Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
2.1.1. The Effect of Ethanol Concentration
2.1.2. The Effect of Solvent to Sample Ratio
2.1.3. The Effect of Extraction Temperature
2.1.4. The Effect of Ultrasonic Power
2.1.5. The Effect of Extraction Time
2.2. Optimization of UAE of Total Flavonoids from Hemerocallis fulva Leaves
2.2.1. Effect of Extraction Variables on Total Flavonoids
2.2.2. Response Surface Analysis
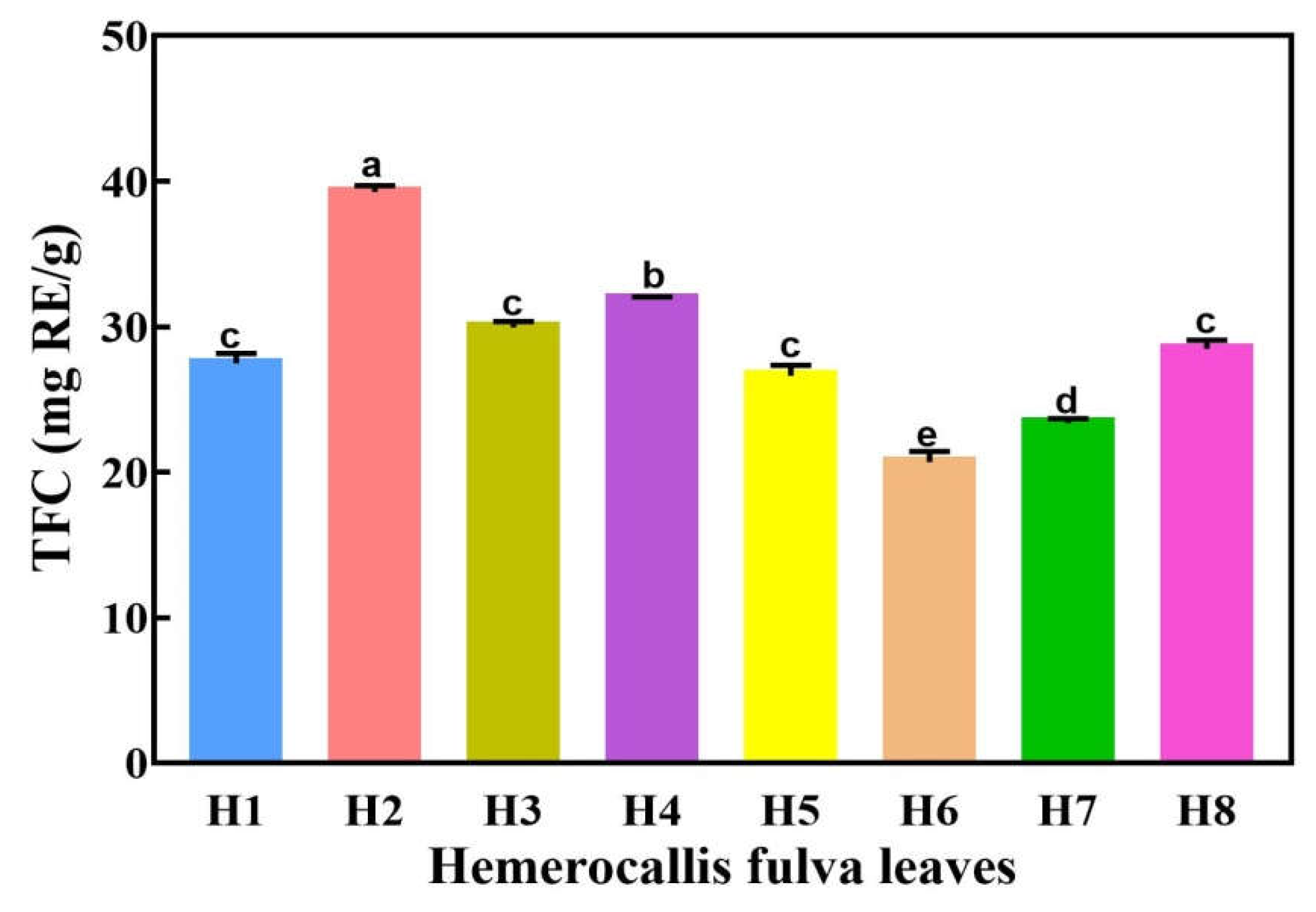
2.3. The TFC in Eight Hemerocallis fulva Varieties
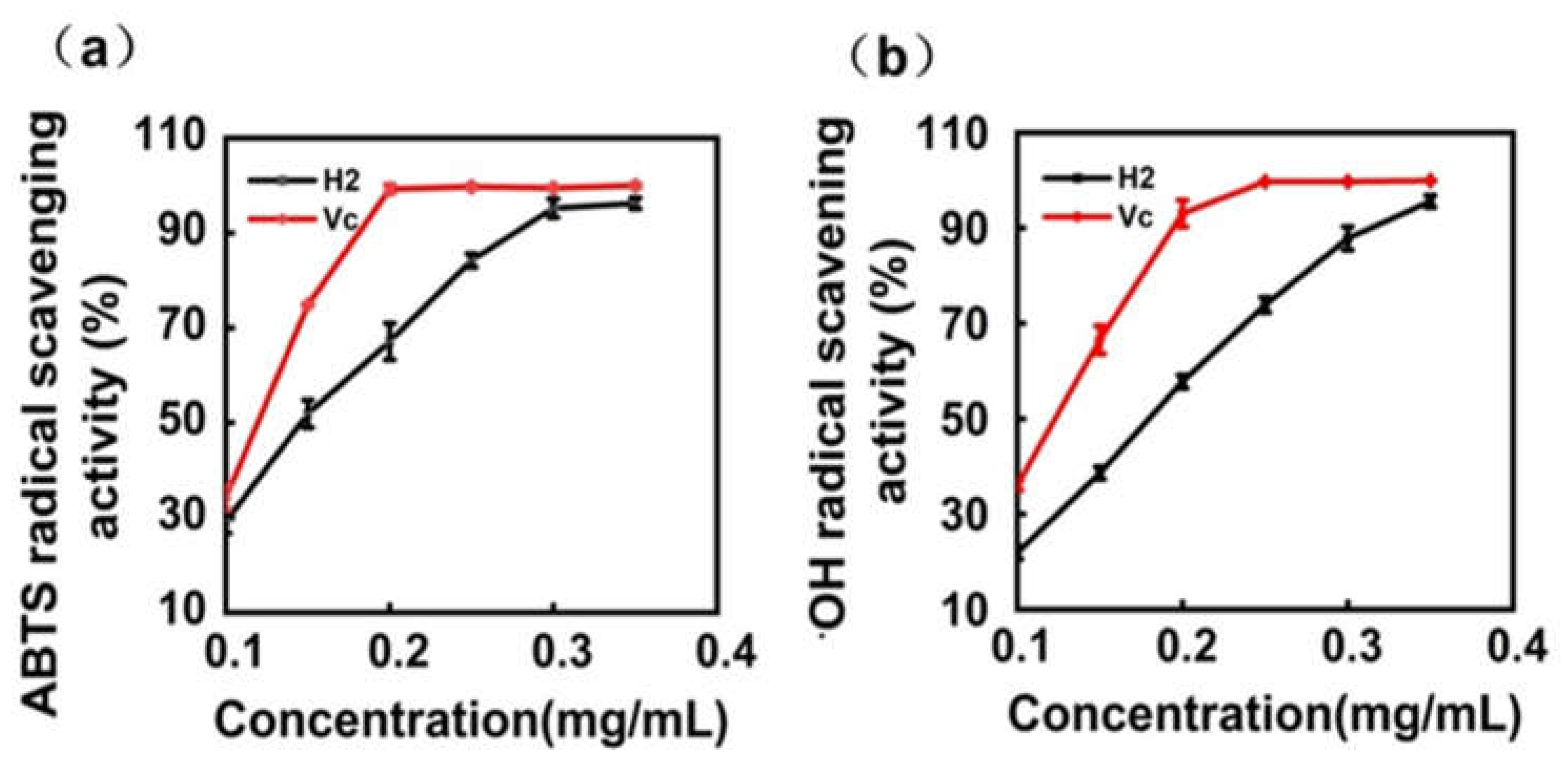
2.4. In Vitro Antioxidant Activity of Total Flavonoid Extracts from H2
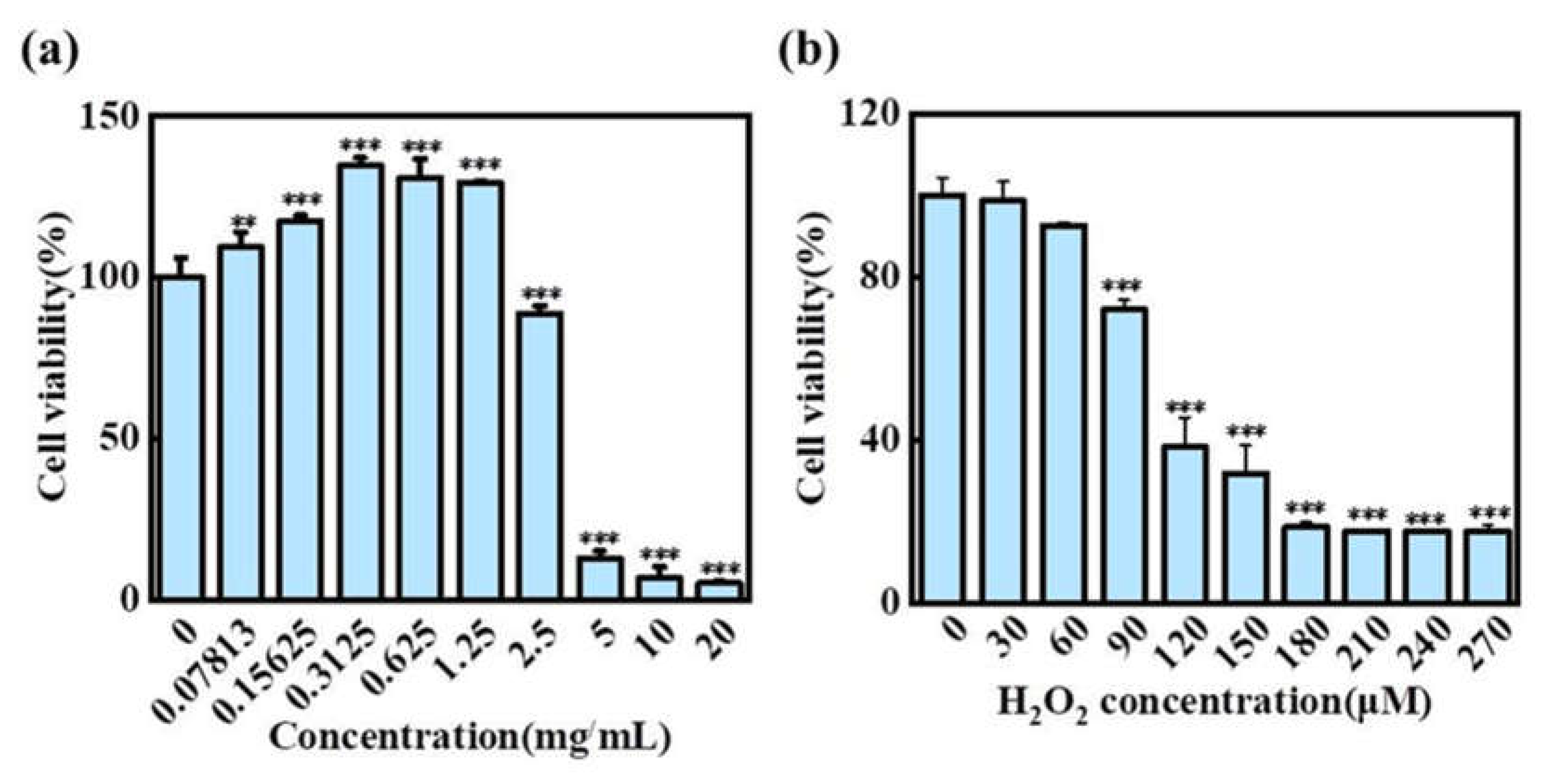
2.5. Cellular Antioxidant Activity of Total Flavonoid Extracts from H2
2.5.1. Effects of H2 and H2O2 on the Activity of HaCaT Cells
2.5.2. Effect of H2 on ROS Level in HaCaT Cells Treated with H2O2
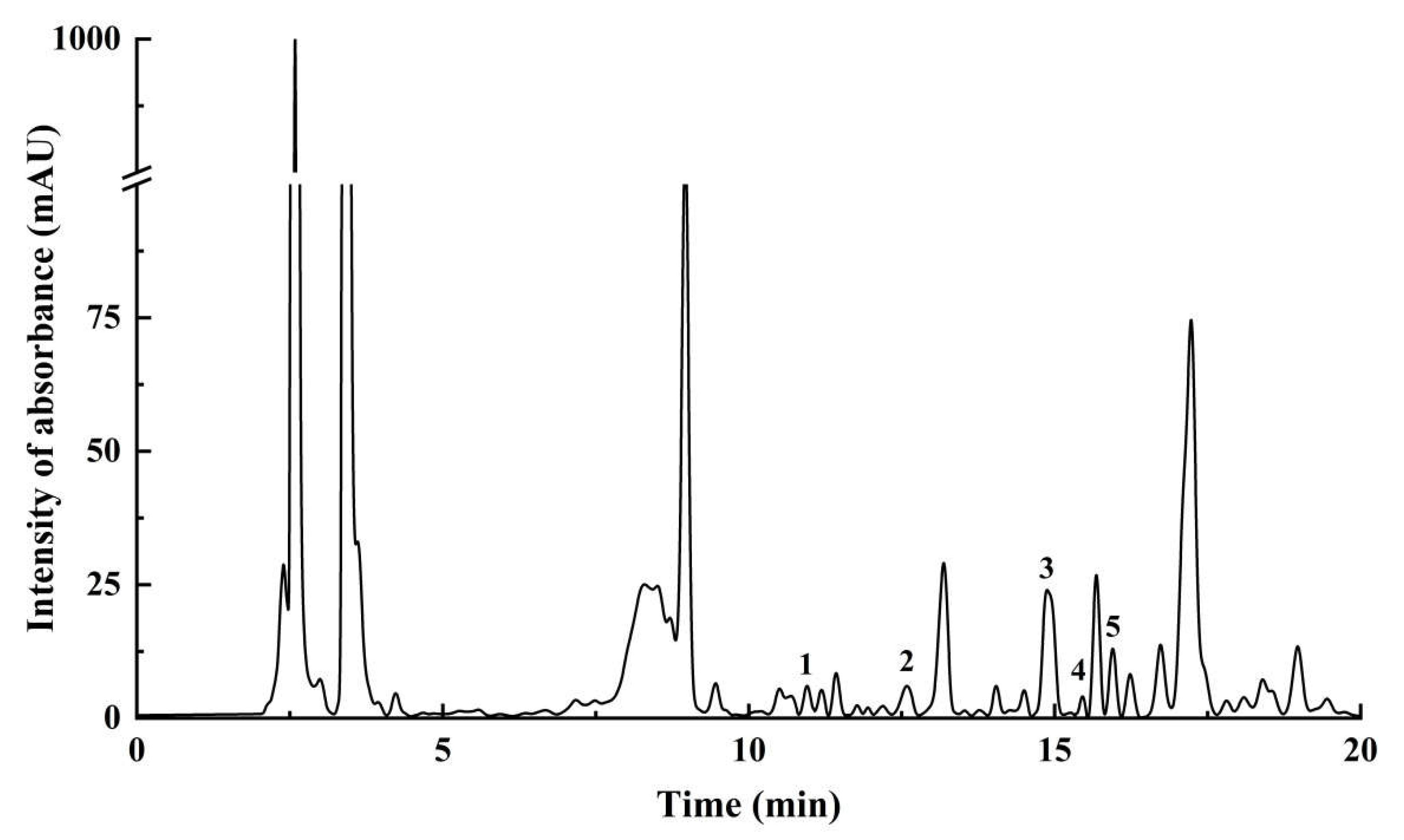
2.6. HPLC Analysis
2.7. Infrared Spectroscopy Analysis
3. Materials and Methods
3.1. Materials
3.2. Chemicals and Reagents
3.3. Ultrasound-Assisted Extraction (UAE)
3.4. Determination of Total Flavonoid Content (TFC)
3.5. Selection of Variables
3.6. RSM Experiment Design
3.7. Antioxidant Assays
3.7.1. Determination of ABTS Radical Scavenging Activity
3.7.2. Determination of Hydroxyl (•OH) Radical Scavenging Activity
3.8. Cellular Antioxidant Assay
3.8.1. Cell Culture
3.8.2. Effects of H2 and H2O2 on the Activity of HaCaT Cells
3.8.3. Effects of H2 on the ROS Level in HaCaT Cells Treated with H2O2
3.9. HPLC Analysis
3.9.1. Preparation of Standard Solution
3.9.2. HPLC Conditions
3.10. Infrared Spectroscopy
3.11. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Gao, Y.; Yuan, L.; Zhang, Q. Molecular Characterization and Expression Patterns of the HkSVP Gene Reveal Distinct Roles in Inflorescence Structure and Floral Organ Development in Hemerocallis fulva. Int. J. Mol. Sci. 2021, 22, 12010. [Google Scholar] [CrossRef]
- Wu, W.-T.; Mong, M.-C.; Yang, Y.-C.; Wang, Z.-H.; Yin, M.-C. Aqueous and Ethanol Extracts of Daylily Flower (Hemerocallis fulva L.) Protect HUVE Cells Against High Glucose. J. Food Sci. 2018, 83, 1463–1469. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, N.; Huang, J.; Guo, A.; Kang, X.; Li, S.; Xing, G. Dynamic changes of pectin epitopes and daylily tepals during flower opening and senescence of Hemerocallis citrina. Sci. Hortic. 2021, 288, 110367. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Sun, J.; Li, G.; Shan, Y.; Chen, P. Study the effects of drying processes on chemical compositions in daylily flowers using flow injection mass spectrometric fingerprinting method and chemometrics. Food Res. Int. 2017, 102, 493–503. [Google Scholar] [CrossRef]
- Matraszek-Gawron, R.; Chwil, M.; Terlecka, P.; Skoczylas, M.M. Recent Studies on Anti-Depressant Bioactive Substances in Selected Species from the Genera Hemerocallis and Gladiolus: A Systematic Review. Pharmaceuticals 2019, 12, 172. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.-M.; Malathy, R.; Kim, S.-H.; Kalaiselvi, K.; Prabakaran, M.; Gopiraman, M. Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J. Adhes. Sci. Technol. 2020, 34, 1483–1506. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Zhang, M.; Geng, P.; Shan, Y.; Li, G.; Zhao, Y.; Chen, P. The analysis of phenolic compounds in daylily using UHPLC-HRMSn and evaluation of drying processing method by fingerprinting and metabolomic approaches. J. Food Process. Preserv. 2018, 42, e13325. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Mladenovic Djordjevic, A.; Loncarevic-Vasiljkovic, N.; Gonos, E.S. Dietary Restriction and Oxidative Stress: Friends or Enemies? Antioxid. Redox Signal. 2021, 34, 421–438. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Cruz, R.G.D.; Beney, L.; Gervais, P.; Lira, S.P.; Vieira, T.; Dupont, S. Comparison of the antioxidant property of acerola extracts with synthetic antioxidants using an in vivo method with yeasts. Food Chem. 2019, 277, 698–705. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Validation of a High-Performance Liquid Chromatography with Photodiode Array Detection Method for the Separation and Quantification of Antioxidant and Skin Anti-Aging Flavonoids from Nelumbo nucifera Gaertn. Stamen Extract. Molecules 2022, 27, 1102. [Google Scholar] [CrossRef]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-Epoxide-Loaded SLNs: Evaluation of Drug Release, Antioxidant Activity, and Cytotoxicity in an HaCaT Cell Line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef] [Green Version]
- Kósa, D.; Pető, Á.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Gonda, S.; Vasas, G.; Fehér, P.; Bácskay, I.; Ujhelyi, Z. Formulation of novel liquid crystal (Lc) formulations with skin-permeation-enhancing abilities of plantago lanceolata (pl) extract and their assessment on hacat cells. Molecules 2021, 26, 1023. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; Garcia, A.; Munoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Pollini, L.; Blasi, F.; Ianni, F.; Grispoldi, L.; Moretti, S.; Di Veroli, A.; Cossignani, L.; Cenci-Goga, B.T. Ultrasound-Assisted Extraction and Characterization of Polyphenols from Apple Pomace, Functional Ingredients for Beef Burger Fortification. Molecules 2022, 27, 1933. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous Optimization for Ultrasound-Assisted Extraction and Antioxidant Activity of Flavonoids from Sophora flavescens Using Response Surface Methodology. Molecules 2019, 24, 112. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Drouet, S.; Kabra, A.; Hano, C. Enrichment in Antioxidant Flavonoids of Stamen Extracts from Nymphaea lotus L. Using Ultrasonic-Assisted Extraction and Macroporous Resin Adsorption. Antioxidants 2020, 9, 576. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of Thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, C.; Yu, Y.-B.; Wu, L.-X.; Chen, T.-T.; Wang, Z.-W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, M.; Weng, H.; Xu, Y.; Zeng, L. Optimization of ultrasound assisted extraction (UAE) of Kinsenoside compound from Anoectochilus roxburghii (Wall.) Lindl by response surface methodology (RSM). Molecules 2020, 25, 193. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Gao, M.; Kong, W.; Yang, B.; Kuang, H.; Yang, B.; Fu, Y.; Cheng, Y.; Li, H. Enhanced and sustainable pretreatment for bioconversion and extraction of resveratrol from peanut skin using ultrasound-assisted surfactant aqueous system with microbial consortia immobilized on cellulose. 3 Biotech 2020, 10, 293. [Google Scholar] [CrossRef]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of Ionic Liquid-Assisted Extraction of Biflavonoids from Selaginella doederleinii and Evaluation of Its Antioxidant and Antitumor Activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef] [Green Version]
- Nuerxiati, R.; Abuduwaili, A.; Mutailifu, P.; Wubulikasimu, A.; Rustamova, N.; Jingxue, C.; Aisa, H.A.; Yili, A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int. J. Biol. Macromol. 2019, 141, 431–443. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.A.; Chen, L.; Fakayode, O.A.; Zhou, C. Synergism of sweeping frequency ultrasound and deep eutectic solvents pretreatment for fractionation of sugarcane bagasse and enhancing enzymatic hydrolysis. Ultrason. Sonochem 2021, 73, 105470. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of extraction conditions on phenolic content, anthocyanin content and antioxidant activity of bran extracts from Thai rice cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Kamairudin, N.; Hoong, S.S.; Abdullah, L.C.; Ariffin, H.; Biak, D.R.A. Optimisation of Epoxide Ring-Opening Reaction for the Synthesis of Bio-Polyol from Palm Oil Derivative Using Response Surface Methodology. Molecules 2021, 26, 648. [Google Scholar] [CrossRef]
- Prabhu, D.; Prabhu, P.R.; Rao, P. Thermodynamics, adsorption, and response surface methodology investigation of the corrosion inhibition of aluminum by Terminalia chebula Ritz. extract in H3PO4. Chem. Pap. 2020, 75, 653–667. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Shah, M.; Jan, H.; Drouet, S.; Tungmunnithum, D.; Shirazi, J.H.; Hano, C.; Abbasi, B.H. Chitosan elicitation impacts flavonolignan biosynthesis in Silybum marianum (L.) Gaertn cell suspension and enhances antioxidant and anti-inflammatory activities of cell extracts. Molecules 2021, 26, 791. [Google Scholar] [CrossRef]
- El Hallal, R.; Lyu, N.; Wang, Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules 2021, 26, 567. [Google Scholar] [CrossRef]
- Suraiya, S.; Lee, J.M.; Cho, H.J.; Jang, W.J.; Kim, D.-G.; Kim, Y.-O.; Kong, I.-S. Monascus spp. fermented brown seaweeds extracts enhance bio-functional activities. Food Biosci. 2018, 21, 90–99. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Mao, X. Chitopentaose protects HaCaT cells against H2O2-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. J. Funct. Foods 2020, 72, 104086. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; An, K.; Xiao, G. Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017, 230, 649–656. [Google Scholar] [CrossRef]
- Szewczyk, K.; Miazga-Karska, M.; Pietrzak, W.; Komsta, L.; Krzeminska, B.; Grzywa-Celinska, A. Phenolic Composition and Skin-Related Properties of the Aerial Parts Extract of Different Hemerocallis Cultivars. Antioxidants 2020, 9, 690. [Google Scholar] [CrossRef]
- Zhang, Y.; Cichewicz, R.H.; Nair, M.G. Lipid peroxidation inhibitory compounds from daylily (Hemerocallis fulva) leaves. Life Sci. 2004, 75, 753–763. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Ahmed, T.; Wu, Z.; Jiang, H.; Luo, J.; Noman, M.; Shahid, M.; Manzoor, I.; Allemailem, K.S.; Alrumaihi, F.; Li, B. Bioinspired Green Synthesis of Zinc Oxide Nanoparticles from a Native Bacillus cereus Strain RNT6: Characterization and Antibacterial Activity against Rice Panicle Blight Pathogens Burkholderia glumae and B. gladioli. Nanomaterials 2021, 11, 884. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Z.; Dong, Y.; Lu, W.; Zhu, D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels 2022, 8, 146. [Google Scholar] [CrossRef]
- Arik, N.; Horzum, N.; Truong, Y.B. Development and Characterizations of Engineered Electrospun Bio-Based Polyurethane Containing Essential Oils. Membranes 2022, 12, 209. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Al Wadaani, F. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO(4)) Nanoparticles. Molecules 2018, 23, 273. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Gao, Y.T.; Wei, J.W.; Chen, Y.F.; Liu, Q.L.; Liu, H.M. Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules 2021, 26, 2494. [Google Scholar] [CrossRef]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.S.; Tae, H.S. In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma. Polymers 2021, 13, 418. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Nikkhah, A.A.; Zilouei, H.; Asadinezhad, A.; Keshavarz, A. Removal of oil from water using polyurethane foam modified with nanoclay. Chem. Eng. J. 2015, 262, 278–285. [Google Scholar] [CrossRef]
- Cao, Q.; Yan, J.; Sun, Z.; Gong, L.; Wu, H.; Tan, S.; Lei, Y.; Jiang, B.; Wang, Y. Simultaneous optimization of ultrasound-assisted extraction for total flavonoid content and antioxidant activity of the tender stem of Triarrhena lutarioriparia using response surface methodology. Food Sci. Biotechnol. 2021, 30, 37–45. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Zengin, G.; Jaradat, N.; Messaoudi, M. Teucrium polium (L.): Phytochemical Screening and Biological Activities at Different Phenological Stages. Molecules 2022, 27, 1561. [Google Scholar] [CrossRef]
- Ulmer, C.Z.; Jones, C.M.; Yost, R.A.; Garrett, T.J.; Bowden, J.A. Optimization of Folch, Bligh-Dyer, and Matyash sample-to-extraction solvent ratios for human plasma-based lipidomics studies. Anal. Chim. Acta 2018, 1037, 351–357. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martinez-Cifuentes, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.; Perez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carmenere Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef] [Green Version]
- Rosello-Soto, E.; Marti-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and pH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Ma, L.; Li, T.; Sun, D.; Hou, J.; Li, A.; Jiang, Z. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process Biochem. 2019, 81, 113–122. [Google Scholar] [CrossRef]
- Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Response Surface Methodology (RSM)-Based Optimization of Ultrasound-Assisted Extraction of Sennoside A, Sennoside B, Aloe-Emodin, Emodin, and Chrysophanol from Senna alexandrina (Aerial Parts): HPLC-UV and Antioxidant Analysis. Molecules 2022, 27, 298. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of Extraction Methods on Polyphenols, Flavonoids, Mineral Elements, and Biological Activities of Essential Oil and Extracts of Mentha pulegium L. Molecules 2021, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.; Shang, Y.; Chen, K. Radical Scavenging Activity of Puerarin: A Theoretical Study. Antioxidants 2019, 8, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.S.; Gao, S.; Chen, Y.; Bao, H.; Li, Z.T.; Yao, Q.P.; Liu, J.T.; Wang, Y.; Qi, Y.X. Platelet-derived microvesicles induce calcium oscillations and promote VSMC migration via TRPV4. Theranostics 2021, 11, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, S.; Wang, L.; Peng, S.; Tian, T.; Li, S.; Xiao, J.; Lin, Y. Tetrahedral framework nucleic acids act as antioxidants in acute kidney injury treatment. Chem. Eng. J. 2021, 413, 127426. [Google Scholar] [CrossRef]
- Kostka, T.; Ostberg-Potthoff, J.J.; Stärke, J.; Guigas, C.; Matsugo, S.; Mirčeski, V.; Stojanov, L.; Veličkovska, S.K.; Winterhalter, P.; Esatbeyoglu, T. Bioactive Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.): Extraction, Chemical Characterization, Fractionation and Cellular Antioxidant Activity. Antioxidants 2022, 11, 467. [Google Scholar] [CrossRef]
- Liu, H.-M.; Xu, P.-F.; Cheng, M.-Y.; Lei, S.-N.; Liu, Q.-L.; Wang, W. Optimization of Fermentation Process of Pomegranate Peel and Schisandra Chinensis and the Biological Activities of Fermentation Broth: Antioxidant Activity and Protective Effect Against H2O2-induced Oxidative Damage in HaCaT Cells. Molecules 2021, 26, 3432. [Google Scholar] [CrossRef]
- Zeng, J.; Guo, Y.; Han, Y.; Li, Z.; Yang, Z.; Chai, Q.; Wang, W.; Zhang, Y.; Fu, C. A Review of the Discriminant Analysis Methods for Food Quality Based on Near-Infrared Spectroscopy and Pattern Recognition. Molecules 2021, 26, 749. [Google Scholar] [CrossRef]





| Level | Factor | ||
|---|---|---|---|
| Ethanol Concentration (X1) (%) | Solvent to Sample Ratio (X2) (mL/g) | Extraction Temperature (X3) (°C) | |
| −1 | 60 | 35 | 50 |
| 0 | 70 | 40 | 60 |
| 1 | 80 | 45 | 70 |
| Runs | Ethanol Concentration (X1) (%) | Solvent to Sample Ratio (X2) (mL/g) | Extraction Temperature (X3) (°C) | TFC (mg RE/g) | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | −1 (60) | 0 (40) | 1 (70) | 21.12 | 21.33 |
| 2 | 0 (70) | −1 (35) | −1 (50) | 19.78 | 19.89 |
| 3 | −1 (60) | 0 (40) | −1 (50) | 21.29 | 21.25 |
| 4 | 0 (70) | 0 (40) | 0 (60) | 23.12 | 23.01 |
| 5 | 0 (70) | 0 (40) | 0 (60) | 23.05 | 23.01 |
| 6 | 1 (80) | −1 (35) | 0 (60) | 19.82 | 19.92 |
| 7 | 0 (70) | 1 (45) | −1 (50) | 22.56 | 22.71 |
| 8 | 1 (80) | 1 (45) | 0 (60) | 20.50 | 20.56 |
| 9 | 1 (80) | 0 (40) | 1 (70) | 20.88 | 20.93 |
| 10 | −1 (60) | −1 (35) | 0 (60) | 19.30 | 19.24 |
| 11 | 0 (70) | 1 (45) | 1 (70) | 22.37 | 22.27 |
| 12 | −1 (60) | 1 (45) | 0 (60) | 22.73 | 22.62 |
| 13 | 1 (80) | 0 (40) | −1 (50) | 20.47 | 20.26 |
| 14 | 0 (70) | 0 (40) | 0 (60) | 23.12 | 23.01 |
| 15 | 0 (70) | 0 (40) | 0 (60) | 22.98 | 23.01 |
| 16 | 0 (70) | −1 (35) | 1 (70) | 21.23 | 21.08 |
| 17 | 0 (70) | 0 (40) | 0 (60) | 22.79 | 23.01 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 27.81 | 9 | 3.09 | 82.43 | <0.0001 *** |
| X1 | 0.96 | 1 | 0.96 | 25.58 | 0.0015 ** |
| X2 | 8.08 | 1 | 8.08 | 215.43 | <0.0001 *** |
| X3 | 0.28 | 1 | 0.28 | 7.40 | 0.0297 * |
| X1X2 | 1.88 | 1 | 1.88 | 50.20 | 0.0002 *** |
| X1X3 | 0.086 | 1 | 0.086 | 2.30 | 0.1728 |
| X2X3 | 0.67 | 1 | 0.67 | 17.76 | 0.0040 ** |
| X12 | 9.29 | 1 | 9.29 | 247.81 | <0.0001 *** |
| X22 | 3.72 | 1 | 3.72 | 99.17 | <0.0001 *** |
| X32 | 1.45 | 1 | 1.45 | 38.55 | 0.0004 *** |
| Residual | 0.26 | 7 | 0.037 | ||
| lack of Fit | 0.19 | 3 | 0.062 | 3.22 | 0.1441 |
| Pure Error | 0.077 | 4 | 0.019 | ||
| Cor Total | 28.07 | 16 | |||
| SD | 0.19 | ||||
| Mean | 21.6 | R2 | 0.9907 | ||
| C.V.% | 0.90 | R2 adj | 0.9786 |
| Analyte | Molecular Formula | Equation | R2 | RT (min) | Concentration (μg/g Dry Weight) |
|---|---|---|---|---|---|
| Rutin | C27H30O16 | y = 20.98727x + 10.52446 | 0.9988 | 14.711 | 1001.316 ± 18.932 |
| Isoquercitrin | C21H20O12 | y = 22.82917x + 4.57751 | 0.9989 | 15.755 | 474.996 ± 5.768 |
| L-Epicatechin | C15H14O6 | y = 6.12946x − 11.33588 | 0.9931 | 12.515 | 423.814 ± 2.330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules 2022, 27, 2916. https://doi.org/10.3390/molecules27092916
Wang W, Zhang X, Liu Q, Lin Y, Zhang Z, Li S. Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules. 2022; 27(9):2916. https://doi.org/10.3390/molecules27092916
Chicago/Turabian StyleWang, Wei, Xiaoli Zhang, Qinglei Liu, Yucheng Lin, Zhiguo Zhang, and Shanshan Li. 2022. "Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves" Molecules 27, no. 9: 2916. https://doi.org/10.3390/molecules27092916
APA StyleWang, W., Zhang, X., Liu, Q., Lin, Y., Zhang, Z., & Li, S. (2022). Study on Extraction and Antioxidant Activity of Flavonoids from Hemerocallis fulva (Daylily) Leaves. Molecules, 27(9), 2916. https://doi.org/10.3390/molecules27092916






