed Farooq Adil, Mujeeb Khan, Mohammad Rafe Hatshan, Adibah Mukhlid Almutairi, and Baji Shaik. 2022. "Green Synthesized ZnO Nanoparticles as Biodiesel Blends and Their Effect on the Performance and Emission of Greenhouse Gases" Molecules 27, no. 9: 2845. https://doi.org/10.3390/molecules27092845
APA StyleKavalli, K., Hebbar, G. S., Shubha, J. P., Adil, S. F., Khan, M., Hatshan, M. R., Almutairi, A. M., & Shaik, B. (2022). Green Synthesized ZnO Nanoparticles as Biodiesel Blends and Their Effect on the Performance and Emission of Greenhouse Gases. Molecules, 27(9), 2845. https://doi.org/10.3390/molecules27092845









