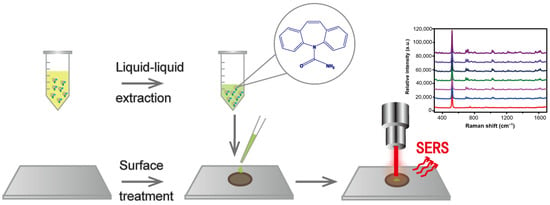
A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Treatment Optimization of Silicon Wafer
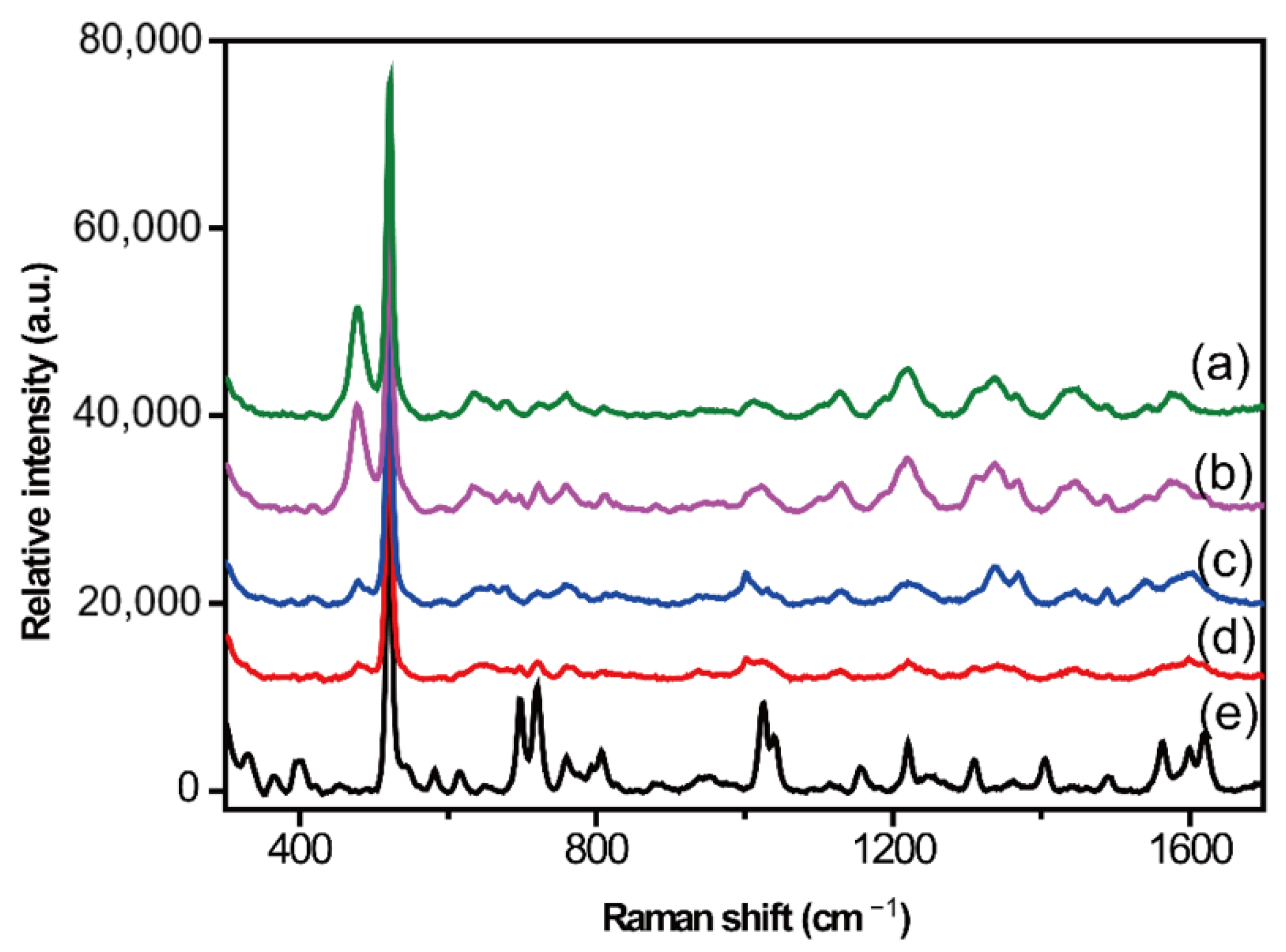
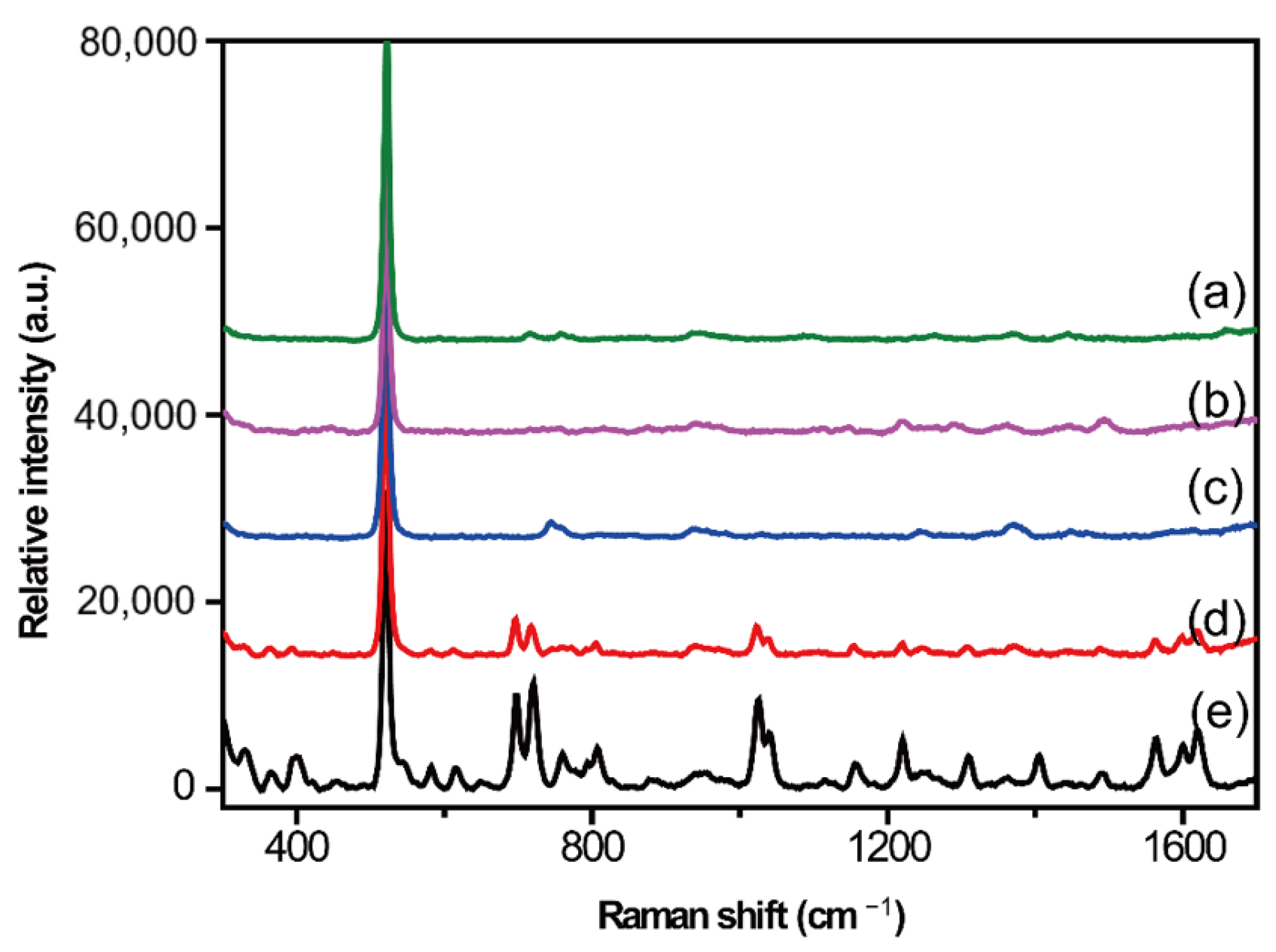
2.2. Optimization of Serum Pretreatment Conditions
Protein Precipitation Method
2.3. Liquid–Liquid Extraction Method
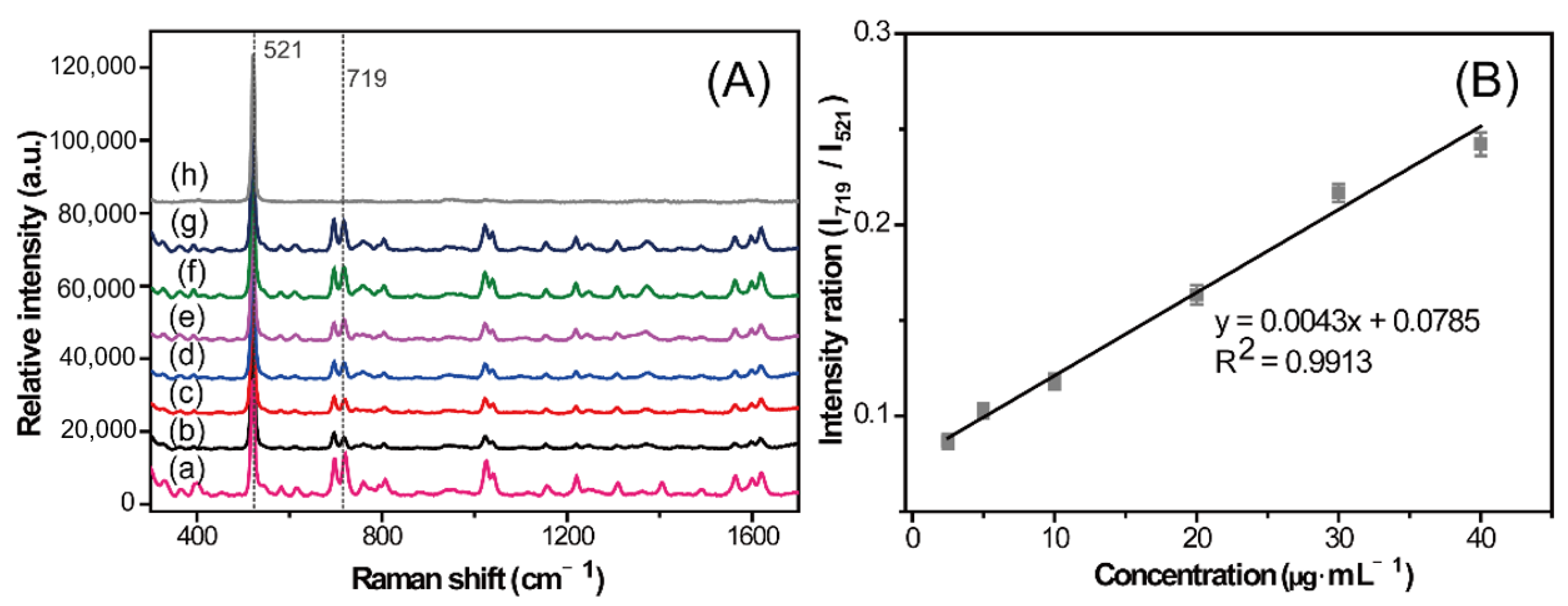
2.4. Detection of Simulated Samples
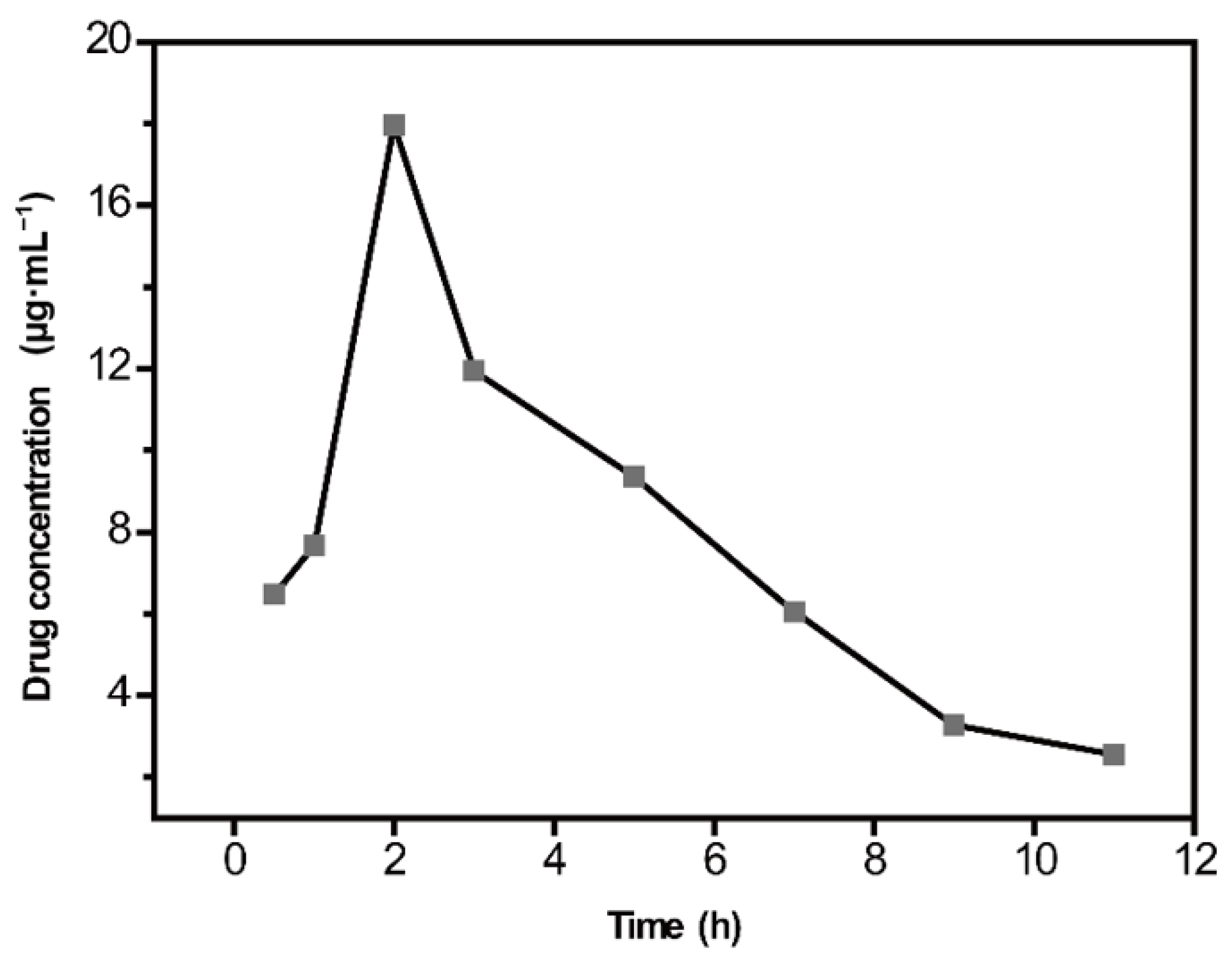
2.5. Quantitative Analysis of CBZ in Rat Serum
3. Materials and Methods
3.1. Chemical Reagents and Materials
3.2. Apparatus and Instruments
3.3. Animals and Treatment
3.4. Serum Collection and Sample Preparation
3.5. Nanoparticle Preparation and Treatment
3.6. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grześk, G.; Stolarek, W.; Kasprzak, M.; Grześk, E.; Rogowicz, D.; Wiciński, M.; Krzyżanowski, M. Therapeutic Drug Monitoring of Carbamazepine: A 20-Year Observational Study. J. Clin. Med. 2021, 10, 5396. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.M.J.; Spyker, D.A.; Brooks, D.E.; Fraser, M.O.; Banner, W. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin. Toxicol. 2016, 10, 1072–1252. [Google Scholar]
- Payette, A.; Ghannoum, M.; Madore, F.; Albert, M.; Troyanov, S.; Bouchard, J. Carbamazepine poisoning treated by multiple extracorporeal treatments. Clin. Nephrol. 2015, 83, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Alves, V.L.; Conceição, C.J.F.; Teixeira, H.M.; Câmara, J.S. Development of MEPS–UHPLC/PDA methodology for the quantification of clozapine, risperidone and their major active metabolites in human urine. Microchem. J. 2015, 123, 90–98. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, X.; Wu, Z.; Lu, F.; Yuan, Y. Antipsychotic drug poisoning monitoring of clozapine in urine by using coffee ring effect based surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2018, 1014, 64–70. [Google Scholar] [CrossRef]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, F.; Hamada, Y.; Kato, H.; Maruyama, T.; Kimura, T. Importance and Reality of TDM for Antibiotics Not Covered by Insurance in Japan. Int. J. Environ. Res. Public Health 2022, 19, 2516. [Google Scholar] [CrossRef]
- Knezevic, C.E.; Clarke, W. Cancer Chemotherapy: The Case for Therapeutic Drug Monitoring. Ther. Drug Monit. 2020, 42, 6–19. [Google Scholar] [CrossRef]
- Turzhitsky, V.; Zhang, L.; Horowitz, G.L.; Vitkin, E.; Khan, U.; Zakharov, Y.; Qiu, L.; Itzkan, I.; Perelman, L.T. Picoanalysis of Drugs in Biofluids with Quantitative Label-Free Surface-Enhanced Raman Spectroscopy. Small 2018, 14, 1802392. [Google Scholar] [CrossRef]
- Sultan, M.A.; Abou El-Alamin, M.M.; Wark, A.W.; Azab, M.M. Detection and quantification of warfarin in pharmaceutical dosage form and in spiked human plasma using surface enhanced Raman scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117533. [Google Scholar] [CrossRef] [PubMed]
- Szaniawska, A.; Kudelski, A. Applications of Surface-Enhanced Raman Scattering in Biochemical and Medical Analysis. Front. Chem. 2021, 9, 664134. [Google Scholar] [CrossRef] [PubMed]
- Trachta, G.; Schwarze, B.; Sägmüller, B.; Brehm, G.; Schneider, S. Combination of high-performance liquid chromatography and SERS detection applied to the analysis of drugs in human blood and urine. J. Mol. Struct. 2004, 693, 175–185. [Google Scholar] [CrossRef]
- Berger, A.G.; Restaino, S.M.; White, I.M. Vertical-flow paper SERS system for therapeutic drug monitoring of flucytosine in serum. Anal. Chim. Acta 2017, 949, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Lin, Y.; Zhao, X.; Gao, J.; Xie, S.; Gong, W.; Yu, Y.; Lin, J. Label-free surface enhanced Raman spectroscopy analysis of blood serum via coffee ring effect for accurate diagnosis of cancers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120605. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Sun, D.-W.; Pu, H. SERS detection of urea and ammonium sulfate adulterants in milk with coffee ring effect. Food Addit. Contam. Part A 2019, 36, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Mampallil, D.; Eral, H.B. A review on suppression and utilization of the coffee-ring effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef]
- Wen, J.T.; Ho, C.M.; Lillehoj, P.B. Coffee ring aptasensor for rapid protein detection. Langmuir ACS J. Surf. Colloids 2013, 29, 8440–8446. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef]
- Tang, S.; Li, Y.; Huang, H.; Li, P.; Guo, Z.; Luo, Q.; Wang, Z.; Chu, P.K.; Li, J.; Yu, X.-F. Efficient Enrichment and Self-Assembly of Hybrid Nanoparticles into Removable and Magnetic SERS Substrates for Sensitive Detection of Environmental Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 7472–7480. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, A.; Bai, S.; Ma, Y.; Su, Q. Surface-enhanced Raman spectra of medicines with large-scale self-assembled silver nanoparticle films based on the modified coffee ring effect. Nanoscale Res. Lett. 2014, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Feng, S.; Lin, J.; Zeng, Y.; Li, L.; Huang, Z.; Li, B.; Zeng, H.; Chen, R. Serum albumin and globulin analysis for hepatocellular carcinoma detection avoiding false-negative results from alpha-fetoprotein test negative subjects. Appl. Phys. Lett. 2013, 103, 204106. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Li, X.; Ong, L.; Soe, Y.G.; Sinsua, N.; Gras, S.L.; Tabor, R.F.; Wang, X.; Shen, W. Trace Analysis and Chemical Identification on Cellulose Nanofibers-Textured SERS Substrates Using the “Coffee Ring” Effect. ACS Sens. 2017, 2, 1060–1067. [Google Scholar] [CrossRef]
- Yang, L.; Li, P.; Liu, H.; Tang, X.; Liu, J. A dynamic surface enhanced Raman spectroscopy method for ultra-sensitive detection: From the wet state to the dry state. Chem. Soc. Rev. 2015, 44, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Z.; Meng, L.; Sun, Y.; Wang, J.; Yang, L.; Liu, J.; Tian, Z. Three-Dimensional and Time-Ordered Surface-Enhanced Raman Scattering Hotspot Matrix. J. Am. Chem. Soc. 2014, 136, 5332–5341. [Google Scholar] [CrossRef]
- Ge, M.; Li, P.; Zhou, G.; Chen, S.; Han, W.; Qin, F.; Nie, Y.; Wang, Y.; Qin, M.; Huang, G.; et al. General Surface-Enhanced Raman Spectroscopy Method for Actively Capturing Target Molecules in Small Gaps. J. Am. Chem. Soc. 2021, 143, 7769–7776. [Google Scholar] [CrossRef]
- Liu, J.; Pan, T.; Woolley, A.T.; Lee, M.L. Surface-Modified Poly(methyl methacrylate) Capillary Electrophoresis Microchips for Protein and Peptide Analysis. Anal. Chem. 2004, 76, 6948–6955. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, Y.; Lu, P.; Wang, L.; Zhang, X.; Chen, H.; Ma, P. Detection of carbamazepine in saliva based on surface-enhanced Raman spectroscopy. Biomed. Opt. Express 2021, 12, 7673. [Google Scholar] [CrossRef]
- Sackmann, M.; Materny, A. Surface enhanced Raman scattering (SERS)—A quantitative analytical tool? J. Raman Spectrosc. 2006, 37, 305–310. [Google Scholar] [CrossRef]
- Peksa, V.; Jahn, M.; Štolcová, L.; Schulz, V.; Proška, J.; Procházka, M.; Weber, K.; Cialla-May, D.; Popp, J. Quantitative SERS Analysis of Azorubine (E 122) in Sweet Drinks. Anal. Chem. 2015, 87, 2840–2844. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Tao, W.; Yu, B.; Du, Y. Quantitative analysis of thymine with surface-enhanced Raman spectroscopy and partial least squares (PLS) regression. Anal. Bioanal. Chem. 2010, 398, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lv, D.Y.; Zhu, Q.X.; Li, H.; Chen, H.; Wu, M.M.; Chai, Y.F.; Lu, F. Chromatographic separation and detection of contaminants from whole milk powder using a chitosan-modified silver nanoparticles surface-enhanced Raman scattering device. Food Chem. 2017, 224, 382–389. [Google Scholar] [CrossRef]
- Aarnoutse, P.J.; Westerhuis, J.A. Quantitative Raman Reaction Monitoring Using the Solvent as Internal Standard. Anal. Chem. 2005, 77, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, H.; Zhu, Q.; Liu, Y.; Lu, F. Analysis of low active-pharmaceutical-ingredient signal drugs based on thin layer chromatography and surface-enhanced Raman spectroscopy. J. Pharm. Biomed. Anal. 2016, 131, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Salkic, S.; Eckler, L.H.; Nee, M.J. Noninvasive monitoring of photocatalytic degradation of X-ray contrast media using Raman spectrometry. J. Raman Spectrosc. 2013, 44, 1746–1752. [Google Scholar] [CrossRef]
- Giovannozzi, A.M.; Rolle, F.; Sega, M.; Abete, M.C.; Marchis, D.; Rossi, A.M. Rapid and sensitive detection of melamine in milk with gold nanoparticles by Surface Enhanced Raman Scattering. Food Chem. 2014, 159, 250–256. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
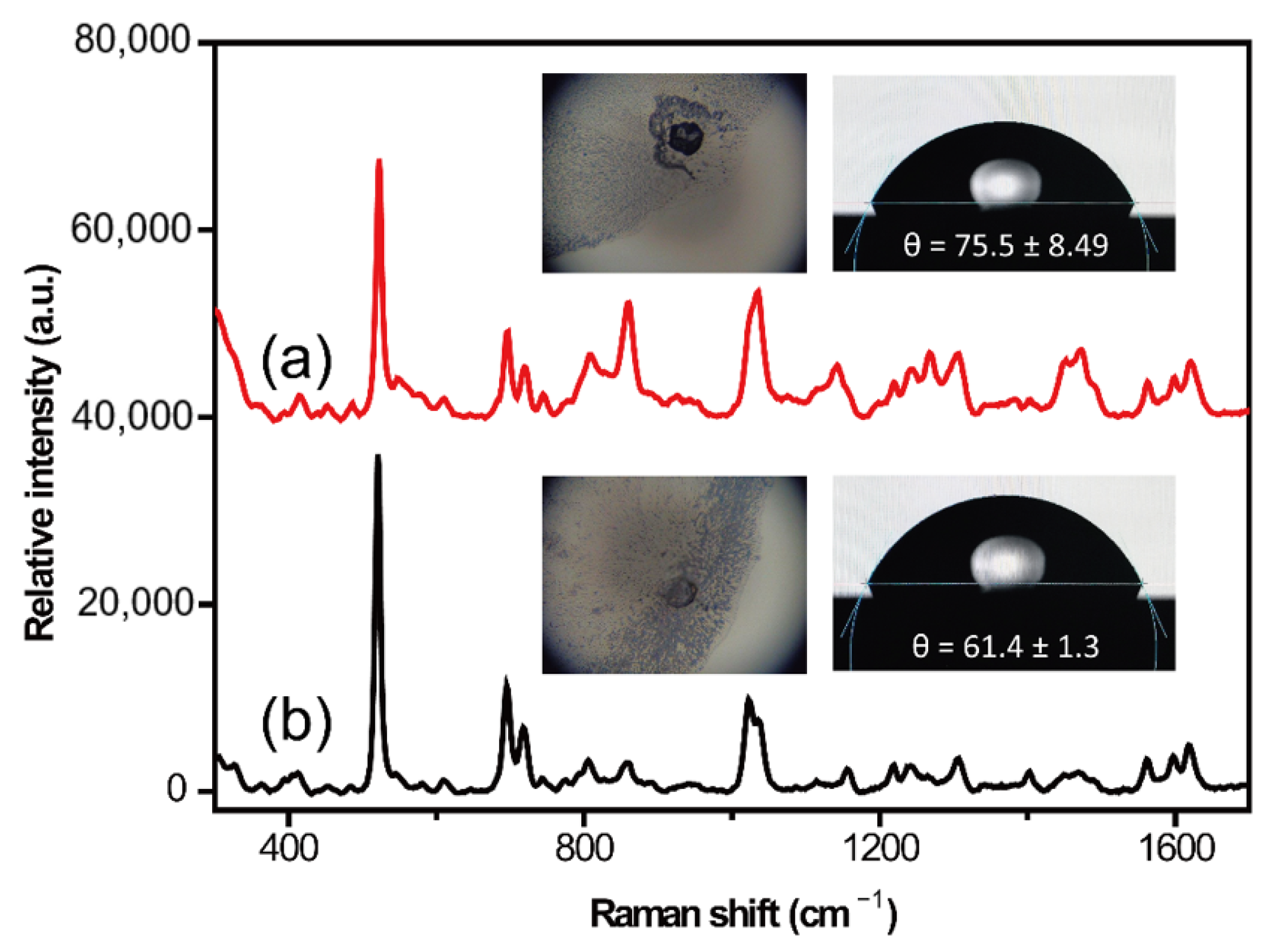

| Cleaning Fluid Number | Cleaning Fluid Composition | Volume Ratio | Incubation Time/Temperature | SERS Detection Performance |
|---|---|---|---|---|
| ① | H2SO4/H2O2 | 3:1 | 10min/75 °C | The edge of the droplet shrinks quickly, unable to successfully detect the signal |
| ② | H2O2/H2O | 1:1 | 10min/75 °C | The edge of the droplet shrinks quickly, unable to successfully detect the signal |
| ③ | NH4OH/H2O2/H2O | 1:1:5 | 10min/75 °C | Edge infiltration, unable to successfully detect the signal |
| ④ | HF/H2O | 1:50 | 15 s/25 °C | Edge infiltration, good SERS signal, noise interference |
| ⑤ | HCL/H2O | 1:6 | 10 min/75 °C | Edge infiltration, good SERS signal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Li, X.; Li, D.; Lu, F.; Zhao, Y.; Yuan, Y. A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy. Molecules 2023, 28, 128. https://doi.org/10.3390/molecules28010128
Zhu Q, Li X, Li D, Lu F, Zhao Y, Yuan Y. A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy. Molecules. 2023; 28(1):128. https://doi.org/10.3390/molecules28010128
Chicago/Turabian StyleZhu, Qingxia, Xinhang Li, Dan Li, Feng Lu, Yunli Zhao, and Yongfang Yuan. 2023. "A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy" Molecules 28, no. 1: 128. https://doi.org/10.3390/molecules28010128
APA StyleZhu, Q., Li, X., Li, D., Lu, F., Zhao, Y., & Yuan, Y. (2023). A Rapid Therapeutic Drug Monitoring Strategy of Carbamazepine in Serum by Using Coffee-Ring Effect Assisted Surface-Enhanced Raman Spectroscopy. Molecules, 28(1), 128. https://doi.org/10.3390/molecules28010128