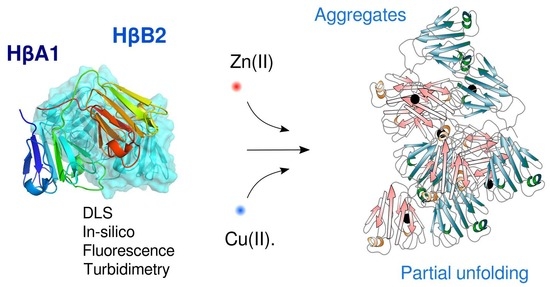
Zinc and Copper Ions Induce Aggregation of Human β-Crystallins
Abstract
:1. Introduction
2. Results
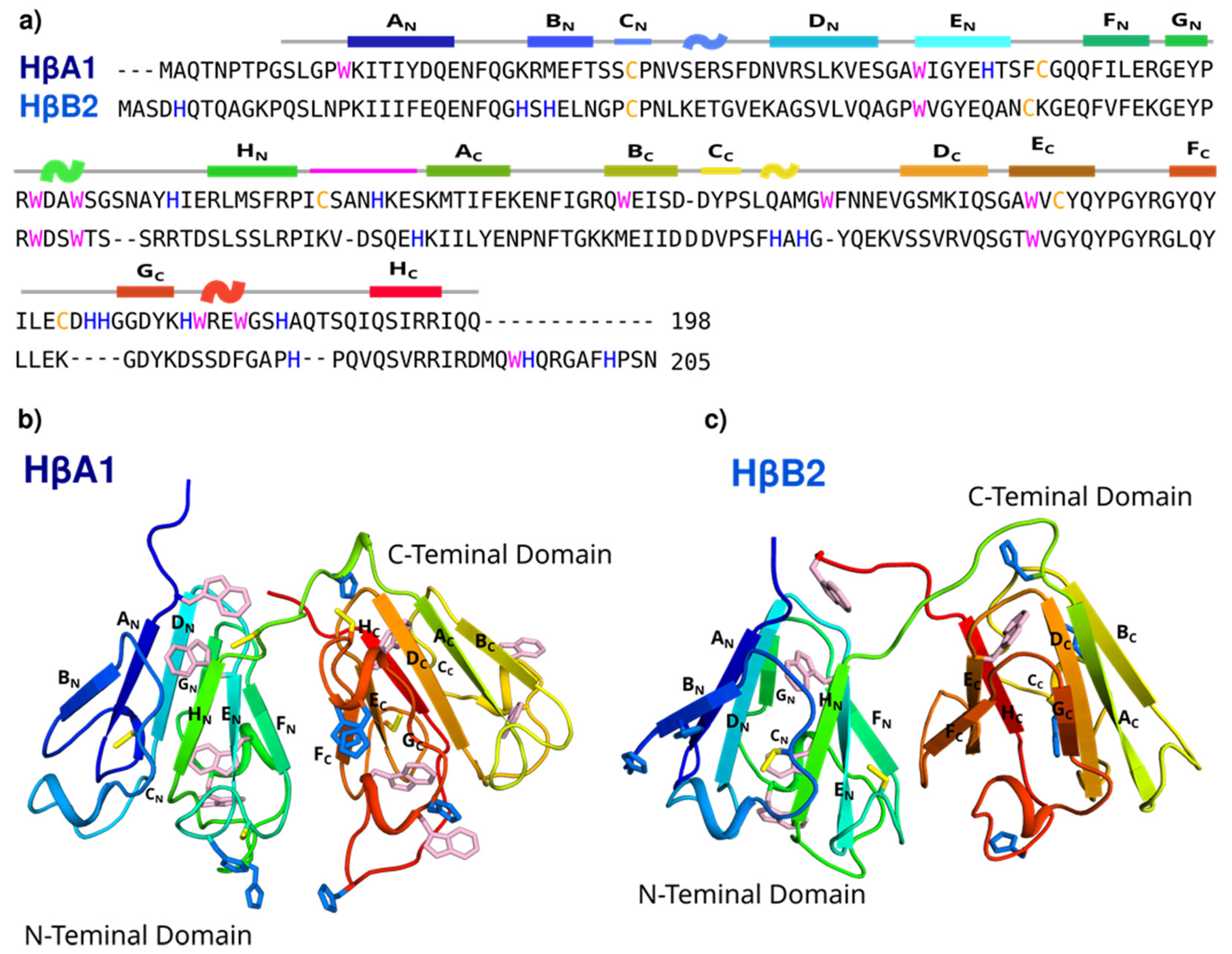
2.1. Bioinformatics and Molecular Modeling
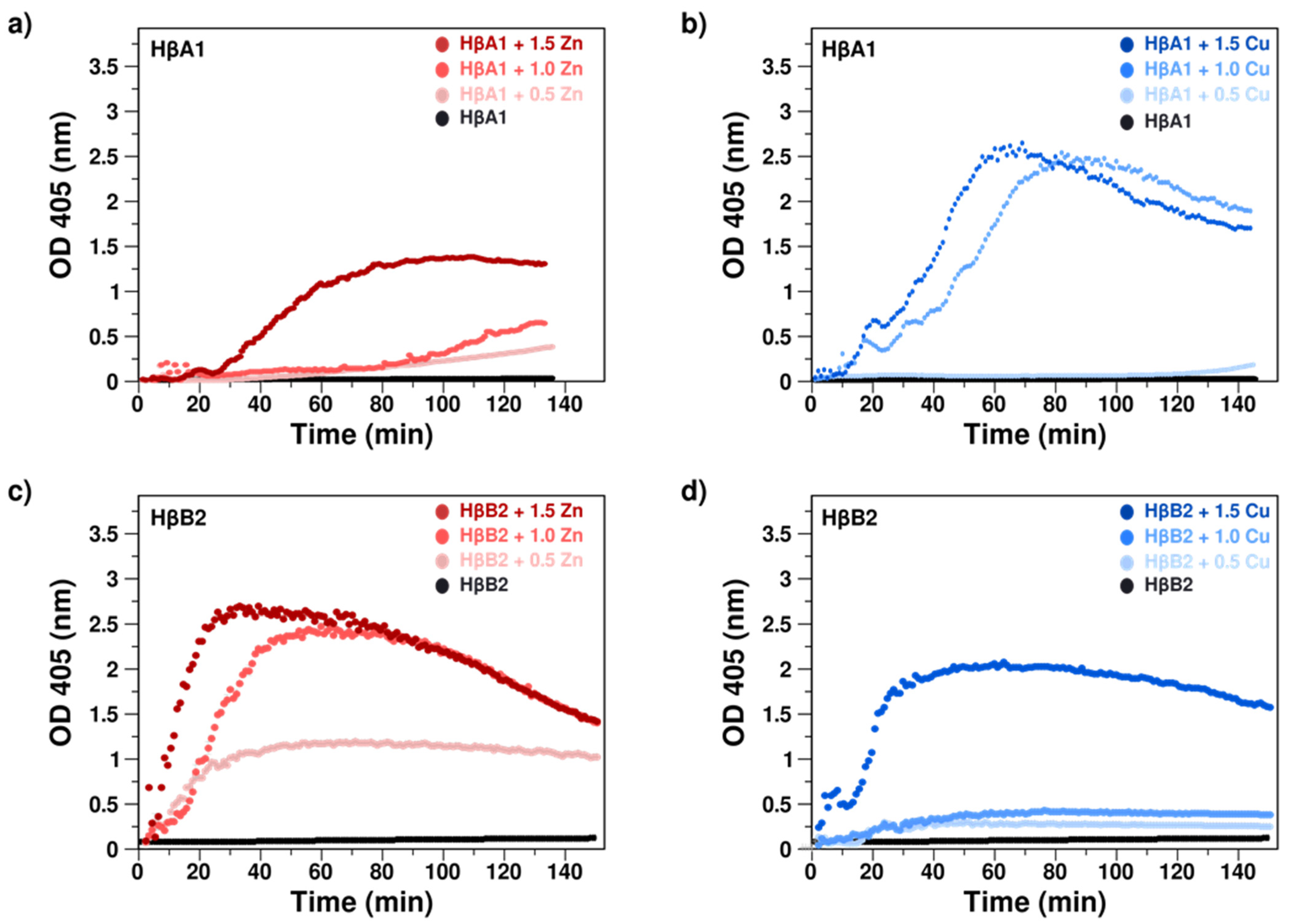
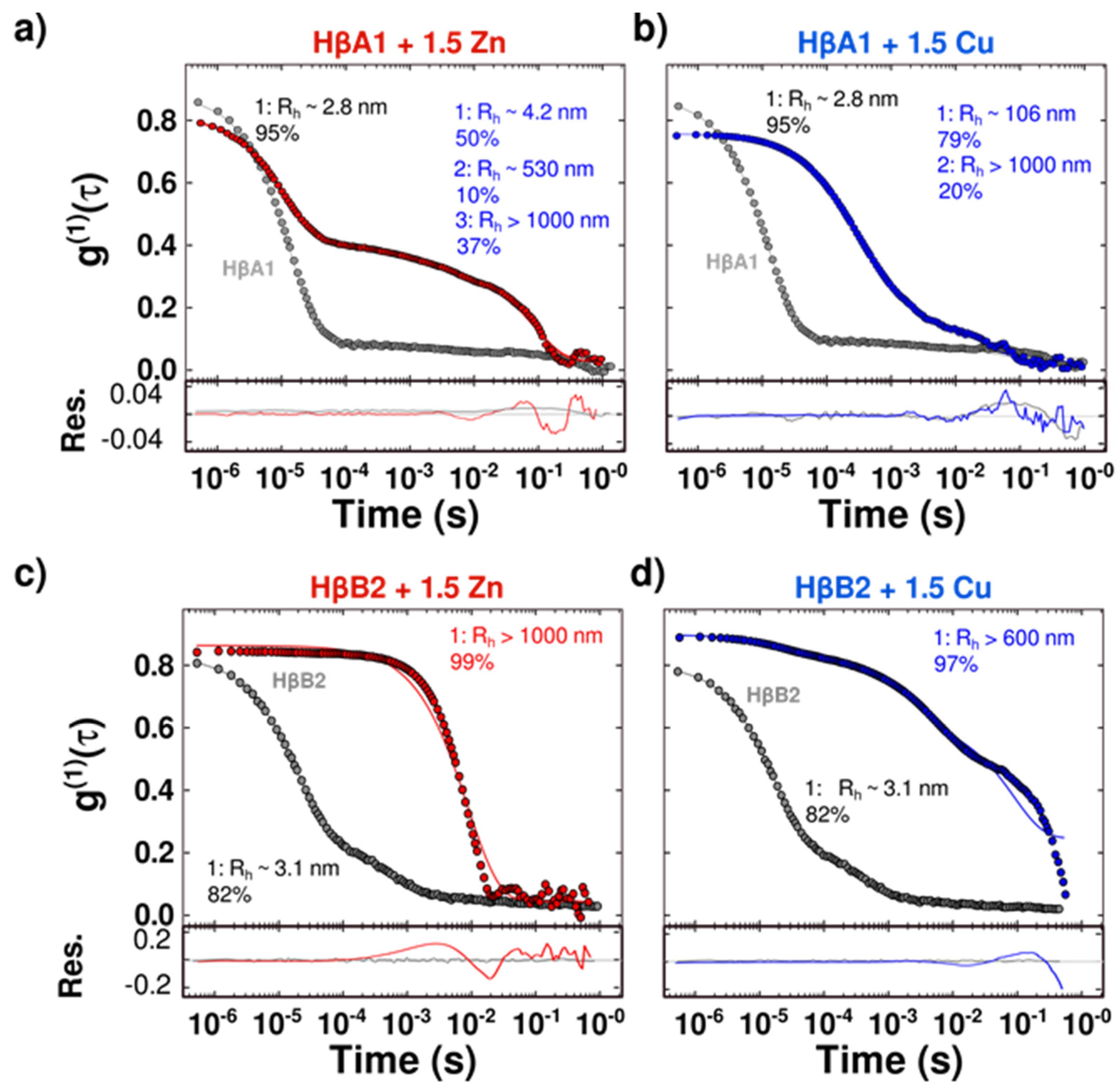
2.2. Cu(II) and Zn(II) Ions Induce Formation of Light-Scattering Aggregates
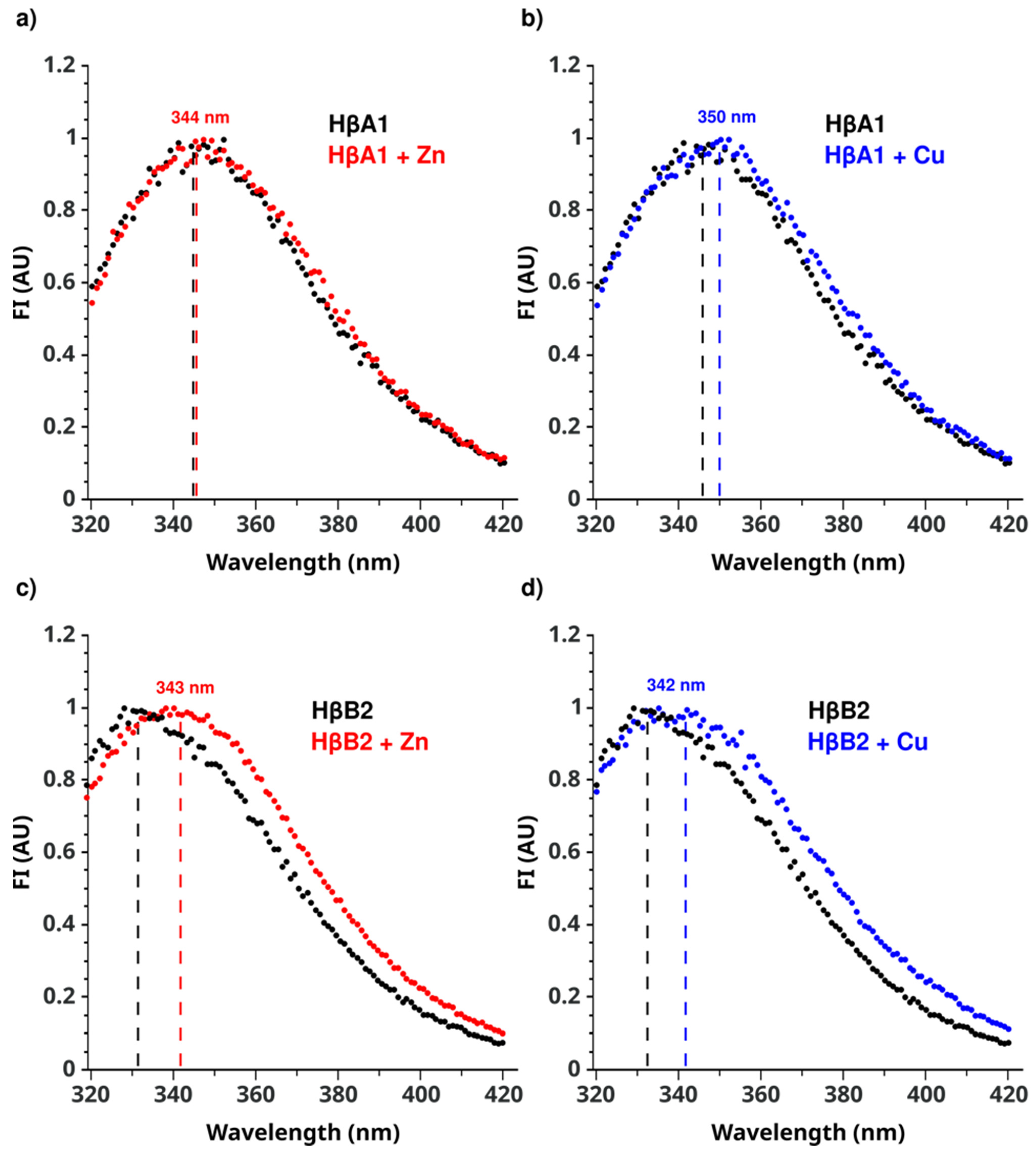
2.3. Cu(II) and Zn(II) Ions Induce Conformational Changes
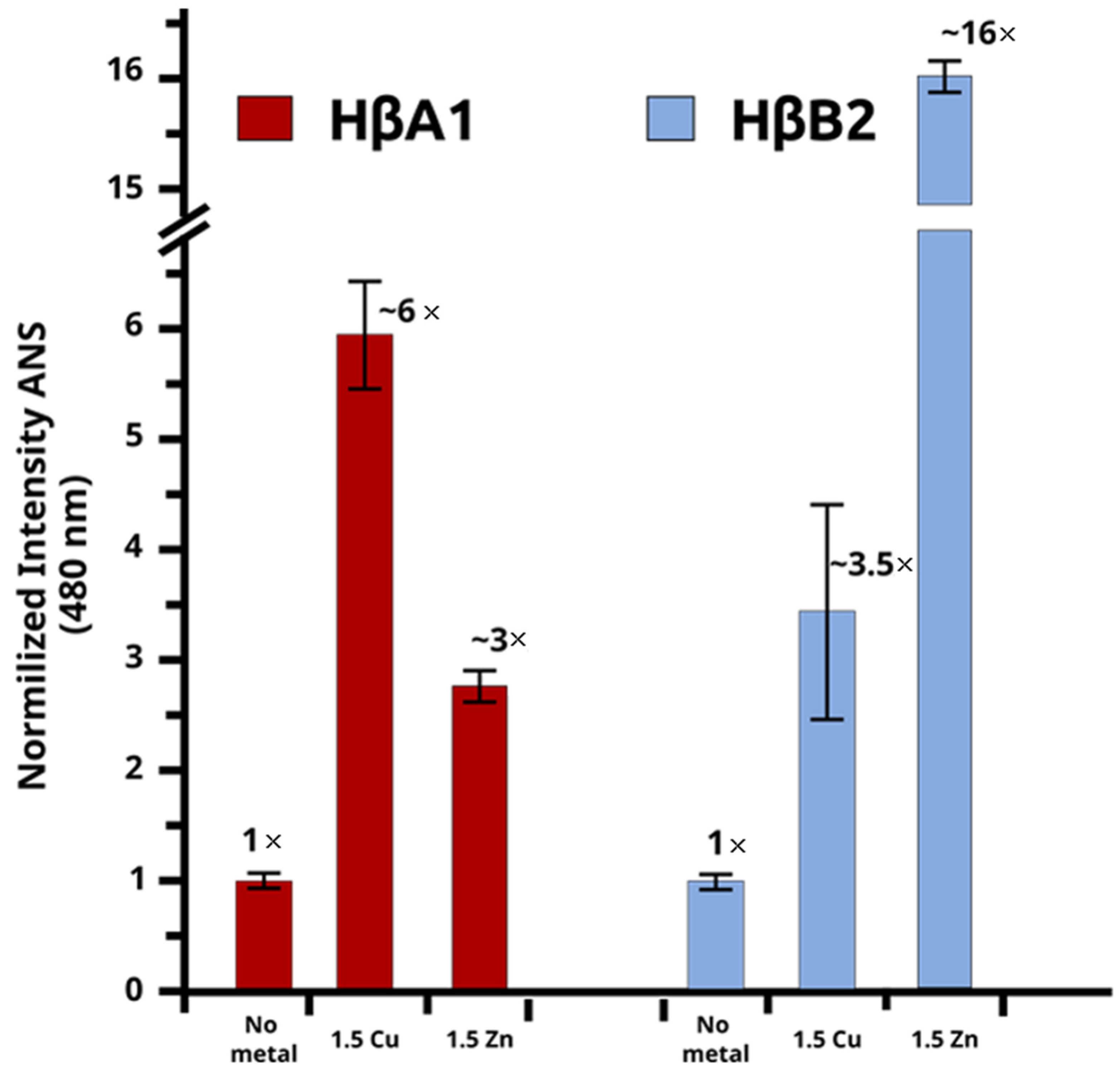
2.4. Changes in Stability Due to Metals
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. UV-Visible Spectroscopy
4.3. Fluorescence
4.4. Fourier Transform Infrared Spectroscopic Analysis
4.5. Dynamic Light Scattering
4.6. Computational Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Steinmetz, J.D.; Saylan, M.; Mersha, A.M.; Weldemariam, A.H.; Wondmeneh, T.G.; Sreeramareddy, C.T.; Pinheiro, M.; Yaseri, M.; Yu, C.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Bourne, R.R.A.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Harshal, W.; Rishi, G.; Niraj, P.; Rajendra, B.; Anand, G.; Tushar, P.; Tirthajit, B. A Comprehensive Review of Molecular Biology and Genetics of Cataract. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 11–20. [Google Scholar] [CrossRef]
- Esteves, J.F.; Pizzol, M.M.D.; Sccoco, C.A.; Roggia, M.F.; Milano, S.B.; Guarienti, J.A.A.; Rodrigues, T.C.; Canani, L.H. Cataract and type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2008, 82, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Robman, L.; Taylor, H. External factors in the development of cataract. Eye 2005, 19, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Eckhert, C.D. Elemental concentrations in ocular tissues of various species. Exp. Eye Res. 1983, 37, 639–647. [Google Scholar] [CrossRef]
- González-Iglesias, H.; Petrash, C.; Rodríguez-Menéndez, S.; García, M.; Álvarez, L.; Fernández-Vega Cueto, L.; Fernández, B.; Pereiro, R.; Sanz-Medel, A.; Coca-Prados, M. Quantitative distribution of Zn, Fe and Cu in the human lens and study of the Zn-metallothionein redox system in cultured lens epithelial cells by elemental MS. J. Anal. At. Spectrom. 2017, 32, 1746–1756. [Google Scholar] [CrossRef]
- Dawczynski, J.; Blum, M.; Winnefeld, K.; Strobel, J. Increased content of zinc and iron in human cataractous lenses. Biol. Trace Elem. Res. 2002, 90, 15–23. [Google Scholar] [CrossRef]
- Aydin, E.; Cumurcu, T.; Özugurlu, F.; Özyurt, H.; Sahinoglu, S.; Mendil, D.; Hasdemir, E. Levels of Iron, Zinc, and Copper in Aqueous Humor, Lens, and Serum in Nondiabetic and Diabetic Patients: Their Relation to Cataract. Biol. Trace Elem. Res. 2005, 108, 033–042. [Google Scholar] [CrossRef]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; González-Iglesias, H.; Coca-Prados, M.; Sanz-Medel, A. Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal. Bioanal. Chem. 2014, 406, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.K.; Varshney, N.; Pandey, D.C. Role of trace elements in senile cataract. Acta Ophthalmol. 2009, 70, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Slingsby, C.; Wistow, G.J.; Clark, A.R. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. 2013, 22, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Bloemendal, H.; de Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and vision: Structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef]
- Rivillas-Acevedo, L.; Fernández-Silva, A.; Amero, C. Function, Structure and Stability of Human Gamma D Crystallins: A Review. In Physical Biology of Proteins and Peptides; Springer: Cham, Switzerland, 2015; pp. 81–98. [Google Scholar] [CrossRef]
- Bateman, O.A.; Sarra, R.; Van Genesen, S.T.; Kappé, G.; Lubsen, N.H.; Slingsby, C. The stability of human acidic β-crystallin oligomers and hetero-oligomers. Exp. Eye Res. 2003, 77, 409–422. [Google Scholar] [CrossRef]
- Sharma, K.K.; Santhoshkumar, P. Lens aging: Effects of crystallins. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Lampi, K.J.; Ma, Z.; Shih, M.; Shearer, T.R.; Smith, J.B.; Smith, D.L.; David, L.L. Sequence analysis of βA3, βB3, and βA4 crystallins completes the identification of the major proteins in young human lens. J. Biol. Chem. 1997, 272, 2268–2275. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Liang, J.J. Protein—protein interactions among human lens acidic and basic b -crystallins. FEBS Lett. 2007, 581, 3936–3942. [Google Scholar] [CrossRef] [Green Version]
- Ueda, Y.; Duncan, M.K.; David, L.L. Lens proteomics: The accumulation of crystallin modifications in the mouse lens with age. Investig. Ophthalmol. Vis. Sci. 2002, 43, 205–215. [Google Scholar]
- Wistow, G. The human crystallin gene families. Hum. Genomics 2012, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Graw, J. Genetics of crystallins: Cataract and beyond. Exp. Eye Res. 2009, 88, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.E.; Lampi, K.J.; Speir, J.P.; Kruppa, G.; Easterling, M.; Robinson, A.B. Quantitative measurement of young human eye lens crystallins by direct injection Fourier transform ion cyclotron resonance mass spectrometry. Mol. Vis. 2006, 12, 704–711. [Google Scholar] [PubMed]
- Van Montfort, R.L.M.; Bateman, O.A.; Lubsen, N.H.; Slingsby, C. Crystal structure of truncated human βB1-crystallin. Protein Sci. 2009, 12, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Bateman, O.A.; Jaenicke, R.; Slingsby, C. Mutation of interfaces in domain-swapped human betaB2-crystallin. Protein Sci. 2007, 16, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.; Lapatto, R.; Nalini, V.; Driessen, H.; Lindley, P.F.; Mahadevan, D.; Blundell, T.L.; Slingsby, C. X-ray analysis of βB2-crystallin and evolution of oligomeric lens proteins. Nature 1990, 347, 776–780. [Google Scholar] [CrossRef]
- Xi, Z.; Whitley, M.J.; Gronenborn, A.M. Human βB2-Crystallin Forms a Face-en-Face Dimer in Solution: An Integrated NMR and SAXS Study. Structure 2017, 25, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Velasco-Bolom, J.L.; Domínguez, L. Exploring the folding process of human βb2-crystallin using multiscale molecular dynamics and the Markov state model. Phys. Chem. Chem. Phys. 2020, 22, 26753–26763. [Google Scholar] [CrossRef]
- Rocha, M.A.; Sprague-Piercy, M.A.; Kwok, A.O.; Roskamp, K.W.; Martin, R.W. Chemical Properties Determine Solubility and Stability in βγ-Crystallins of the Eye Lens. ChemBioChem 2021, 22, 1329–1346. [Google Scholar] [CrossRef]
- Ramkumar, S.; Fan, X.; Wang, B.; Yang, S.; Monnier, V.M. Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 3595–3604. [Google Scholar] [CrossRef]
- Chauhan, P.; Brinda, S.; Anand, M.; Velappan, B. Inhibition of copper—mediated aggregation of human γ D–crystallin by Schiff bases. JBIC J. Biol. Inorg. Chem. 2017, 22, 505–517. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Huang, W.; Zhang, J. Physiological And Pathological Functions Of Βb2-Crystallins In Multiple Organs: A Systematic Review. Aging (Albany NY) 2021, 13, 15674–15687. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Silva, A.; French-Pacheco, L.; Rivillas-Acevedo, L.; Amero, C. Aggregation pathways of human γ D crystallin induced by metal ions revealed by time dependent methods. PeerJ 2020, 2020, e9178. [Google Scholar] [CrossRef] [PubMed]
- Roskamp, K.W.; Azim, S.; Kassier, G.; Norton-Baker, B.; Sprague-Piercy, M.A.; Miller, R.J.D.; Martin, R.W. Human γS-Crystallin-Copper Binding Helps Buffer against Aggregation Caused by Oxidative Damage. Biochemistry 2020, 59, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Pal, D. Inferring metal binding sites in flexible regions of proteins. Proteins Struct. Funct. Bioinform. 2021, 89, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Cheng, C.W.; Shih, C.S.; Hwang, J.K.; Yu, C.S.; Lu, C.H. MIB: Metal Ion-Binding Site Prediction and Docking Server. J. Chem. Inf. Model. 2016, 56, 2287–2291. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Aparicio, J.E.; Tiessler-Sala, L.; Velasco-Carneros, L.; Roldán-Martín, L.; Sciortino, G.; Maréchal, J.D. BioMetAll: Identifying Metal-Binding Sites in Proteins from Backbone Preorganization. J. Chem. Inf. Model. 2021, 61, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.J.; Fleming, K.G. HullRad: Fast Calculations of Folded and Disordered Protein and Nucleic Acid Hydrodynamic Properties. Biophys. J. 2018, 114, 856–869. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Sadat, A.; Joye, I.J. Peak fitting applied to fourier transform infrared and raman spectroscopic analysis of proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Domínguez-Calva, J.A.; Haase-Pettingell, C.; Serebryany, E.; King, J.A.; Quintanar, L. A Histidine Switch for Zn-Induced Aggregation of γ-Crystallins Reveals a Metal-Bridging Mechanism That Is Relevant to Cataract Disease. Biochemistry 2018, 57, 4959–4962. [Google Scholar] [CrossRef]
- Quintanar, L.; Domínguez-Calva, J.A.; Serebryany, E.; Rivillas-Acevedo, L.; Haase-Pettingell, C.; Amero, C.; King, J.A.; Dom, J.A.; Serebryany, E.; Rivillas-Acevedo, L.; et al. Copper and Zinc Ions Specifically Promote Nonamyloid Aggregation of the Highly Stable Human γ-D Crystallin. ACS Chem. Biol. 2016, 11, 263–272. [Google Scholar] [CrossRef]
- Leal, S.S.; Botelho, H.M.; Gomes, C.M. Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord. Chem. Rev. 2012, 256, 2253–2270. [Google Scholar] [CrossRef]
- Ota, C.; Tanaka, S.I.; Takano, K. Revisiting the rate-limiting step of the ans–protein binding at the protein surface and inside the hydrophobic cavity. Molecules 2021, 26, 420. [Google Scholar] [CrossRef]
- Glasgow, B.; Gasymov, O. ANS Fluorescence: Potential to Augment the Identification of the External Binding Sites of Proteins. Biochim. Biophys. Acta. 2014, 23, 403–411. [Google Scholar]
- Evans, P.; Slingsby, C.; Wallace, B.A. Association of partially folded lens βB2-crystallins with the α-crystallin molecular chaperone. Biochem. J. 2008, 409, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Liang, J.J.-N. Unfolding of human lens recombinant βB2- and γC-crystallins. J. Struct. Biol. 2002, 139, 191–198. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Gabrielson, J.P.; Randolph, T.W.; Kendrick, B.S.; Stoner, M.R. Sedimentation velocity analytical ultracentrifugation and SEDFIT/c(s): Limits of quantitation for a monoclonal antibody system. Anal. Biochem. 2007, 361, 24–30. [Google Scholar] [CrossRef]
- Pundir, S.; Martin, M.J.; O’Donovan, C. UniProt Tools. Curr. Protoc. Bioinform. 2016, 53, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, 252–258. [Google Scholar] [CrossRef]
| Protein Recovered −EDTA | Protein Recovered +EDTA | |
|---|---|---|
| HβA1 | 100% | 100% |
| HβA1 + 1.5 eq Zn(II) | 18 ± 8% | 70 ± 5% |
| HβA1 + 1.5 eq Cu(II) | 18 ± 5% | 20 ± 5% |
| HβB2 | 100% | 100% |
| HβB2 + 1.5 eq Zn(II) | 10 ± 5% | 20 ± 5% |
| HβB2 + 1.5 eq Cu(II) | 18 ± 7% | 35 ± 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Bello, V.; Martinez-Seoane, J.; Fernández-Silva, A.; Amero, C. Zinc and Copper Ions Induce Aggregation of Human β-Crystallins. Molecules 2022, 27, 2970. https://doi.org/10.3390/molecules27092970
Ramirez-Bello V, Martinez-Seoane J, Fernández-Silva A, Amero C. Zinc and Copper Ions Induce Aggregation of Human β-Crystallins. Molecules. 2022; 27(9):2970. https://doi.org/10.3390/molecules27092970
Chicago/Turabian StyleRamirez-Bello, Vanesa, Javier Martinez-Seoane, Arline Fernández-Silva, and Carlos Amero. 2022. "Zinc and Copper Ions Induce Aggregation of Human β-Crystallins" Molecules 27, no. 9: 2970. https://doi.org/10.3390/molecules27092970
APA StyleRamirez-Bello, V., Martinez-Seoane, J., Fernández-Silva, A., & Amero, C. (2022). Zinc and Copper Ions Induce Aggregation of Human β-Crystallins. Molecules, 27(9), 2970. https://doi.org/10.3390/molecules27092970