Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer
Abstract
:1. Introduction
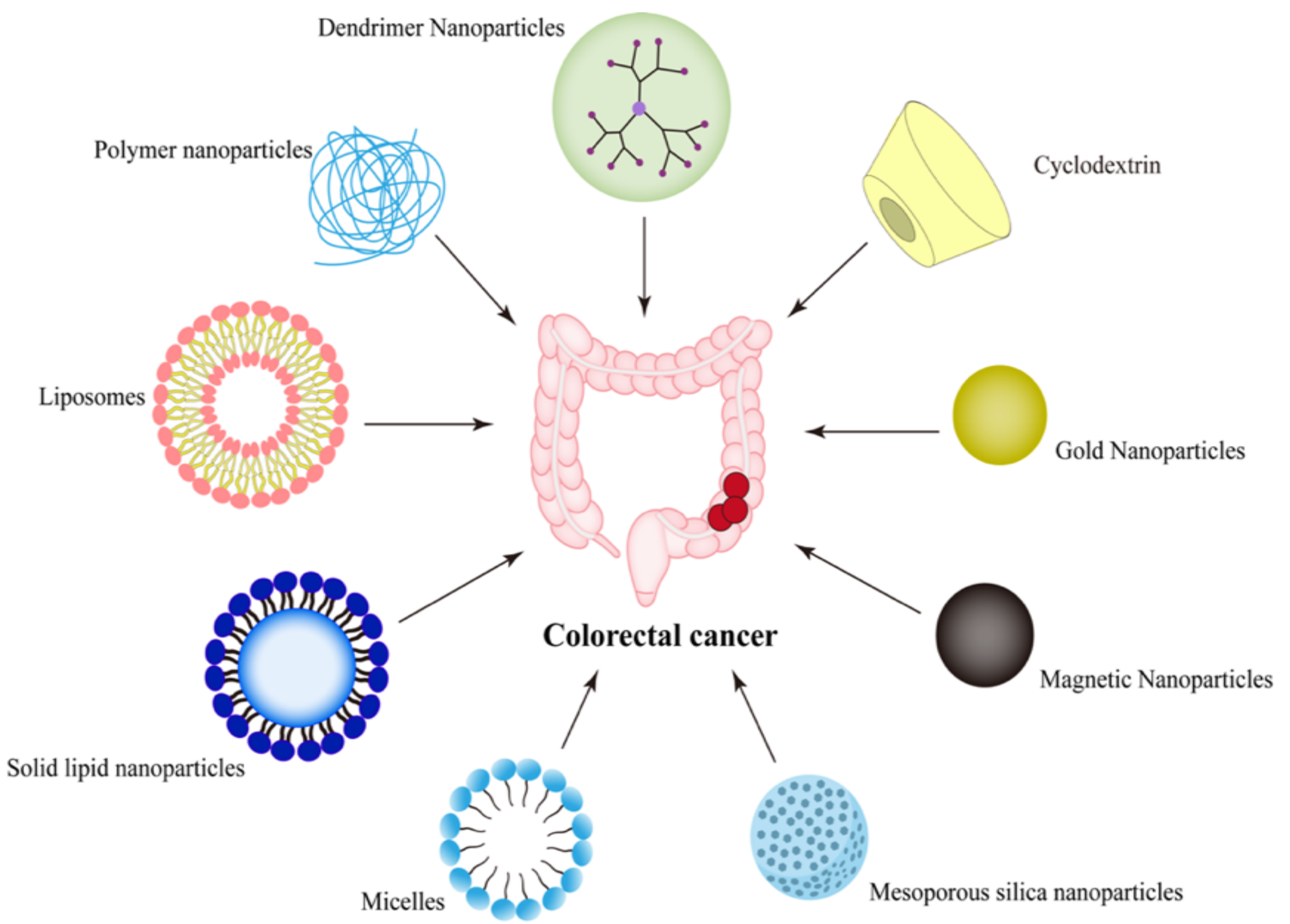
2. Nano-Drug Delivery Systems Targeting CRC
2.1. Passive Targeting Nanoparticles
2.2. Active Targeting Nanoparticles
2.2.1. Nanoparticles Targeting EpCAM
2.2.2. Nanoparticles Targeting Folate Receptor
2.2.3. Nanoparticles Targeting EGFR
2.2.4. Nanoparticles Targeting CD44
2.2.5. Biomimetic Nano Delivery Systems
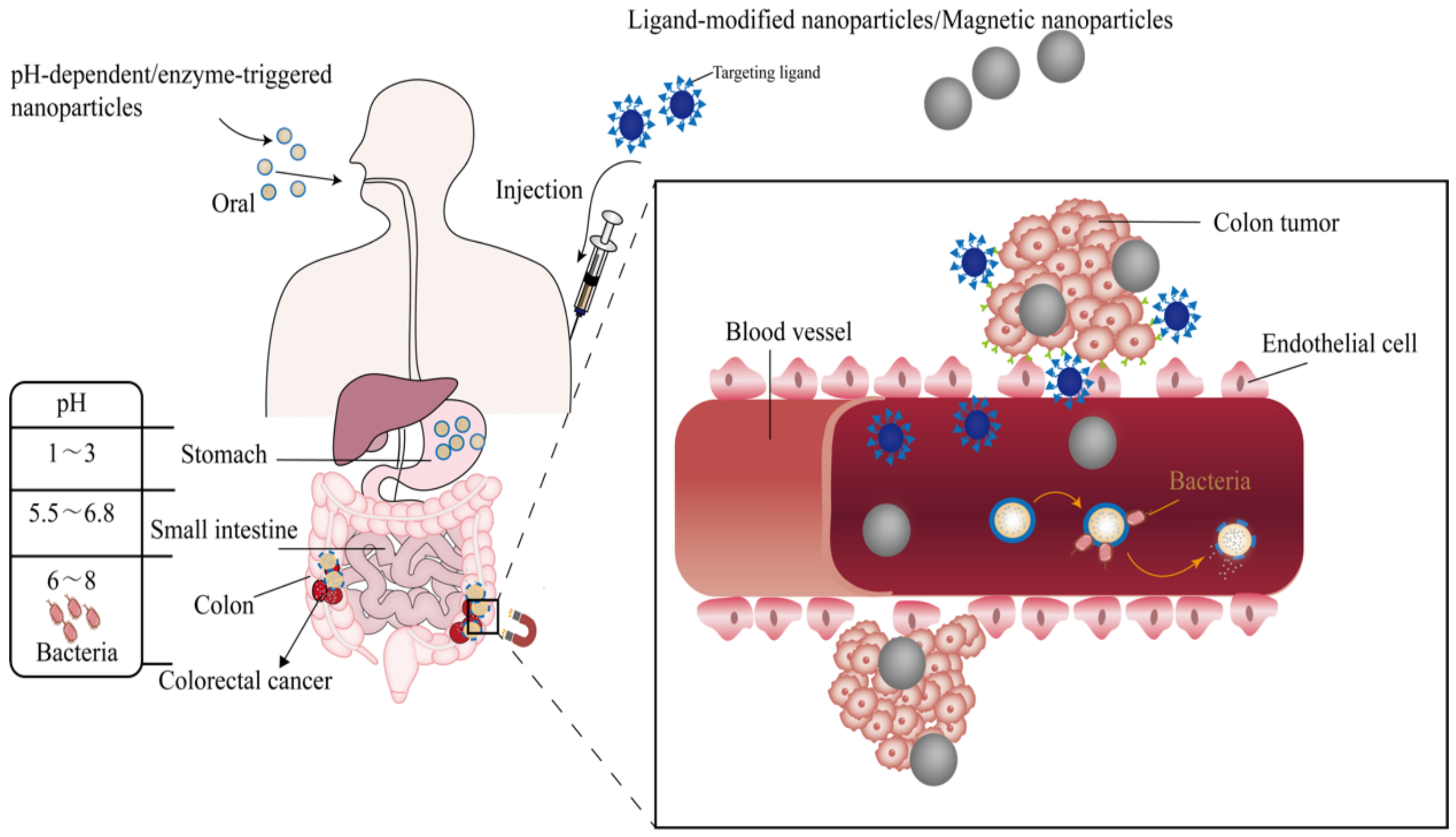
3. Nano-Drug Delivery Systems in Response to Environmental Signals
3.1. Nanoparticles Based on Stimulus Response
3.2. Oral Colon-Targeted Nano Delivery Systems
3.2.1. pH-Dependent Nano Platforms
3.2.2. Enzyme-Triggered Nanoparticles
4. Multifunctional Targeted Nanoparticles
5. Challenges of Nano-Drug Delivery Systems in CRC
6. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| ESMO | European Society for Medical Oncology |
| EGFR | epithelial growth factor |
| EPR | enhanced permeability and retention |
| EpCAM | epithelial cell adhesion molecule |
| 5-FU | 5-fluorouracil |
| MSN | mesoporous silica nanoparticles |
| SLB | supported lipid bilayer |
| HA | hyaluronic acid |
| ROS | reactive oxygen species |
| NIR | near infrared |
| SLNs | solid lipid nanoparticles |
| ICG | indocyanine green |
| DOX | doxorubicin |
| SIM | simvastatin |
| LRM | lactobacillus reuteri biofilm |
References
- Douaiher, J.; Ravipati, A.; Grams, B.; Chowdhury, S.; Alatise, O.; Are, C. Colorectal cancer-global burden, trends, and geographical variations. J. Surg. Oncol. 2017, 115, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Concise update on colorectal cancer epidemiology. Ann. Transl. Med. 2019, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Alomrani, A.; Badran, M.; Harisa, G.I.; Alshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Tabah-Fisch, I.; Bleiberg, H.; de Gramont, A.; Tournigand, C.; Andre, T.; Rothenberg, M.L.; Green, E.; Sargent, D.J. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J. Clin. Oncol. 2006, 24, 4085–4091. [Google Scholar] [CrossRef]
- Son, H.S.; Lee, W.Y.; Lee, W.S.; Yun, S.H.; Chun, H.K. Compliance and effective management of the hand-foot syndrome in colon cancer patients receiving capecitabine as adjuvant chemotherapy. Yonsei Med. J. 2009, 50, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.; Haustermans, K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res. 2014, 203, 189–201. [Google Scholar] [CrossRef]
- Masago, K.; Imamichi, F.; Masuda, Y.; Ariga, N.; Fujitomi, K.; Fukumine, Y.; Hatakenaka, K.; Fujita, S.; Katakami, N. Team Management of Skin Rash Associated with Use of Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors. Asia Pac. J. Oncol. Nurs. 2018, 5, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Szabados, B.; Leung, C.; Sahdev, A. Adverse effects and radiological manifestations of new immunotherapy agents. Br. J. Radiol. 2019, 92, 20180164. [Google Scholar] [CrossRef]
- Feldmann, S.M. Cancer immunotherapy in routine cost-effective cancer care? EMBO Mol. Med. 2018, 10, e9660. [Google Scholar] [CrossRef]
- Skalickova, S.; Loffelmann, M.; Gargulak, M.; Kepinska, M.; Docekalova, M.; Uhlirova, D.; Stankova, M.; Fernandez, C.; Milnerowicz, H.; Ruttkay-Nedecky, B.; et al. Zinc-Modified Nanotransporter of Doxorubicin for Targeted Prostate Cancer Delivery. Nanomaterials 2017, 7, 435. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Maeda, H.; Bharate, G.Y.; Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 2009, 71, 409–419. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Qiu, T.; Tang, M.; Zhang, X.; Dong, W. In vitro and in vivo combinatorial anticancer effects of oxaliplatin- and resveratrol-loaded N,O-carboxymethyl chitosan nanoparticles against colorectal cancer. Eur. J. Pharm. Sci. 2021, 163, 105864. [Google Scholar] [CrossRef]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Sivaram, A.J.; Mony, U.; Jayakumar, R. Combinatorial nanomedicines for colon cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 151–159. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor Targeted Curcumin Delivery by Folate-Modified MPEG-PCL Self-Assembly Micelles for Colorectal Cancer Therapy. Int. J. Nanomed. 2020, 15, 1239–1252. [Google Scholar] [CrossRef] [Green Version]
- Dabkeviciene, D.; Jonusiene, V.; Zitkute, V.; Zalyte, E.; Grigaitis, P.; Kirveliene, V.; Sasnauskiene, A. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med. Oncol. 2015, 32, 258. [Google Scholar] [CrossRef]
- Yu, D.H.; Lu, Q.; Xie, J.; Fang, C.; Chen, H.Z. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials 2010, 31, 2278–2292. [Google Scholar] [CrossRef]
- Jain, A.K.; Massey, A.; Yusuf, H.; McDonald, D.M.; McCarthy, H.O.; Kett, V.L. Development of polymeric-cationic peptide composite nanoparticles, a nanoparticle-in-nanoparticle system for controlled gene delivery. Int. J. Nanomed. 2015, 10, 7183–7196. [Google Scholar] [CrossRef] [Green Version]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.H.; Ku, S.K.; et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Bhattacharya, S. Anti-EGFR-mAb and 5-Fluorouracil Conjugated Polymeric Nanoparticles for Colorectal Cancer. Recent Pat. Anti-Cancer Drug Discov. 2021, 16, 84–100. [Google Scholar] [CrossRef]
- Ge, P.J.; Niu, B.N.; Wu, Y.H.; Xu, W.X.; Li, M.Y.; Sun, H.S.; Zhou, H.; Zhang, X.K.; Xie, J.J. Enhanced cancer therapy of celastrol in vitro and in vivo by smart dendrimers delivery with specificity and biosafety. Chem. Eng. J. 2020, 383, 123228. [Google Scholar] [CrossRef]
- Xu, M.; Wen, Y.Y.; Liu, Y.N.; Tan, X.J.; Chen, X.; Zhu, X.F.; Wei, C.F.; Chen, L.M.; Wang, Z.; Liu, J. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale 2019, 11, 9661–9678. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Matin, M.M.; Danesh, N.M.; Bahrami, A.R.; Abnous, K.; Taghdisi, S.M. Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR-21. Carbohydr. Polym. 2021, 266, 118111. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.Z.; Wang, J.W.; Phan, C.U.; Chen, Q.; Hu, X.R.; Shao, G.Q.; Zhou, J.; Lai, L.H.; Tang, G.P. Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment. Nat. Commun. 2021, 12, 759. [Google Scholar] [CrossRef]
- Pan, D.C.; Krishnan, V.; Salinas, A.K.; Kim, J.; Sun, T.; Ravid, S.; Peng, K.; Wu, D.; Nurunnabi, M.; Nelson, J.A.; et al. Hyaluronic acid-doxorubicin nanoparticles for targeted treatment of colorectal cancer. Bioeng. Transl. Med. 2021, 6, e10166. [Google Scholar] [CrossRef]
- Pandey, A.N.; Rajpoot, K.; Jain, S.K. Using 5-fluorouracil-encored plga nanoparticles for the treatment of colorectal cancer: The in-vitro characterization and cytotoxicity studies. Nanomed. J. 2020, 7, 211–224. [Google Scholar] [CrossRef]
- Wei, Y.H.; Gu, X.L.; Sun, Y.P.; Meng, F.H.; Storm, G.; Zhong, Z.Y. Transferrin-binding peptide functionalized polymersomes mediate targeted doxorubicin delivery to colorectal cancer in vivo. J. Control. Release 2020, 319, 407–415. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, M.M.; Verma, R.K.; Khan, R. Aminocellulose-Grafted Polymeric Nanoparticles for Selective Targeting of CHEK2-Deficient Colorectal Cancer. ACS Appl. Bio. Mater. 2021, 4, 5324–5335. [Google Scholar] [CrossRef]
- Serna, N.; Alamo, P.; Ramesh, P.; Vinokurova, D.; Sanchez-Garcia, L.; Unzueta, U.; Gallardo, A.; Cespedes, M.V.; Vazquez, E.; Villaverde, A.; et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4(+) colorectal cancer stem cells. J. Control. Release 2020, 320, 96–104. [Google Scholar] [CrossRef]
- Lee, K.J.; Ko, E.J.; Park, Y.Y.; Park, S.S.; Ju, E.J.; Park, J.; Shin, S.H.; Suh, Y.A.; Hong, S.M.; Park, I.J.; et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials 2020, 255, 120151. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Shi, Y.; Shan, S.F.; Li, C.H.; Song, X.J.; Zhang, C.X.; Chen, J.Y.; You, J.; Xiong, J.Y. Application of the Tumor Site Recognizable and Dual-Responsive Nanoparticles for Combinational Treatment of the Drug-Resistant Colorectal Cancer. Pharm. Res. 2020, 37, 72. [Google Scholar] [CrossRef]
- DuRoss, A.N.; Landry, M.R.; Thomas, C.R.; Neufeld, M.J.; Sun, C. Fucoidan-coated nanoparticles target radiation-induced P-selectin to enhance chemoradiotherapy in murine colorectal cancer. Cancer Lett. 2021, 500, 208–219. [Google Scholar] [CrossRef]
- Ramzy, L.; Metwally, A.A.; Nasr, M.; Awad, G.A.S. Novel thymoquinone lipidic core nanocapsules with anisamide-polymethacrylate shell for colon cancer cells overexpressing sigma receptors. Sci. Rep. 2020, 10, 10987. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ibrahim, M.A.; Amin, M.A.; Maswadeh, H.; Alwehaibi, M.N.; Al-Harbi, S.N.; Alharbi, Z.A.; Mohammed, H.A.; Mehany, A.B.M.; Saleem, I. Cetuximab Conjugated with Octreotide and Entrapped Calcium Alginate-beads for Targeting Somatostatin Receptors. Sci. Rep. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Jinka, S.; Rachamalla, H.K.; Bhattacharyya, T.; Sridharan, K.; Jaggarapu, M.; Yakati, V.; Banerjee, R. Glucocorticoid receptor-targeted liposomal delivery system for delivering small molecule ESC8 and anti-miR-Hsp90 gene construct to combat colon cancer. Biomed. Mater. 2021, 16, 024105. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.; de Leij, L.M.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef]
- Herlyn, M.; Steplewski, Z.; Herlyn, D.; Koprowski, H. Colorectal carcinoma-specific antigen: Detection by means of monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1979, 76, 1438–1442. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.M.; Speicher, D.W. Determination of disulfide bond assignments and N-glycosylation sites of the human gastrointestinal carcinoma antigen GA733-2 (CO17-1A, EGP, KS1-4, KSA, and Ep-CAM). J. Biol. Chem. 2001, 276, 5804–5813. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.Y.; Xu, J.J.; Le, V.M.; Gong, Q.Y.; Li, S.Y.; Gao, F.; Ni, L.; Liu, J.W.; Liang, X. EpCAM Aptamer-Functionalized Cationic Liposome-Based Nanoparticles Loaded with miR-139-5p for Targeted Therapy in Colorectal Cancer. Mol. Pharm. 2019, 16, 4696–4710. [Google Scholar] [CrossRef]
- Li, C.; Cai, G.; Song, D.; Gao, R.; Teng, P.; Zhou, L.; Ji, Q.; Sui, H.; Cai, J.; Li, Q.; et al. Development of EGFR-targeted evodiamine nanoparticles for the treatment of colorectal cancer. Biomater Sci. 2019, 7, 3627–3639. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. Cetuximab-conjugated chitosan-pectinate (modified) composite nanoparticles for targeting colon cancer. Int. J. Pharm. 2019, 572, 118775. [Google Scholar] [CrossRef]
- Pedrosa, P.; Corvo, M.L.; Ferreira-Silva, M.; Martins, P.; Carvalheiro, M.C.; Costa, P.M.; Martins, C.; Martins, L.; Baptista, P.V.; Fernandes, A.R. Targeting Cancer Resistance via Multifunctional Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hallal, R.; Lyu, N.; Wang, Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules 2021, 26, 567. [Google Scholar] [CrossRef] [PubMed]
- Prewett, M.; Bassi, R.; Carrick, F.; Finnerty, B.; Ellis, L.M.; Witte, L.; Tonra, J.R.; Hicklin, D.J.; Fan, F. Cetuximab (ERBITUX (R)) enhances clinical efficacy of oxaliplatin in human colon carcinoma xenografts and reverses oxaliplatin resistance. Clin. Cancer Res. 2005, 11, 9048S. [Google Scholar]
- Prewett, M.C.; Hooper, A.T.; Bassi, R.; Ellis, L.M.; Waksal, H.W.; Hicklin, D.J. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin. Cancer Res. 2002, 8, 994–1003. [Google Scholar]
- Chen, R.; Huang, Y.; Wang, L.; Zhou, J.; Tan, Y.; Peng, C.; Yang, P.; Peng, W.; Li, J.; Gu, Q.; et al. Cetuximab functionalization strategy for combining active targeting and antimigration capacities of a hybrid composite nanoplatform applied to deliver 5-fluorouracil: Toward colorectal cancer treatment. Biomater. Sci. 2021, 9, 2279–2294. [Google Scholar] [CrossRef]
- Medrano-González, P.A.; Rivera-Ramírez, O.; Montaño, L.F.; Rendón-Huerta, E.P. Proteolytic Processing of CD44 and Its Implications in Cancer. Stem Cells Int. 2021, 2021, 6667735. [Google Scholar] [CrossRef]
- Wang, X.L.; Ouyang, X.M.; Chen, J.L.; Hu, Y.; Sun, X.Y.; Yu, Z.W. Nanoparticulate photosensitizer decorated with hyaluronic acid for photodynamic/photothermal cancer targeting therapy. Nanomedicine 2019, 14, 151–167. [Google Scholar] [CrossRef]
- Lokeshwar, B.L.; Lokeshwar, V.B.; Block, N.L. Expression of CD44 in prostate cancer cells: Association with cell proliferation and invasive potential. Anticancer Res. 1995, 15, 1191–1198. [Google Scholar]
- Huang, J.; Zhang, H.; Yu, Y.; Chen, Y.; Wang, D.; Zhang, G.; Zhou, G.; Liu, J.; Sun, Z.; Sun, D.; et al. Biodegradable self-assembled nanoparticles of poly (D,L-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 2014, 35, 550–566. [Google Scholar] [CrossRef]
- Gao, S.; Wang, J.; Tian, R.; Wang, G.; Zhang, L.; Li, Y.; Li, L.; Ma, Q.; Zhu, L. Construction and Evaluation of a Targeted Hyaluronic Acid Nanoparticle/Photosensitizer Complex for Cancer Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 32509–32519. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhang, D.D.; Fang, G.Z.; Wang, S. Erythrocyte membrane bioinspired near-infrared persistent luminescence nanocarriers for in vivo long-circulating bioimaging and drug delivery. Biomaterials 2018, 165, 39–47. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Zhao, N.; Li, C.Y.; Lv, S.W.; Hu, Y.Z.; Lv, H.; Wang, D.; Wang, S. Cancer Cell Macrophage Membrane Camouflaged Persistent Luminescent Nanoparticles for Imaging-Guided Photothermal Therapy of Colorectal Cancer. ACS Appl. Nano Mater. 2020, 3, 7105–7118. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Yang, F.E.; Hu, Y.Z.; Lv, H.; Wang, S. Tailor-Made Cell-Based Biomimetic Nanoprobes for Fluorescence Imaging Guided Colorectal Cancer Chemo-immunotherapy. ACS Appl. Bio Mater. 2021, 4, 1920–1931. [Google Scholar] [CrossRef]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.Y.; Ali, R.R.; Siew, K.K.; Chan, H.Y.; Wong, M.M.T.; Lim, W.L.; Kuca, K. 5-Fluorouracil loaded magnetic cellulose bionanocomposites for potential colorectal cancer treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, L.X.; Zhang, W.Y.; Xu, S.M.; Yang, Y.; Yan, Q.Y.; Yang, G.S. A biocompatible superparamagnetic chitosan-based nanoplatform enabling targeted SN-38 delivery for colorectal cancer therapy. Carbohydr. Polym. 2021, 274, 118641. [Google Scholar] [CrossRef]
- Dabaghi, M.; Quaas, R.; Hilger, I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers 2020, 12, 2562. [Google Scholar] [CrossRef]
- Xing, R.; Mustapha, O.; Ali, T.; Rehman, M.; Zaidi, S.S.; Baseer, A.; Batool, S.; Mukhtiar, M.; Shafique, S.; Malik, M.; et al. Development, Characterization, and Evaluation of SLN-Loaded Thermoresponsive Hydrogel System of Topotecan as Biological Macromolecule for Colorectal Delivery. Biomed Res. Int. 2021, 2021, 9968602. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, F.Z.; Li, S.S.; Liu, R.F.; Jin, T.T.; Dou, Y.; Zhou, Z.H.; Zhang, J.X. A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment. Theranostics 2019, 9, 3732–3753. [Google Scholar] [CrossRef]
- Kumar, B.; Murali, A.; Bharath, A.B.; Giri, S. Guar gum modified upconversion nanocomposites for colorectal cancer treatment through enzyme-responsive drug release and NIR-triggered photodynamic therapy. Nanotechnology 2019, 30, 315102. [Google Scholar] [CrossRef]
- Yadav, S.; Ramesh, K.; Kumar, P.; Jo, S.-H.; Yoo, S.I.; Gal, Y.-S.; Park, S.-H.; Lim, K.T. Near-Infrared Light-Responsive Shell-Crosslinked Micelles of Poly(d,l-lactide)-b-poly((furfuryl methacrylate)-co-(N-acryloylmorpholine)) Prepared by Diels–Alder Reaction for the Triggered Release of Doxorubicin. Materials 2021, 14, 7913. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Ramesh, K.; Kim, M.; Hyun, K.; Lim, K.T. Near-infrared light-responsive alginate hydrogels based on diselenide-containing cross-linkage for on demand degradation and drug release. Carbohydr. Polym. 2019, 223, 115070. [Google Scholar] [CrossRef]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2017, 150, 352–361. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef]
- Taymouri, S.; Ahmadi, Z.; Mirian, M.; Tavakoli, N. Simvastatin nanosuspensions prepared using a combination of pH-sensitive and timed-release approaches for potential treatment of colorectal cancer. Pharm. Dev. Technol. 2021, 26, 335–348. [Google Scholar] [CrossRef]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Nazar, M.F.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. Int. J. Pharm. 2020, 586, 119605. [Google Scholar] [CrossRef]
- Mohamed, J.M.; Alqahtani, A.; Ahmad, F.; Krishnaraju, V.; Kalpana, K. Pectin co-functionalized dual layered solid lipid nanoparticle made by soluble curcumin for the targeted potential treatment of colorectal cancer. Carbohydr. Polym. 2021, 252, 117180. [Google Scholar] [CrossRef]
- Bijari, N.; Ghobadi, S.; Derakhshandeh, K. -lactoglobulin-irinotecan inclusion complex as a new targeted nanocarrier for colorectal cancer cells. Res. Pharm. Sci. 2019, 14, 216–227. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Meneguin, A.B.; Akhter, D.T.; Fletcher, N.; Houston, Z.H.; Bell, C.; Thurecht, K.J.; Gremia, M.P.D. Understanding the role of colon-specific microparticles based on retrograded starch/pectin in the delivery of chitosan nanoparticles along the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2021, 158, 371–378. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap((R)) System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Fattal, E.; Youssef, M.; Couvreur, P.; Andremont, A. Treatment of experimental salmonellosis in mice with ampicillin-bound nanoparticles. Antimicrob. Agents Chemother. 1989, 33, 1540–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, A.; Dev, A.; Mohanbhai, S.J.; Shrimali, N.; Kapasiya, M.; Kushwaha, A.C.; Choudhury, S.R.; Guchhait, P.; Karmakar, S. Disulfide-Bridged Chitosan-Eudragit S-100 Nanoparticles for Colorectal Cancer. ACS Appl. Nano Mater. 2019, 2, 6409–6417. [Google Scholar] [CrossRef]
- Wang, T.R.; Ma, X.Y.; Lei, Y.; Luo, Y.C. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcumin. Colloids Surf. B-Biointerfaces 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, E.; Elalmis, Y.B.; Ikizler, B.K.; Yucel, S. Novel organic/inorganic hybrid nanoparticles as enzyme-triggered drug delivery systems: Dextran and Dextran aldehyde coated silica aerogels. J. Drug Deliv. Sci. Technol. 2020, 56, 101517. [Google Scholar] [CrossRef]
- Gibson, S.A.; McFarlan, C.; Hay, S.; MacFarlane, G.T. Significance of microflora in proteolysis in the colon. Appl. Env. Microbiol. 1989, 55, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hao, W.S.; Zhang, L.; Zhu, Y.N.; Chen, K.; Ma, S.; Cheng, X.F.; Zhao, J.S. Lipid-polymer nano core-shell type hybrid system for colon specific drug delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102540. [Google Scholar] [CrossRef]
- Rajpoot, K.; Jain, S.K. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach. Int. J. Biol. Macromol. 2020, 151, 830–844. [Google Scholar] [CrossRef]
- Wang, Z.H.; Liu, J.M.; Li, C.Y.; Wang, D.; Lv, H.; Lv, S.W.; Zhao, N.; Ma, H.; Wang, S. Bacterial Biofilm Bioinspired Persistent Luminescence Nanoparticles with Gut-Oriented Drug Delivery for Colorectal Cancer Imaging and Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 36409–36419. [Google Scholar] [CrossRef]
- Ma, Y.M.; Thurecht, K.J.; Coombes, A.G.A. Development of enteric-coated, biphasic chitosan/HPMC microcapsules for colon-targeted delivery of anticancer drug-loaded nanoparticles. Int. J. Pharm. 2021, 607, 121026. [Google Scholar] [CrossRef]
- Valencia, P.M.; Basto, P.A.; Zhang, L.F.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-Step Assembly of Homogenous Lipid—Polymeric and Lipid—Quantum Dot Nanoparticles Enabled by Microfluidic Rapid Mixing. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.C.; Lin, D.; Gout, P.W.; Collins, C.C.; Xu, Y.; Wang, Y.Z. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv. Drug Deliv. Rev. 2014, 79–80, 222–237. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.Z.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical Translation of Nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, N.; Wu, J.; Xu, X.Y.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Lee, S.; Chen, X.Y. Nanotheranostics for personalized medicine. Expert Rev. Mol. Diagn. 2013, 13, 257–269. [Google Scholar] [CrossRef]
| Ligand | Receptor | Nano Delivery System | Active Agents | Size (nm) | Charge (mv) | Administration Route | Ref. |
|---|---|---|---|---|---|---|---|
| Folate | Folate Receptor | Self-assembled nanoparticles | Bortezomib | 196 | 28 | IV | [26] |
| Anti-EGFR mAb | Epidermal Growth Factor Receptor | PLGA and PEG-based polymeric nanoparticles | 5-Fluorouracil (5-FU) | 252 | −31 | IV | [27] |
| EpCAM aptamer | Epithelial cell adhesion molecule | PAMAM dendrimers | Celastrol | 300 | −6 | IV | [28] |
| SS-Fc | Carcinoembryonic antigen | PEGylated hollow mesoporous ruthenium nanoparticles | [Ru(bpy)2(tip)]2+, RBT | 110 | 22 | IV | [29] |
| AS1411 aptamer | Nucleolin | Silica nanoparticles coated with chitosan | AntimiR-21, doxorubicin (DOX) | 87 | 16 | IV | [30] |
| Mannose | Mannose receptor | Cyclodextrin-based host–guest complexes | Regorafenib | 100 | \ | IV | [31] |
| Hyaluronic acid | RHAMM, CD44 | Hyaluronic Acid–Doxorubicin nanoparticles | DOX | 175 | −5 | IV | [32] |
| Wheat germ agglutinin | N-acetyl-d-glucosamine, sialic acid | PLGA nanoparticles | 5-FU | 156 | −18 | \ | [33] |
| Transferrin | Transferrin receptor | Polymersomes | DOX | 72 | −2 | IV | [34] |
| LRP-1 targeting peptide | Lipoprotein receptor-related protein-1 | Human serum albumin nanoparticles | 5-FU | 208 | −13 | IV | [37] |
| MUC1 aptamer | MUC1 | Mesenchymal-stem-cell-derived exosomes | DOX | 50 | −80 | IV | [38] |
| Tumor-homing peptide tLyp-1 | NRP-1 | Nanoparticles | Paclitaxel, chlorin e6 | 107 | −25 | IV | [39] |
| Fucoidan | P-selectin | Nanoscale metal organic framework | Talazoparib, temozolomide | 84 | −18 | IV | [40] |
| Anisamide | Sigma receptors | Lipidic core nanocapsules | Thymoquinone | 217 | −36 | \ | [41] |
| Dexamethasone | Glucocorticoid receptor | Cationic liposomes | ESC8, anti-Hsp90 plasmid | 251 | 28 | IV | [43] |
| Formulations | Ingredients | Targeting Strategy | Ref. |
|---|---|---|---|
| Polymeric nanoparticles | Chitosan and thiolated chitosan, Eudragit L100, genipin | pH responsive, mucoadhesiveness | [75] |
| Solid lipid nanoparticles | Pectin, skimmed milk powder, lipid | pH responsive | [78] |
| Beta-lactoglobulin nanoparticles | Beta-lactoglobulin | pH responsive | [79] |
| Polymeric coated capsule, nanosuspension | Ethyl cellulose, Eudragit S100 | pH responsive, time-dependent | [76] |
| Polymeric nanoparticles | Dextran, bifunctional telechelic oligoester | Enzyme responsive | [77] |
| Microparticle | Chitosan, retrograded starch, pectin | Enzyme responsive | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Shen, R.; Meng, T.; Hu, F.; Yuan, H. Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules 2022, 27, 2981. https://doi.org/10.3390/molecules27092981
Wang K, Shen R, Meng T, Hu F, Yuan H. Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules. 2022; 27(9):2981. https://doi.org/10.3390/molecules27092981
Chicago/Turabian StyleWang, Ke, Ruoyu Shen, Tingting Meng, Fuqiang Hu, and Hong Yuan. 2022. "Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer" Molecules 27, no. 9: 2981. https://doi.org/10.3390/molecules27092981
APA StyleWang, K., Shen, R., Meng, T., Hu, F., & Yuan, H. (2022). Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules, 27(9), 2981. https://doi.org/10.3390/molecules27092981







