Insight into Spodium–π Bonding Characteristics of the MX2⋯π (M = Zn, Cd and Hg; X = Cl, Br and I) Complexes—A Theoretical Study
Abstract
:1. Introduction
2. Theoretical Methods
3. Results and Discussion
3.1. Molecular Electrostatic Potential of Monomers
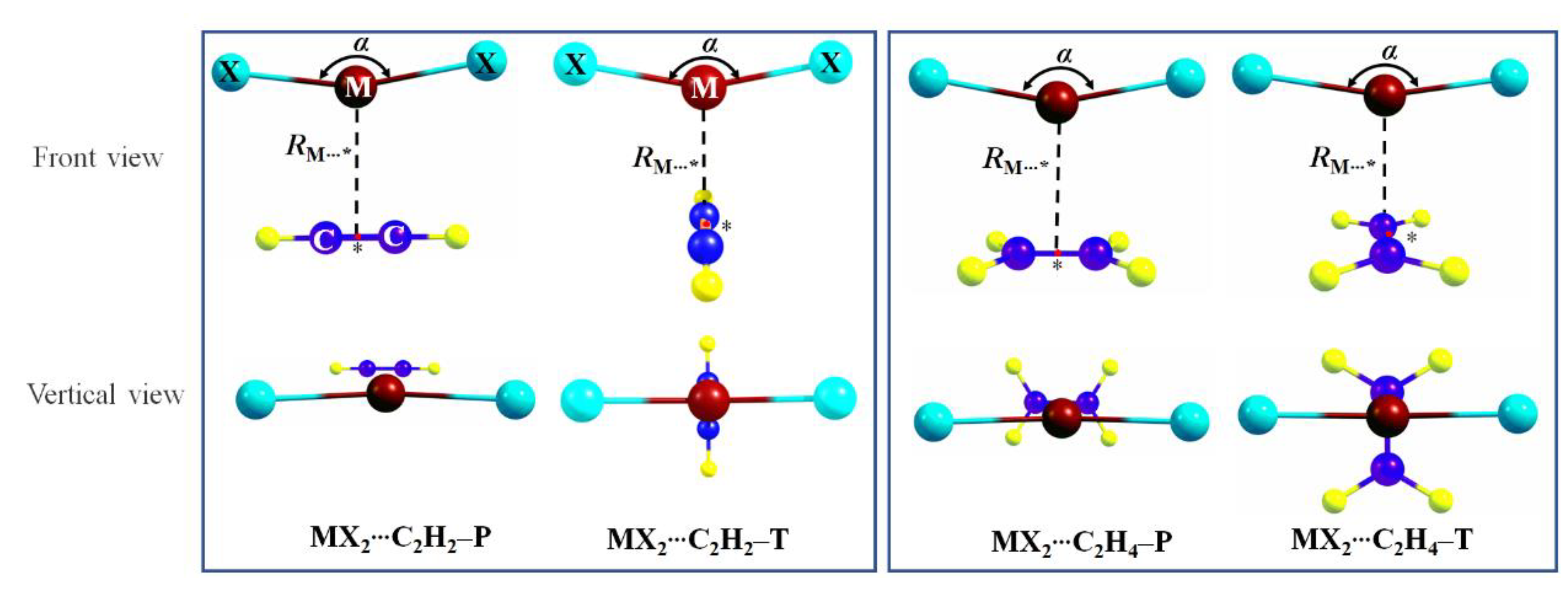
3.2. Geometrics and Interaction Energies
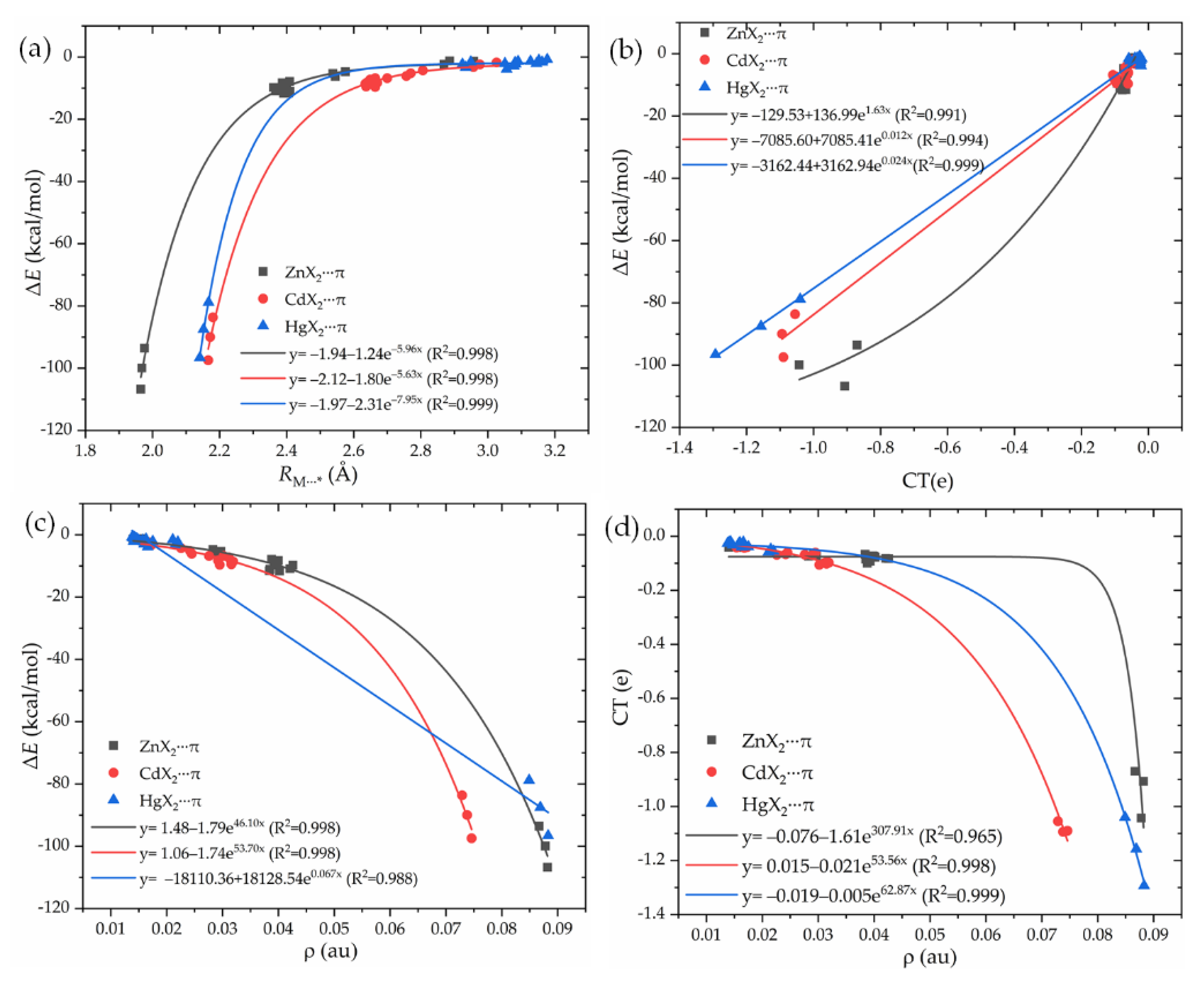
3.3. Substituent Effect
3.4. AIM
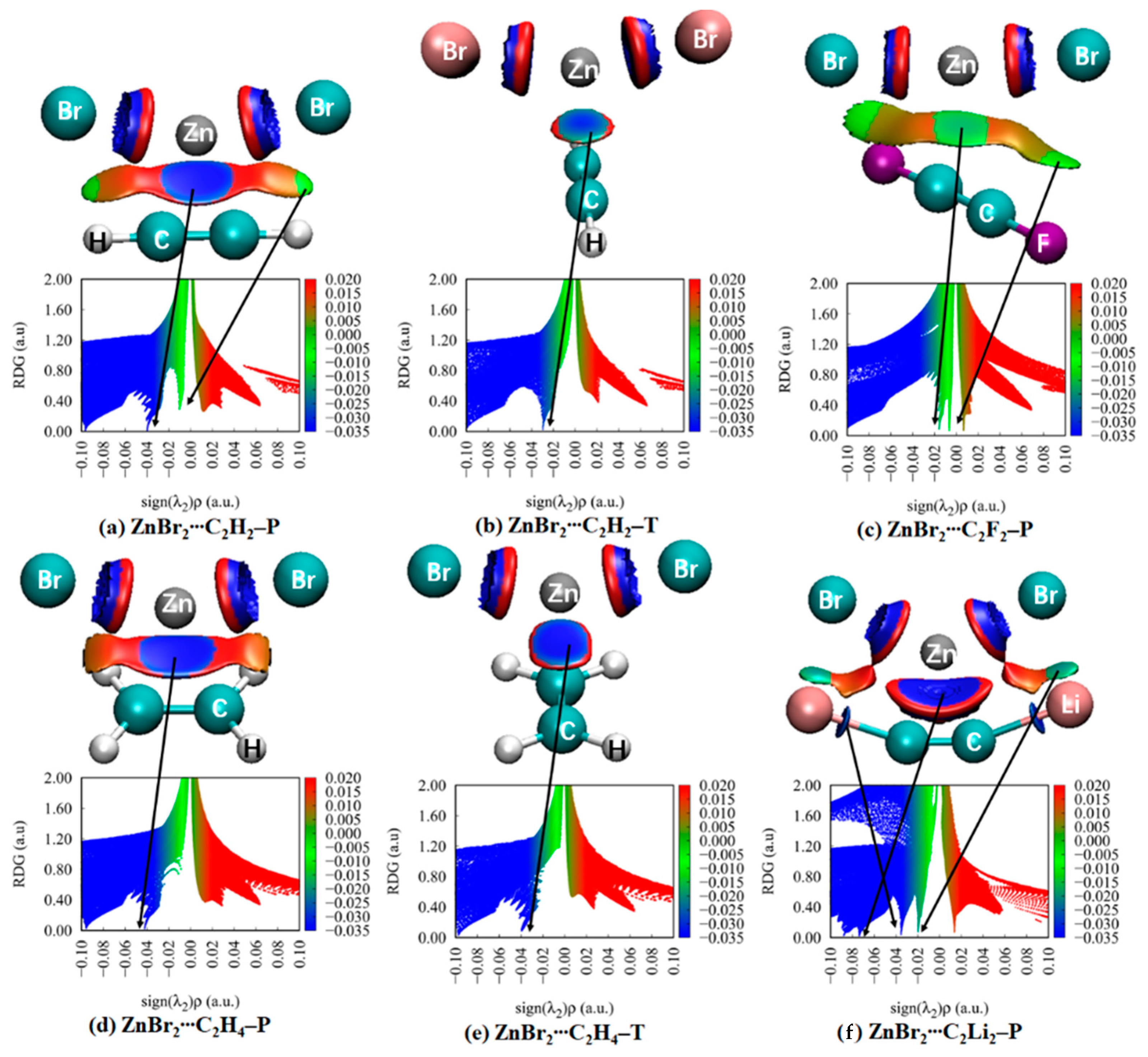
3.5. NCI Analyses
3.6. Electron Density Shift
3.7. Energy Decomposition
3.8. Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bauzá, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Lawrence, S.E.; Cisterna, J.; Cardenas, A.; Brito, I.; Frontera, A.; Safin, D.A. A new spodium bond driven coordination polymer constructed from mercury(II) azide and 1,2-bis (pyridin-2-ylmethylene) hydrazine. New J. Chem. 2020, 44, 21100–21107. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Masoudiasl, A.; Babashkina, M.G.; Frontera, A.; Doert, T.; White, J.M.; Zangrando, E.; Zubkov, F.I.; Safin, D.A. On the importance of π-hole spodium bonding in tricoordinated HgII complexes. Dalton Trans. 2020, 49, 17547–17551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Banerjee, S.; Radha, A.; Firdoos, T.; Sahoo, S.C.; Pandey, S.K. Role of non-covalent interactions in the supramolecular architectures of mercury(II) diphenyldithiophosphates: An experimental and theoretical investigation. New J. Chem. 2021, 45, 2249–2263. [Google Scholar] [CrossRef]
- Karmakar, M.; Frontera, A.; Chattopadhyay, S.; Mooibroek, T.J.; Bauzá, A. Intramolecular Spodium Bonds in Zn(II) Complexes: Insights from Theory and Experiment. Int. J. Mol. Sci. 2020, 21, 7091. [Google Scholar] [CrossRef]
- Basak, T.; Gomila, R.M.; Frontera, A.; Chattopadhyay, S. Differentiating intramolecular spodium bonds from coordination bonds in two polynuclear zinc(II) Schiff base complexes. CrystEngComm 2021, 23, 2703–2710. [Google Scholar] [CrossRef]
- Gomila, R.M.; Bauzá, A.; Mooibroek, T.J.; Frontera, A. Spodium bonding in five coordinated Zn(II): A new player in crystal engineering? CrystEngComm 2021, 23, 3084–3093. [Google Scholar] [CrossRef]
- Gomila, R.M.; Bauzá, A.; Mooibroek, T.J.; Frontera, A. π-Hole spodium bonding in tri-coordinated Hg(II) complexes. Dalton Trans. 2021, 50, 7545–7553. [Google Scholar] [CrossRef]
- Samie, A.; Salimi, A.; Garrison, J.C. Coordination chemistry of mercury(II) halide complexes: A combined experimental, theoretical and (ICSD & CSD) database study on the relationship between inorganic and organic units. Dalton Trans. 2020, 49, 11859–11877. [Google Scholar]
- Biswal, H.S.; Kumar Sahu, A.; Frontera, A.; Bauzá, A. Spodium Bonds in Biological Systems: Expanding the Role of Zn in Protein Structure and Function. J. Chem. Inf. Model. 2021, 61, 3945–3954. [Google Scholar] [CrossRef]
- Llull, R.; Montalbán, G.; Vidal, I.; Gomila, R.M.; Bauzá, A.; Frontera, A. Theoretical study of spodium bonding in the active site of three Zn-proteins and several model systems. Phys. Chem. Chem. Phys. 2021, 23, 16888–16896. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Li, D.; Cheng, L.J. Theoretical analysis of the spodium bonds in HgCl2⋯L (L = ClR, SR2, and PR3) dimers. Chem. Phys. 2020, 539, 110978. [Google Scholar] [CrossRef]
- Echeverría, J.; Cirera, J.; Alvarez, S. Mercurophilic interactions: A theoretical study on the importance of ligands. Phys. Chem. Chem. Phys. 2017, 19, 11645–11654. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Wang, Y.; Yu, Z. The Structures of ZnCl2-Ethanol Mixtures, a Spectroscopic and Quantum Chemical Calculation Study. Molecules 2021, 26, 2498. [Google Scholar] [CrossRef]
- Jabłoński, M. Study of Beryllium, Magnesium, and Spodium Bonds to Carbenes and Carbodiphosphoranes. Molecules 2021, 26, 2275. [Google Scholar] [CrossRef]
- Jabłoński, M. Theoretical Study of N-Heterocyclic-Carbene-ZnX2 (X = H, Me, Et) Complexes. Materials 2021, 14, 6147. [Google Scholar] [CrossRef]
- Wysokiński, R.; Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Anion⋯Anion Attraction in Complexes of MCl3− (M=Zn, Cd, Hg) with CN−. ChemPhysChem 2020, 21, 1119–1125. [Google Scholar] [CrossRef]
- Wysokiński, R.; Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Crystallographic and Theoretical Evidences of Anion⋯Anion Interaction. ChemPhysChem 2021, 22, 818–821. [Google Scholar] [CrossRef]
- Wysokiński, R.; Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Anionanion (MX3−)2 dimers (M = Zn, Cd, Hg; X = Cl, Br, I) in different environments. Phys. Chem. Chem. Phys. 2021, 23, 13853–13861. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.Z. Group 12 Carbonates and their Binary Complexes with Nitrogen Bases and FH2Z Molecules (Z=P, As, Sb): Synergism in Forming Ternary Complexes. ChemPhysChem 2021, 22, 1698–1705. [Google Scholar] [CrossRef]
- Liu, N.; Xie, X.; Li, Q. Chalcogen Bond Involving Zinc(II)/Cadmium(II) Carbonate and Its Enhancement by Spodium Bond. Molecules 2021, 26, 6443. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Q.; Scheiner, S. Spodium and tetrel bonds involving Zn(II)/Cd(II) and their interplay. Chem. Phys. 2022, 556, 111470. [Google Scholar] [CrossRef]
- Gao, M.; Yang, X.; Cheng, J.B.; Li, Q.Z.; Li, W.Z.; Loffredo, R.E. Interplay between metal⋯π interactions and hydrogen bonds: Some unusual synergetic effects of coinage metals and substituents. ChemPhysChem 2013, 14, 3341–3347. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Cheng, J.B.; Li, W.Z.; Xiao, B.; Li, Q.Z. The aerogen–π bonds involving π systems. Chem. Phys. Lett. 2016, 651, 50–55. [Google Scholar] [CrossRef]
- Frontera, A. σ- and π-Hole Interactions. Crystals 2020, 10, 721. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. Origin of the Attraction and Directionality of the NH/π Interaction: Comparison with OH/π and CH/π Interactions. J. Am. Chem. Soc. 2000, 122, 11450–11458. [Google Scholar] [CrossRef]
- McDowell, S.A.C. Isotope effects in X–H⋯π type hydrogen-bonded complexes (X = F, Cl or Br). Phys. Chem. Chem. Phys. 2001, 3, 2754–2757. [Google Scholar] [CrossRef]
- Biswal, H.S.; Wategaonkar, S. Sulfur, not too far behind O, N, and C: SH⋯π hydrogen bond. J. Phys. Chem. A 2009, 113, 12774–12782. [Google Scholar] [CrossRef]
- Andersen, J.; Heimdal, J.; Nelander, B.; Wugt Larsen, R. Competition between weak OH⋯π and CH⋯O hydrogen bonds: THz spectroscopy of the C2H2–H2O and C2H4–H2O complexes. J. Chem. Phys. 2017, 146, 194302. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.Y.; Su, H.; Wang, H.Y.; Wang, H. Ab initio calculations, structure, NBO and NCI analyses of X–H⋯π interactions. Chem. Phys. Lett. 2018, 693, 202–209. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, Y.; Liu, Y.; Zhang, J.; Lv, L.; Zhang, J. π Type Lithium Bond Interaction between Ethylene, Acetylene, or Benzene and Amido-lithium. Chin. J. Chem. 2009, 27, 697–702. [Google Scholar] [CrossRef]
- Aarabi, M.; Gholami, S.; Grabowski, S.J. Hydrogen and Lithium Bonds-Lewis Acid Units Possessing Multi-Center Covalent Bonds. Molecules 2021, 26, 6939. [Google Scholar] [CrossRef] [PubMed]
- Ammal, S.S.C.; Venuvanalingam, P. π-systems as lithium/hydrogen bond acceptors: Some theoretical observations. J. Chem. Phys. 1998, 109, 9820–9830. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, E.F.; Mó, O.; Yáñez, M. On the existence and characteristics of π-beryllium bonds. Phys. Chem. Chem. Phys. 2014, 16, 17531–17536. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Zhang, X.T.; Li, H.X.; Zhu, Y.C.; Yang, X.P. Theoretical observations of π-systems as sodium bond donors. Chem. Phys. Lett. 2011, 510, 273–277. [Google Scholar] [CrossRef]
- Li, S.Y.; Wu, D.; Li, Y.; Yu, D.; Liu, J.Y.; Li, Z.R. Insight into structural and π–magnesium bonding characteristics of the X2Mg⋯Y (X = H, F; Y = C2H2, C2H4 and C6H6) complexes. RSC Adv. 2016, 6, 102754–102761. [Google Scholar] [CrossRef]
- Dias, H.V.; Flores, J.A.; Wu, J.; Kroll, P. Monomeric copper(I), silver(I), and gold(I) alkyne complexes and the coinage metal family group trends. J. Am. Chem. Soc. 2009, 131, 11249–11255. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Regium-π bonds: An Unexplored Link between Noble Metal Nanoparticles and Aromatic Surfaces. Chem. Eur. J. 2018, 24, 7228–7234. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.Z.; Li, R.; Li, W.Z.; Cheng, J.B. Prediction and characterization of HCCH⋯AuX (X = OH, F, Cl, Br, CH3, CCH, CN, and NC) complexes: A π Au-bond. J. Chem. Phys. 2011, 135, 074304. [Google Scholar] [CrossRef]
- Piña, M.L.N.; Frontera, A.; Bauzá, A. Regium-π Bonds Are Involved in Protein-Gold Binding. J. Phys. Chem. Lett. 2020, 11, 8259–8263. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X-C⋯π (X = F, Cl, Br, CN) carbon bond. J. Phys. Chem. A 2014, 118, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel Bonds with π-Electrons Acting as Lewis Bases-Theoretical Results and Experimental Evidences. Molecules 2018, 23, 1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Suryaprasad, B.; Ramanathan, N.; Sundararajan, K. Dominance of unique P⋯π phosphorus bonding with π donors: Evidence using matrix isolation infrared spectroscopy and computational methodology. Phys. Chem. Chem. Phys. 2020, 22, 20771–20791. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, P.-P.; Wang, Y. Chalcogen⋯π Bonding Catalysis. Angew. Chem. Int. Ed. 2021, 60, 9395–9400. [Google Scholar] [CrossRef]
- Li, R.Y.; Li, Z.R.; Wu, D.; Li, Y.; Chen, W.; Sun, C.C. Study of π halogen bonds in complexes C2H4-nFn-ClF (n = 0–2). J. Phys. Chem. A 2005, 109, 2608–2613. [Google Scholar] [CrossRef]
- Dougherty, D.A. The cation-π interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S. Cation-π Interactions in Organic Synthesis. Chem. Rev. 2018, 118, 11353–11432. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Wang, S.R.; Arrowsmith, M.; Braunschweig, H.; Dewhurst, R.D.; Domling, M.; Mattock, J.D.; Pranckevicius, C.; Vargas, A. Monomeric 16-Electron π-Diborene Complexes of Zn(II) and Cd(II). J. Am. Chem. Soc. 2017, 139, 10661–10664. [Google Scholar] [CrossRef] [Green Version]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Lopes, K.C.; Pereira, F.S.; Ramos, M.N.; de Araúijo, R.C.M.U. An ab-initio study of the C3H6-HX, C2H4-HX and C2H2-HX hydrogen-bonded complexes with X = F or Cl. Spectrochim. Acta A 2001, 57, 1339–1346. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ghosh, S.; Wategaonkar, S. O-H stretching frequency red shifts do not correlate with the dissociation energies in the dimethylether and dimethylsulfide complexes of phenol derivatives. Phys. Chem. Chem. Phys. 2021, 23, 5718–5739. [Google Scholar] [CrossRef] [PubMed]
- Biswal, H.S.; Bhattacharyya, S.; Wategaonkar, S. Molecular-level understanding of ground- and excited-state O-H⋯O hydrogen bonding involving the tyrosine side chain: A combined high-resolution laser spectroscopy and quantum chemistry study. ChemPhysChem 2013, 14, 4165–4176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.L.; Zhang, X.Y.; Li, X.Y.; Zheng, S.J.; Meng, L.P. Ab initio and AIM studies on typical π-type and pseudo-π-type halogen bonds: Comparison with hydrogen bonds. Int. J. Quantum Chem. 2011, 111, 3725–3740. [Google Scholar] [CrossRef]
- Halldin Stenlid, J.; Johansson, A.J.; Brinck, T. σ-Holes and σ-lumps direct the Lewis basic and acidic interactions of noble metal nanoparticles: Introducing regium bonds. Phys. Chem. Chem. Phys. 2018, 20, 2676–2692. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1990, 19, 553–566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Su, P.F.; Li, H. Energy decomposition analysis of covalent bonds and intermolecular interactions. J. Chem. Phys. 2009, 131, 014102. [Google Scholar] [CrossRef] [Green Version]
- Barca, G.M.J.; Bertoni, C.; Carrington, L.; Datta, D.; De Silva, N.; Deustua, J.E.; Fedorov, D.G.; Gour, J.R.; Gunina, A.O.; Guidez, E.; et al. Recent developments in the general atomic and molecular electronic structure system. J. Chem. Phys. 2020, 152, 154102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Lu, T.; Chen, Q.X. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo [18]carbon: Focusing on molecular adsorption and stacking. Carbon 2021, 171, 514–523. [Google Scholar] [CrossRef]
- Li, Z.D.; Liu, Y.L.; Li, X.M.; Li, Q.Z.; Li, X.Y. Theoretical investigation of the nature of π(B≡B)⋯M interactions in coinage metal π-diborene complexes. New J. Chem. 2021, 45, 13380–13388. [Google Scholar] [CrossRef]
- Joy, J.; Jemmis, E.D. Contrasting Behavior of the Z Bonds in X–Z⋯Y Weak Interactions: Z = Main Group Elements Versus the Transition Metals. Inorg. Chem. 2017, 56, 1132–1143. [Google Scholar] [CrossRef]
- Joy, J.; Jemmis, E.D. Designing M-bond (X–M⋯Y, M = transition metal): σ-hole and radial density distribution. J. Chem. Sci. 2019, 131, 117. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Frenking, G. Transition-Metal Chemistry of the Heavier Alkaline Earth Atoms Ca, Sr, and Ba. Acc. Chem. Res. 2021, 54, 3071–3082. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Ford, G.P. Relationship between olefinic π complexes and three-membered rings. J. Am. Chem. Soc. 1979, 101, 783–791. [Google Scholar] [CrossRef]
- Rozas, I.; Alkorta, I.; Elguero, J. Behavior of Ylides Containing N, O, and C Atoms as Hydrogen Bond Acceptors. J. Am. Chem. Soc. 2000, 122, 11154–11161. [Google Scholar] [CrossRef]
- Koch, U.; Popelie, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Arnold, W.D.; Oldfield, E. The Chemical Nature of Hydrogen Bonding in Proteins via NMR: J-Couplings, Chemical Shifts, and AIM Theory. J. Am. Chem. Soc. 2000, 122, 12835–12841. [Google Scholar] [CrossRef]
- Llusar, R.; Beltrán, A.; Andrés, J.; Noury, S.; Silvi, B. Topological Analysis of Electron Density in Depleted Homopolar Chemical Bonds. J. Comput. Chem. 1999, 20, 1517–1526. [Google Scholar] [CrossRef]
- Bianchi, R.; Gervasio, G.; Marabello, D. Experimental electron density analysis of Mn2(CO)10: Metal-metal and metal-ligand bond characterization. Inorg. Chem. 2000, 39, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- An, X.L.; Li, R.; Li, Q.Z.; Liu, X.F.; Li, W.Z.; Cheng, J.B. Substitution, cooperative, and solvent effects on π pnicogen bonds in the FH2P and FH2As complexes. J. Mol. Model. 2012, 18, 4325–4332. [Google Scholar] [CrossRef]
- Nziko Vde, P.; Scheiner, S. S⋯π Chalcogen Bonds between SF2 or SF4 and C-C Multiple Bonds. J. Phys. Chem. A 2015, 119, 5889–5897. [Google Scholar] [CrossRef]
- Zheng, B.S.; Liu, Y.; Wang, Z.X.; Zhou, F.X.; Jiao, Y.C.; Liu, Y.; Ding, X.L.; Li, Q.Z. Comparison of halide donators based on pi⋯M (M = Cu, Ag, Au), pi⋯H and pi⋯halogen bonds. Theor. Chem. Acc. 2018, 137, 179. [Google Scholar] [CrossRef]
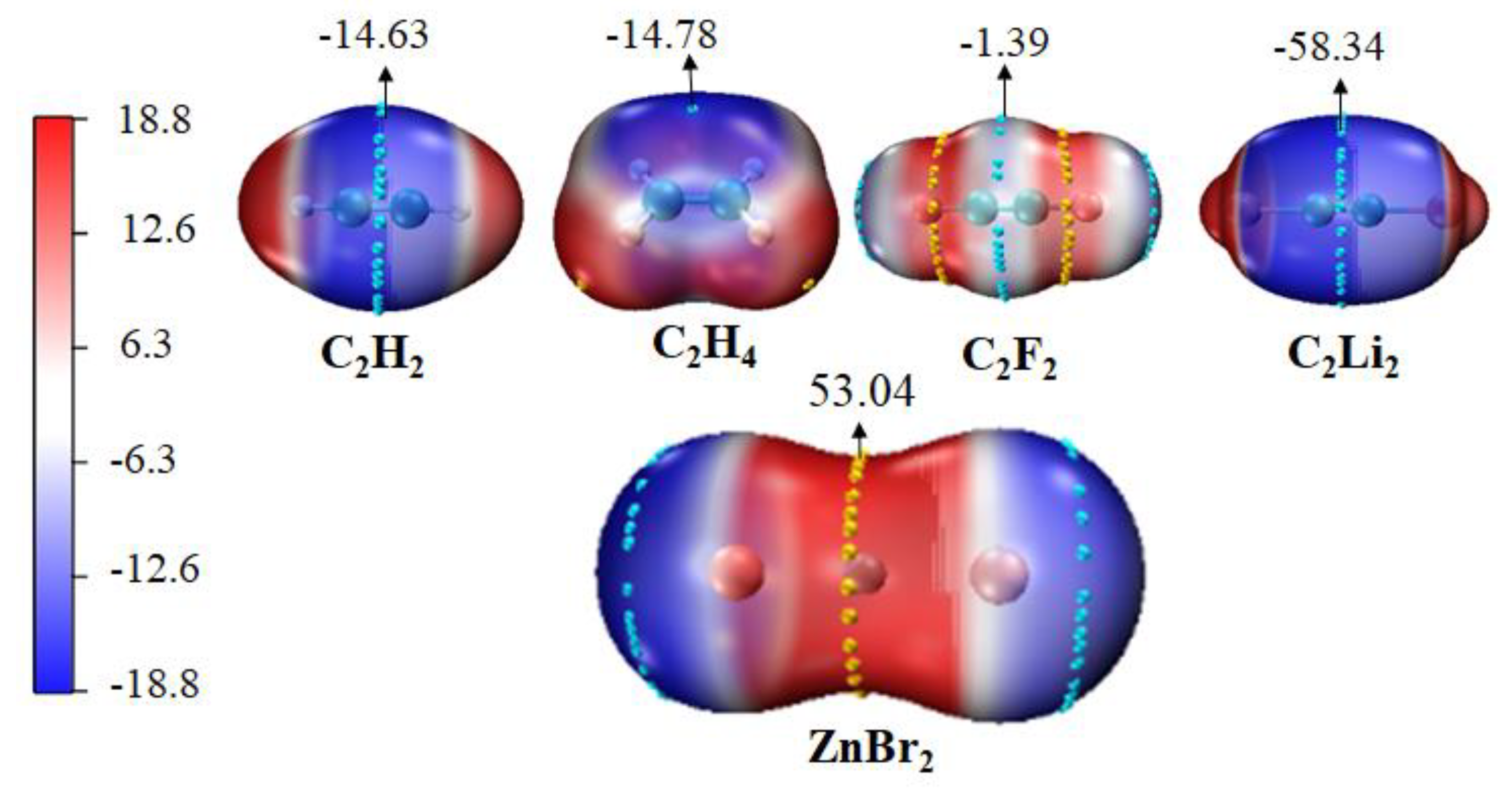

| Monomers | Vmax | Monomers | Vmin |
|---|---|---|---|
| ZnCl2 | 59.24 | C2H2 | −14.63 |
| ZnBr2 | 53.04 | C2H4 | −14.78 |
| ZnI2 | 45.56 | C2F2 | −1.39 |
| CdCl2 | 63.29 | C2Li2 | −58.34 |
| CdBr2 | 58.61 | ||
| CdI2 | 51.82 | ||
| HgCl2 | 44.54 | ||
| HgBr2 | 41.46 | ||
| HgI2 | 37.45 |
| Complexes | RM⋯* | RM–X | RC–C | α | θ | ΔE |
|---|---|---|---|---|---|---|
| ZnBr2⋯C2H2–P | 2.387 | 2.222 | 1.219 | 149 | 0 | −9.72 |
| CdBr2⋯C2H2–P | 2.670 | 2.401 | 1.218 | 161 | 0 | −8.26 |
| HgBr2⋯C2H2–P | 3.078 | 2.363 | 1.215 | 175 | 0 | −2.96 |
| ZnBr2⋯C2H2–T | 2.538 | 2.204 | 1.216 | 158 | 83 | −5.29 |
| CdBr2⋯C2H2–T | 2.770 | 2.389 | 1.216 | 165 | 83 | −5.24 |
| HgBr2⋯C2H2–T | 3.083 | 2.359 | 1.214 | 176 | 84 | −1.78 |
| ZnBr2⋯C2H4–P | 2.367 | 2.225 | 1.347 | 146 | 0 | −10.89 |
| CdBr2⋯C2H4–P | 2.635 | 2.406 | 1.344 | 157 | 0 | −8.58 |
| HgBr2⋯C2H4–P | 3.051 | 2.364 | 1.338 | 174 | 0 | −2.12 |
| ZnBr2⋯C2H4–T | 2.400 | 2.216 | 1.346 | 153 | 82 | −9.51 |
| CdBr2⋯C2H4–T | 2.640 | 2.401 | 1.344 | 163 | 82 | −8.17 |
| HgBr2⋯C2H4–T | 2.924 | 2.365 | 1.339 | 175 | 82 | −2.30 |
| ZnBr2⋯C2F2–P | 2.886 | 2.184 | 1.199 | 171 | 42 | −1.26 |
| CdBr2⋯C2F2–P | 2.976 | 2.376 | 1.199 | 174 | 40 | −2.39 |
| HgBr2⋯C2F2–P | 3.152 | 2.355 | 1.197 | 179 | 46 | −1.03 |
| ZnBr2⋯C2Li2–P | 1.968 | 2.348 | 1.276 | 119 | 0 | −99.94 |
| CdBr2⋯C2Li2–P | 2.172 | 2.534 | 1.279 | 132 | 0 | −89.97 |
| HgBr2⋯C2Li2–P | 2.152 | 2.543 | 1.289 | 126 | 0 | −87.51 |
| Complexes | ρ | ▽2ρ | H |
|---|---|---|---|
| ZnBr2⋯C2H2–P | 0.0398 | 0.1023 | −0.0076 |
| CdBr2⋯C2H2–P | 0.0292 | 0.0863 | −0.0018 |
| HgBr2⋯C2H2–P | 0.0160 | 0.0480 | 0.0009 |
| ZnBr2⋯C2H2–T | 0.0297 | 0.0754 | −0.0037 |
| CdBr2⋯C2H2–T | 0.0242 | 0.0708 | −0.0006 |
| HgBr2⋯C2H2–T | 0.0157 | 0.0468 | 0.0009 |
| ZnBr2⋯C2H4–P | 0.0421 | 0.0893 | −0.0091 |
| CdBr2⋯C2H4–P | 0.0319 | 0.0817 | −0.0030 |
| HgBr2⋯C2H4–P | 0.0175 | 0.0463 | 0.0005 |
| ZnBr2⋯C2H4–T | 0.0393 | 0.0843 | −0.0078 |
| CdBr2⋯C2H4–T | 0.0315 | 0.0807 | −0.0028 |
| HgBr2⋯C2H4–T | 0.0220 | 0.0570 | −0.0003 |
| ZnBr2⋯C2F2–P | 0.0158 | 0.0412 | 0.0001 |
| CdBr2⋯C2F2–P | 0.0168 | 0.0486 | 0.0005 |
| HgBr2⋯C2F2–P | 0.0140 | 0.0423 | 0.0010 |
| ZnBr2⋯C2Li2–P | 0.0878 | 0.2251 | −0.0309 |
| CdBr2⋯C2Li2–P | 0.0738 | 0.2095 | −0.0189 |
| HgBr2⋯C2Li2–P | 0.0869 | 0.2212 | −0.0255 |
| Complexes | ES | EX | REP | POL | DISP |
|---|---|---|---|---|---|
| ZnBr2⋯C2H2–P | −31.95 | −51.86 | 96.03 | −18.31 | −5.66 |
| CdBr2⋯C2H2–P | −23.41 | −38.14 | 69.28 | −11.88 | −5.65 |
| HgBr2⋯C2H2–P | −10.75 | −20.25 | 35.06 | −3.99 | −4.70 |
| ZnBr2⋯C2H2–T | −18.09 | −34.58 | 62.46 | −11.21 | −5.46 |
| CdBr2⋯C2H2–T | −14.02 | −27.28 | 48.73 | −8.27 | −5.60 |
| HgBr2⋯C2H2–T | −7.14 | −17.03 | 29.18 | −3.34 | −5.11 |
| ZnBr2⋯C2F2–P | −7.24 | −18.32 | 32.41 | −4.09 | −5.88 |
| CdBr2⋯C2F2–P | −6.89 | −17.67 | 31.40 | −4.21 | −6.34 |
| HgBr2⋯C2F2–P | −4.47 | −13.30 | 23.02 | −2.17 | −6.04 |
| ZnBr2⋯C2Li2–P | −171.59 | −175.81 | 340.82 | −86.87 | −10.41 |
| CdBr2⋯C2Li2–P | −157.82 | −166.35 | 322.57 | −81.82 | −11.45 |
| HgBr2⋯C2Li2–P | −169.45 | −211.53 | 410.98 | −118.60 | −10.72 |
| ZnBr2⋯C2H4–P | −33.38 | −57.61 | 106.78 | −20.80 | −7.76 |
| CdBr2⋯C2H4–P | −24.55 | −43.50 | 79.30 | −14.31 | −7.45 |
| HgBr2⋯C2H4–P | −11.32 | −24.16 | 41.93 | −4.88 | −6.11 |
| ZnBr2⋯C2H4–T | −29.13 | −49.86 | 92.49 | −17.69 | −7.18 |
| CdBr2⋯C2H4–T | −22.86 | −40.02 | 73.28 | −13.14 | −7.27 |
| HgBr2⋯C2H4–T | −13.64 | −28.38 | 50.07 | −6.44 | −6.75 |
| Complexes | R | ΔE | Method | Types | Reference |
|---|---|---|---|---|---|
| FH⋯C2H2 | 0.931 | −2.88 | MP2/aug-cc-pVTZ | π–hydrogen | [30] |
| FH⋯C2H4 | 0.932 | −2.87 | ΔEint(BSSE) | ||
| FLi⋯C2H2 | 2.356 | −7.73 | MP2/6-311++G(d,p) | π–lithium | [33] |
| FLi⋯C2H4 | 2.325 | −7.72 | ΔEint(BSSE) | ||
| FNa⋯C2H2 | 2.760 | −5.20 | MP2/6-311++G(d,p) | π–sodium | [35] |
| FNa⋯C2H4 | 2.808 | −5.23 | ΔEint(BSSE) | ||
| F2Be⋯C2H2 | - | 14.11 | CCSD(T)/aug-cc-pVTZ//MP2/aug-cc-pVTZ | π–beryllium | [34] |
| F2Be⋯C2H4 | - | 13.16 | ΔECCSD(T) | ||
| F2Mg⋯C2H2 | 2.460 | −15.00 | MP2/aug-cc-pVTZ | π–magnesium | [36] |
| F2Mg⋯C2H4 | 2.523 | −13.16 | ΔEint(BSSE) | ||
| F3Al⋯C2H2 | 2.437 | −18.7 | MP2/aug-cc-pVTZ | π–triel | [41] |
| F3Al⋯C2H4 | 2.467 | −20.1 | ΔEint(BSSE) | ||
| FH3Ge⋯C2H2 | 3.299 | −2.80 | MP2/aug-cc-pVTZ | π–tetrel | [42] |
| FH3Ge⋯C2H4 | 3.269 | −2.53 | ΔEbind(BSSE) | ||
| FH2As⋯C2H2 | 3.013 | −4.03 | MP2/aug-cc-pVTZ | π–pnictogen | [81] |
| FH2As⋯C2H4 | 2.907 | −4.60 | ΔEint(BSSE) | ||
| F2S⋯C2H2 | 2.988 | 3.79 | MP2/aug-cc-pVTZ | π–chalcogen | [82] |
| F2S⋯C2H4 | 2.904 | 4.47 | ΔEbind(BSSE) | ||
| FBr⋯C2H2 | 2.813 | −4.90 | CCSD(T)/aug-cc-pVTZ//ωB97XD/aug-cc-pVTZ | π–halogen | [83] |
| FBr⋯C2H4 | 2.681 | −6.69 | ΔECCSD(T) | ||
| FAu⋯C2H2 | 2.008 | −54.30 | CCSD(T)/aug-cc-pVTZ//ωB97XD/aug-cc-pVTZ | regium–π | [83] |
| FAu⋯C2H4 | 2.017 | −58.79 | ΔECCSD(T) | ||
| F2OXe⋯C2H2 | 3.073 | −6.6 | MP2/aug-cc-pVTZ | aerogen–π | [24] |
| F2OXe⋯C2H4 | 3.020 | −6.2 | ΔEint(BSSE) | ||
| Cl2Zn⋯C2H2–P | 2.406 | −11.60 | MP2/aug-cc-pVTZ | spodium–π | Our results |
| Cl2Zn⋯C2H4–P | 2.391 | −11.68 | ΔEint(BSSE) |
| Lewis Acid | Lewis Base | R | ΔE | Reference |
|---|---|---|---|---|
| ZnBr2L2 (L = thiourea) | CO | 3.79 | −2.2 a | [1] |
| CdCl2L2 (L = thiourea) | H2CS | 2.95 | −8.9 a | |
| CdCO3 | NCH | 2.146 | −31.84 b | [20] |
| HgCO3 | NHCH2 | 2.047 | −56.68 b | |
| HgCl3− | HgCl3− | 3.616 | −1.88 c | [18] |
| ZnMe2 | cyclopropenylidene | 2.192 | 10.3 d | [15] |
| ZnF2 | (NH3)2C | 1.879 | 78.8 d | |
| HgI2 | C2F2 | 3.177 | −0.79 | Our results |
| ZnCl2 | C2Li2 | 1.965 | −106.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zhao, Q.; Yu, H.; Fu, M.; Li, Q. Insight into Spodium–π Bonding Characteristics of the MX2⋯π (M = Zn, Cd and Hg; X = Cl, Br and I) Complexes—A Theoretical Study. Molecules 2022, 27, 2885. https://doi.org/10.3390/molecules27092885
Gao M, Zhao Q, Yu H, Fu M, Li Q. Insight into Spodium–π Bonding Characteristics of the MX2⋯π (M = Zn, Cd and Hg; X = Cl, Br and I) Complexes—A Theoretical Study. Molecules. 2022; 27(9):2885. https://doi.org/10.3390/molecules27092885
Chicago/Turabian StyleGao, Meng, Qibo Zhao, Hao Yu, Min Fu, and Qingzhong Li. 2022. "Insight into Spodium–π Bonding Characteristics of the MX2⋯π (M = Zn, Cd and Hg; X = Cl, Br and I) Complexes—A Theoretical Study" Molecules 27, no. 9: 2885. https://doi.org/10.3390/molecules27092885
APA StyleGao, M., Zhao, Q., Yu, H., Fu, M., & Li, Q. (2022). Insight into Spodium–π Bonding Characteristics of the MX2⋯π (M = Zn, Cd and Hg; X = Cl, Br and I) Complexes—A Theoretical Study. Molecules, 27(9), 2885. https://doi.org/10.3390/molecules27092885






