Detection of Ovine or Bovine Milk Components in Commercial Camel Milk Powder Using a PCR-Based Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DNA Extraction and Quality Evaluation
2.2.1. DNA Extraction Method of Milk Powder
2.2.2. Evaluation of DNA Quality
2.3. Qualitative Detection of Bovine Milk and Ovine Milk in Camel Milk Powders
2.3.1. Validation of Primers
2.3.2. Qualitative Detection of Camel Milk Powder
2.3.3. Detection of Commercial Camel Milk Powder
2.4. Qualitative Detection of Bovine Milk and Ovine Milk in Camel Milk Powders
2.4.1. Validation of Primers
2.4.2. Preparation of Quantitative Calibration Curve
2.4.3. Analysis of Adulterated Simulations
3. Results and Discussion
3.1. Quality of DNA Extracted from Milk Powders
3.1.1. DNA Yield and Purity
3.1.2. DNA Integrity
3.2. Results of Qualitative Detection
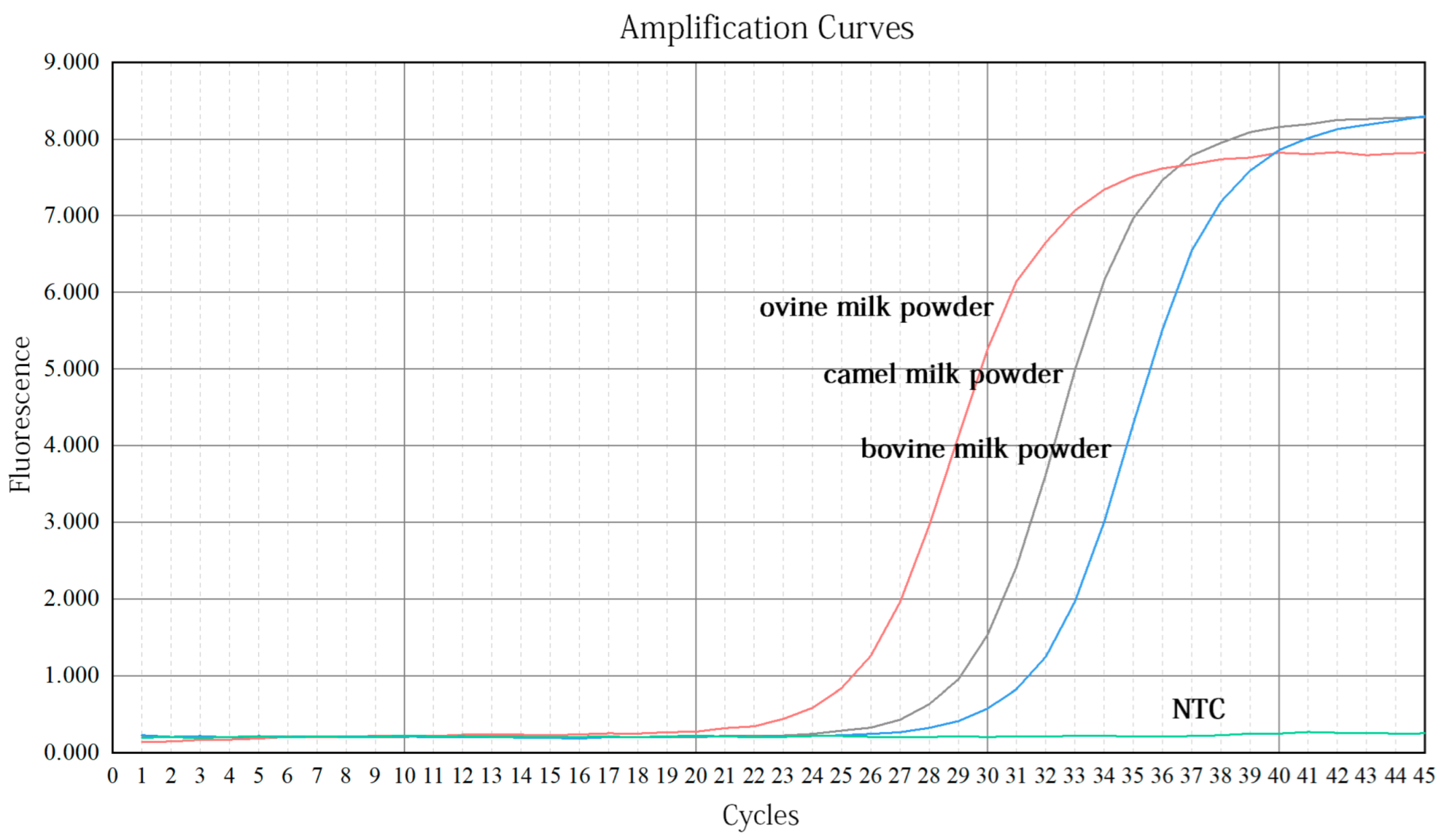
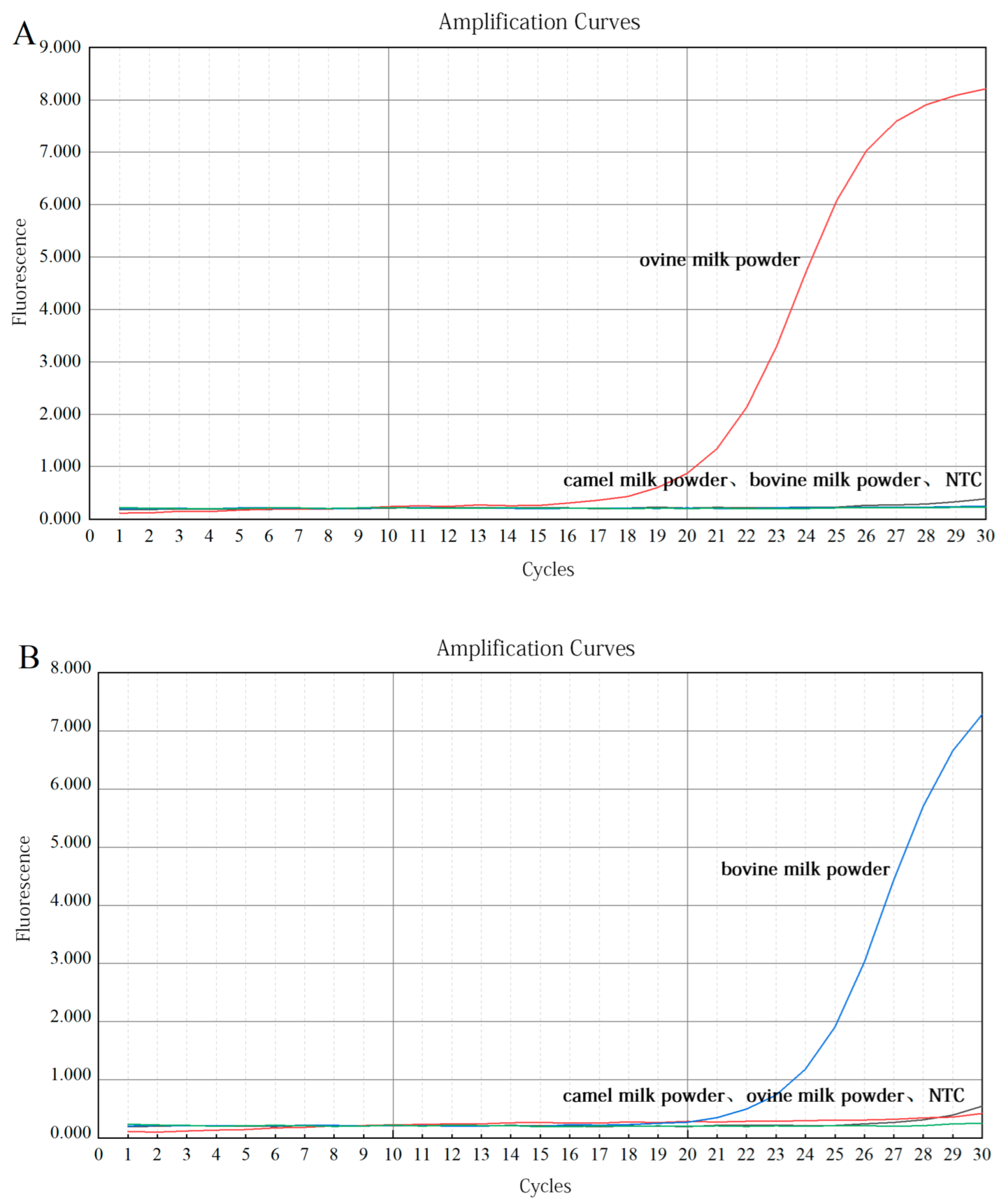
3.2.1. Validation of Primers
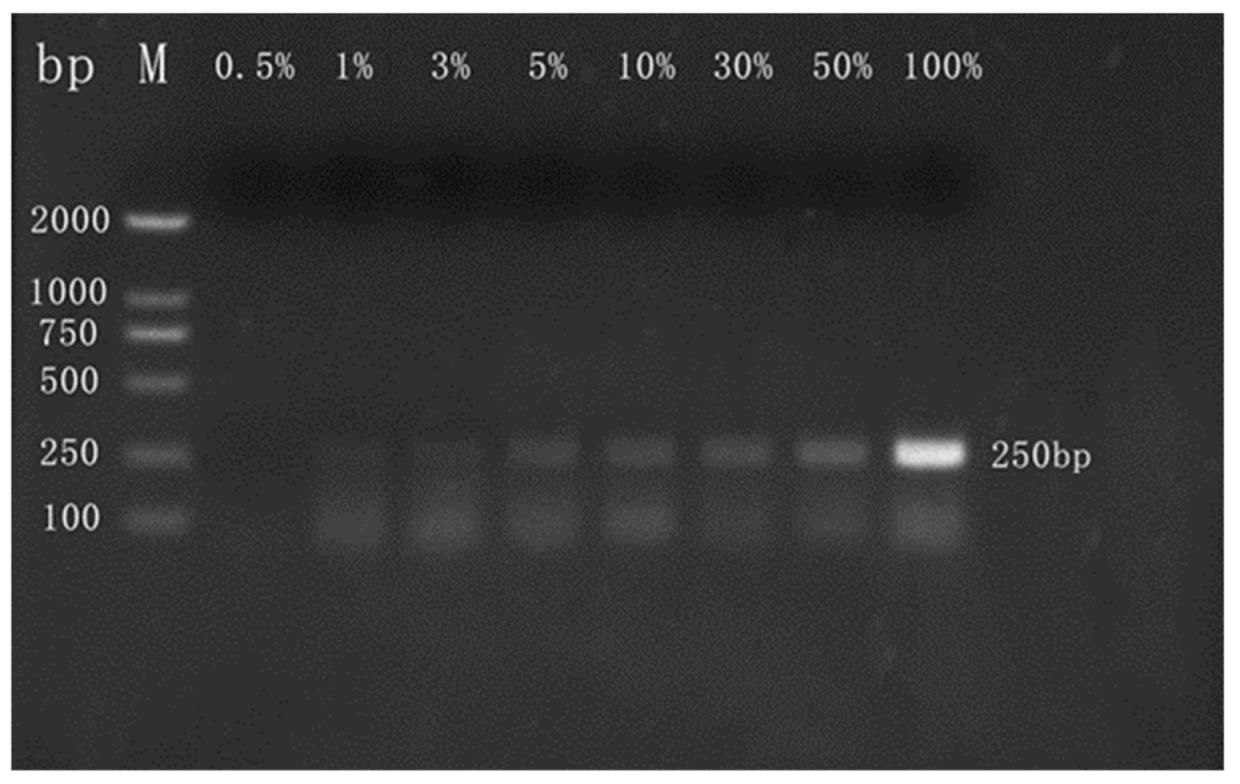
3.2.2. Qualitative Detection of Camel Milk Powder
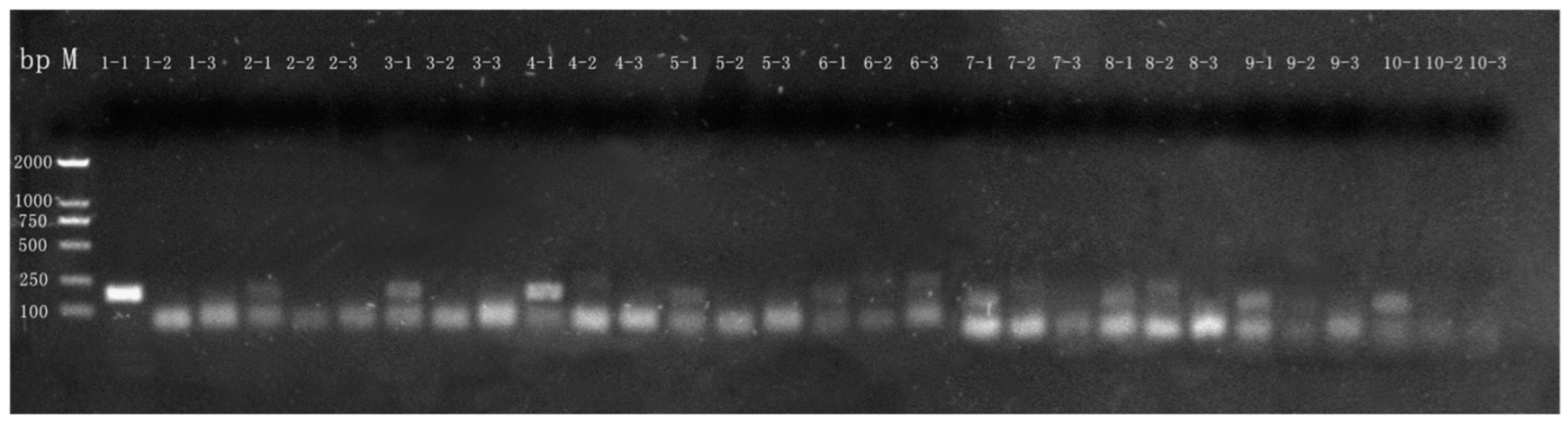
3.2.3. Detection of Commercial Camel Milk Powder
3.3. Results of Quantitative Detection
3.3.1. Validation of Primers
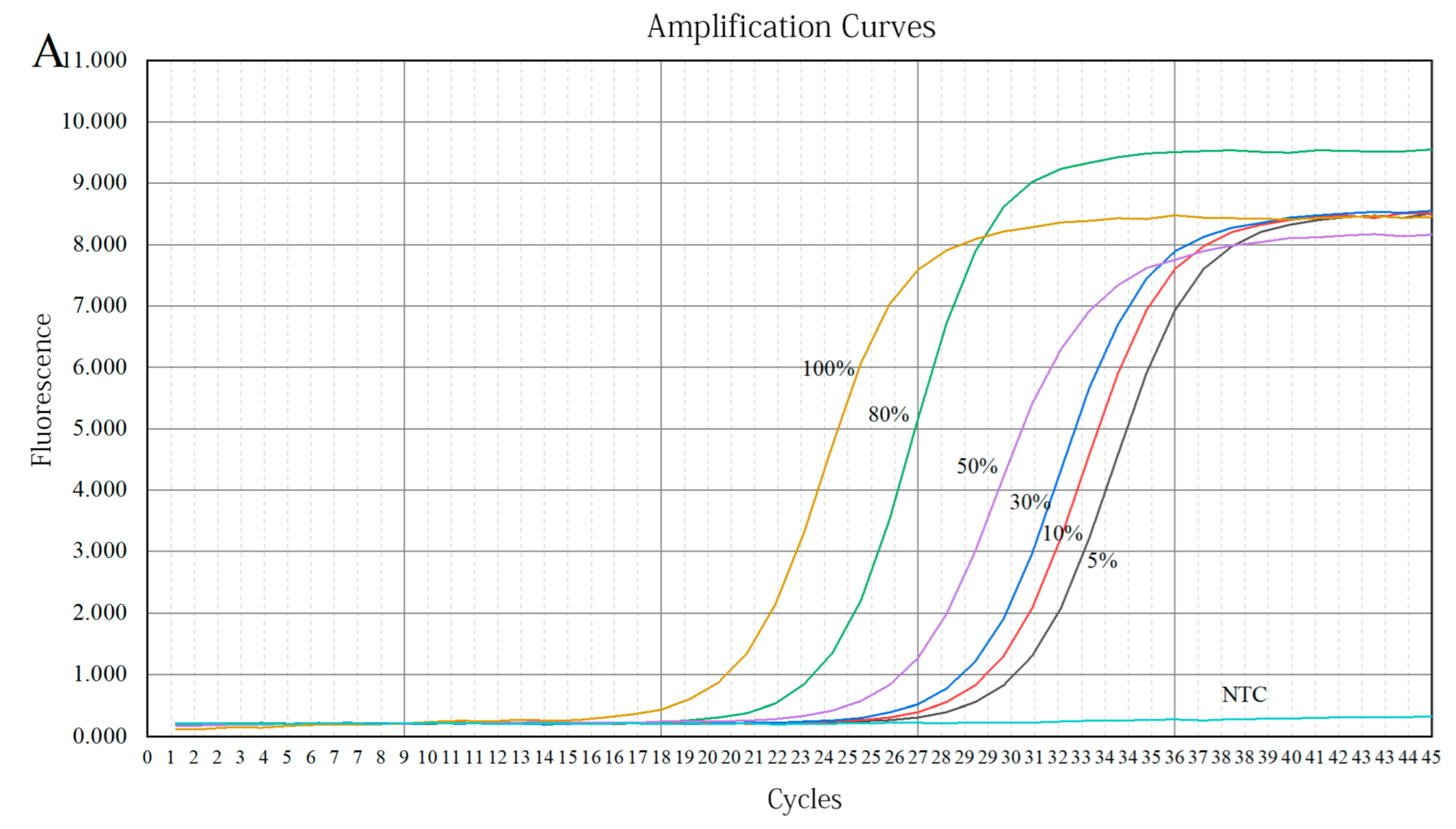
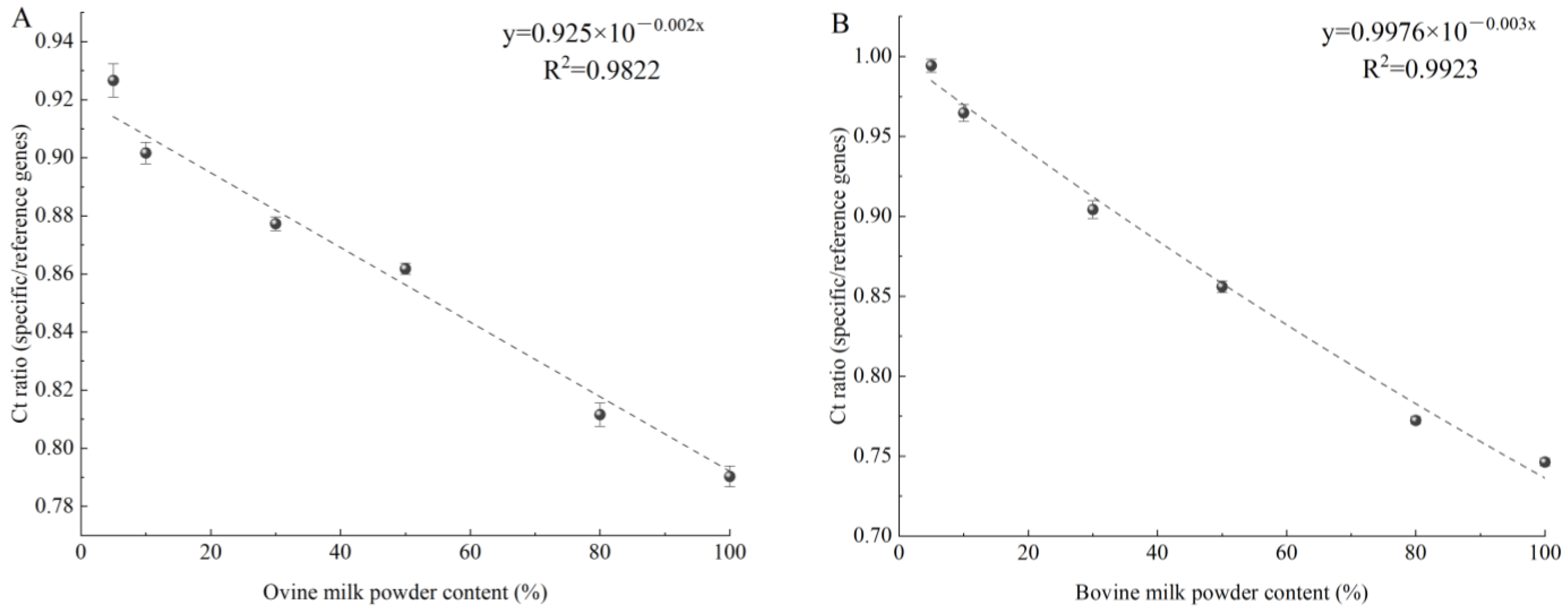
3.3.2. Preparation of Quantitative Calibration Curve
3.3.3. Analysis of Adulterated Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saljooghi, S.; Mansouri-Najand, L.; Ebrahim-Nejad, H.; Doostan, F.; Askari, N. Microbiological, biochemical and organoleptic properties of fermented probiotic drink produced from camel milk. Vet. Res. Forum 2017, 8, 313–317. [Google Scholar] [PubMed]
- Soliman, M.M.; Hassan, M.Y.; Mostafa, S.A.H.; Ali, H.A.M.; Saleh, O.M. Protective effects of camel milk against pathogenicity induced by Escherichia coli and Staphylococcus aureus in Wistar rats. Mol. Med. Rep. 2015, 12, 8306–8312. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography-mass spectrometry-ScienceDirect. J. Dairy Sci. 2021, 105, 1687–1700. [Google Scholar] [CrossRef]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.P.; Kelly, A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. J. Dairy Sci. 2012, 79, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, P.; Thomas, S.; Marko, O.; Juhasz, J. Milk production, raw milk quality and fertility of Dromedary camels (Camelus Dromedarius) under intensive management. Acta Weterinaria Hung. 2013, 61, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Salama, S.A.; Abdelghany, T.M.; Omar, H.A.; Arafa, E.S.A.; Alrobaian, M.A.; Maghrabi, I.A. Camel Milk Attenuates Rheumatoid Arthritis Via Inhibition of Mitogen Activated Protein Kinase Pathway. Cell. Physiol. Biochem. 2017, 43, 540–552. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, F.; Zhang, M.; Fang, B.; Zhao, L.; Dong, L.; Zhou, X.; Ge, S. Hypoglycemic effect of camel milk powder in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Food Sci Nutr 2021, 9, 4461–4472. [Google Scholar] [CrossRef]
- Krishnankutty, R.; Iskandarani, A.; Therachiyil, L.; Uddin, S.; Azizi, F.; Kulinski, M.; Bhat, A.A.; Mohammad, R.M. Anticancer Activity of Camel Milk via Induction of Autophagic Death in Human Colorectal and Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2018, 19, 3501–3509. [Google Scholar] [CrossRef] [Green Version]
- Windarsih, A.; Rohman, A.; Riyanto, S. The Combination of Vibrational Spectroscopy and Chemometrics for Analysis of Milk Products Adulteration. Int. J. Food Sci. 2021, 2021, 8853358. [Google Scholar] [CrossRef]
- Trimboli, F.; Costanzo, N.; Lopreiato, V.; Ceniti, C.; Morittu, V.M.; Spina, A.; Britti, D. Detection of buffalo milk adulteration with cow milk by capillary electrophoresis analysis. J. Dairy Sci. 2019, 102, 5962–5970. [Google Scholar] [CrossRef]
- Pizzano, R.; Salimei, E. Isoelectric focusing and ELISA for detecting adulteration of donkey milk with cow milk. J. Agric. Food Chem. 2014, 62, 5853–5858. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, N.; Soyeurt, H.; Yang, Y.; Wang, J. Detection of plant protein in adulterated milk using nontargeted nano-high-performance liquid chromatography-tandem mass spectroscopy combined with principal component analysis. Food Sci. Nutr. 2019, 7, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, M.; FitzGerald, R.J. Investigation of the flowability, thermal stability and emulsification properties of two milk protein concentrates having different levels of native whey proteins. Food Res. Int. 2021, 147, 110576. [Google Scholar] [CrossRef]
- Liao, J.; Liu, Y.F.; Ku, T.; Liu, M.H.; Huang, Y. Qualitative and quantitative identification of adulteration of milk powder using DNA extracted with a novel method. J. Dairy Sci. 2017, 100, 1657–1663. [Google Scholar] [CrossRef] [Green Version]
- Maudet, C.; Taberlet, P. Detection of cows’ milk in goats’ cheeses inferred from mitochondrial DNA polymorphism. J. Dairy Res. 2001, 68, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.D.; Conversano, M.C.; Forte, V.T.; Novello, L.; Tantillo, G.M. Detection of cow milk in buffalo “mozzarella” by polymerase chain reaction (PCR) assay. J. Food Qual. 2010, 27, 428–435. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, B.; Liu, M.; Han, J.; Chen, Y. A Real-Time PCR Method Targeting Camel Ingredient for Food Authentication. J. AOAC Int. 2015, 98, 1640–1644. [Google Scholar] [CrossRef]
- Wajahat, W.; Azad, Z.; Nazir, S.; Nasir, G. Real Time-PCR coupled with melt curve analysis for detecting the authenticity of camel milk. J. Food Sci. Technol. 2022, 59, 1538–1548. [Google Scholar] [CrossRef]
- Arya, M.; Shergill, I.S.; Williamson, M.; Gommersall, L.; Arya, N.; Patel, H.R. Basic principles of real-time quantitative PCR. Expert Rev. Mol. Diagn. 2005, 5, 209–219. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Yu, W.; Qiao, L.; Liu, R.; Li, S.; Zhao, Y.; Yang, S.; Chen, A. Determination of content of camel milk in adulterated milk samples by normalized real-time polymerase chain reaction system based on single-copy nuclear genes. J. Sci. Food Agric. 2020, 100, 3465–3470. [Google Scholar] [CrossRef]
- Zheng, W.W. Establishment of Real-Time PCR Detection Method for Bovine and Ovine Derived Materials in Feedstuff. Master’s Thesis, Jilin University, Jilin, China, 2009. (In Chinese). [Google Scholar]
- Fan, L.; Li, P.; Ding, H.; Jin, P.; Chun, L. Detection for bovine-derived ingredients in foods with real-time polymerase chain reaction method. Sci. Technol. Food Ind. China 2013, 34, 65–85. (In Chinese) [Google Scholar] [CrossRef]
- Luo, J.Q.; Wang, J.Q.; Bu, D.P.; Dan, L.I.; Wang, L.; Wei, H.Y.; Zhou, L.Y. Development and Application of a PCR Approach for Detection of Bovis, Sheep, Pig, and Chicken Derived Materials in Feedstuff. Agric. Sci. China 2008, 7, 1260–1266. [Google Scholar] [CrossRef]
- Li, T.T.; Jalbani, Y.M.; Zhang, G.L.; Zhao, Z.Y.; Wang, Z.Y.; Zhao, X.Y.; Chen, A.L. Detection of goat meat adulteration by real-time PCR based on a reference primer. Food Chem. 2019, 277, 554–557. [Google Scholar] [CrossRef]
- Sales, K.G.D.S.; Miranda, D.E.O.; Silva, F.J.D.; Otranto, D.; Figueredo, L.A.; Dantas-Torres, F. Evaluation of different storage times and preservation methods on phlebotomine sand fly DNA concentration and purity. Parasites Vectors 2020, 13, 399–404. [Google Scholar] [CrossRef]
- Koshy, L.; Anju, A.L.; Harikrishnan, S.; Kutty, V.R.; Jissa, V.T.; Kurikesu, I.; Jayachandran, P.; Jayakumaran, N.A.; Gangaprasad, A.; Nair, G.M.; et al. Evaluating genomic DNA extraction methods from human whole blood using endpoint and real-time PCR assays. Mol. Biol. Rep. 2017, 44, 97–108. [Google Scholar] [CrossRef]
- Bottero, M.T.; Civera, T.; Anastasio, A.; Turi, R.M.; Rosati, S. Identification of Cow’s Milk in “Buffalo” Cheese by Duplex Polymerase Chain Reaction. J. Food Prot. 2002, 65, 362–366. [Google Scholar] [CrossRef]
- Garafutdinov, R.R.; Galimova, A.A.; Sakhabutdinova, A.R. The influence of quality of primers on the formation of primer dimers in PCR. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1251–1269. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Mafra, I.; Oliveira, M.B. Quantitative detection of poultry meat adulteration with pork by a duplex PCR assay. Meat Sci. 2010, 85, 531–536. [Google Scholar] [CrossRef]
- Deng, L.; Li, A.; Gao, Y.; Shen, T.; Yue, H.; Miao, J.; Li, R.; Yang, J. Detection of the Bovine Milk Adulterated in Camel, Horse, and Goat Milk Using Duplex PCR. Food Anal. Methods 2020, 13, 560–567. [Google Scholar] [CrossRef]
- Bustin, S.A.; Mueller, R.; Nolan, T. Parameters for Successful PCR Primer Design. Methods Mol. Biol. 2020, 2065, 5–22. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Vishnuraj, M.R.; Narender, R.G.; Srinivas, C. Authentication of camel meat using species-specific PCR and PCR-RFLP. J. Food Sci. Technol. 2021, 58, 3882–3889. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Chen, S.D.; Weng, C.F. Investigation of Goats’ Milk Adulteration with Cows’ Milk by PCR. Asian Australas. J. Anim. Sci. 2006, 19, 1503–1507. [Google Scholar] [CrossRef]
- Psifidi, A.; Dovas, C.I.; Banos, G. A comparison of six methods for genomic DNA extraction suitable for PCR-based genotyping applications using ovine milk samples. Mol. Cell. Probes. 2010, 24, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhao, L.; Wang, Z.; Li, T.; Chen, A.; Yang, S. Quantitative detection of donkey hide gelatin (colla corii asini) adulteration by real-time PCR based on single-copy nuclear genes. J. Food Prot. 2020, 84, 194–199. [Google Scholar] [CrossRef]
- Hayden, R.T.; Gu, Z.; Ingersoll, J.; Abdul-Ali, D.; Shi, L.; Pounds, S.; Caliendo, A.M. Comparison of Droplet Digital PCR to Real-Time PCR for Quantitative Detection of Cytomegalovirus. J. Clin. Microbiol. 2013, 51, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Agrimonti, C.; Pirondini, A.; Marmiroli, M.; Marmiroli, N. A quadruplex PCR (qxPCR) assay for adulteration in dairy products. Food Chem. 2015, 187, 58–64. [Google Scholar] [CrossRef]
- Cerkvenik-Flajs, V.; Milcinski, L.; Süssinger, A.; Hodoscek, L.; Danaher, M.; Antonić, J. Trace analysis of endectocides in milk by high performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2010, 663, 165–171. [Google Scholar] [CrossRef]




| Species | Primer Sequence (5′-3′) | Gene | Amplicons (Bp) | Origin of Primer Sequences |
|---|---|---|---|---|
| camel | F: CATTATCACGGCTCTAGTGGC | Cytb | 182 | Sangon Biotechnology Co. Ltd. |
| R: CTGGTGAGAATAATACGAGGATAAG | ||||
| ovine | F: GAGTAATCCTCCTATTTGCGAC | Cytb | 240 | Sangon Biotechnology Co. Ltd. |
| R: GAACTATGGCGAGGGCTGC | ||||
| bovine | F: GTACTATTTGCGCCCAACCTCC | Cytb | 250 | Sangon Biotechnology Co. Ltd. |
| R: AGAACAGGCATTGGCTGAGCA | ||||
| ovine | F: CAGCCTTCCTGTTAACTTTCAATAG | 12S rRNA | 106 | Zheng et al. [21] |
| R: RGTGCTTGATACCTGCTCCTTTTAG | ||||
| bovine | F: CAACAGGAATCTCCTCAGACGTAGA | Cytb | 91 | Fan et al. [22] |
| R: GCTAGAATTAGTAAGAGGGCCCCTAA | ||||
| ruminant | F: AAGACGAGAAGACCCTTGGACTTTA | 16S rRNA | 234–262 | Luo et al. [23] |
| R: GATTGCGCTGTTATCCCTAGGGTA |
| Actual Content (%) | Calculated Content (%) | Mean ± SD (%) | CV (%) | Recovery (%) |
|---|---|---|---|---|
| 30 | 27.28 | 26.40 ± 1.45 | 5.51 | 88.0 |
| 24.72 | ||||
| 27.20 | ||||
| 50 | 41.73 | 41.84 ± 0.19 | 0.45 | 83.68 |
| 42.05 | ||||
| 41.73 | ||||
| 80 | 70.82 | 71.57 ± 2.51 | 3.50 | 89.46 |
| 74.36 | ||||
| 69.52 |
| Actual Content (%) | Calculated Content (%) | Mean ± SD (%) | CV (%) | Recovery (%) |
|---|---|---|---|---|
| 30 | 30.40 | 32.55 ± 1.96 | 6.01 | 108.51 |
| 33.04 | ||||
| 34.22 | ||||
| 50 | 49.50 | 50.96 ± 1.38 | 2.71 | 101.93 |
| 51.15 | ||||
| 52.24 | ||||
| 80 | 85.05 | 85.16 ± 1.01 | 1.19 | 106.45 |
| 86.22 | ||||
| 84.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Na, Q.; Hao, S.; Ji, R.; Ming, L. Detection of Ovine or Bovine Milk Components in Commercial Camel Milk Powder Using a PCR-Based Method. Molecules 2022, 27, 3017. https://doi.org/10.3390/molecules27093017
Wu X, Na Q, Hao S, Ji R, Ming L. Detection of Ovine or Bovine Milk Components in Commercial Camel Milk Powder Using a PCR-Based Method. Molecules. 2022; 27(9):3017. https://doi.org/10.3390/molecules27093017
Chicago/Turabian StyleWu, Xiaoyun, Qin Na, Shiqi Hao, Rimutu Ji, and Liang Ming. 2022. "Detection of Ovine or Bovine Milk Components in Commercial Camel Milk Powder Using a PCR-Based Method" Molecules 27, no. 9: 3017. https://doi.org/10.3390/molecules27093017
APA StyleWu, X., Na, Q., Hao, S., Ji, R., & Ming, L. (2022). Detection of Ovine or Bovine Milk Components in Commercial Camel Milk Powder Using a PCR-Based Method. Molecules, 27(9), 3017. https://doi.org/10.3390/molecules27093017






