Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection
Abstract
:1. Introduction
2. Chemical and Vibrational Properties of the CPs/GO Composites
2.1. Chemical and Vibrational Properties of GO/PIm Composites
2.2. Chemical and Vibrational Properties of GO/PPy Composites
2.3. Chemical and Vibrational Properties of GO/PEDOT Composites
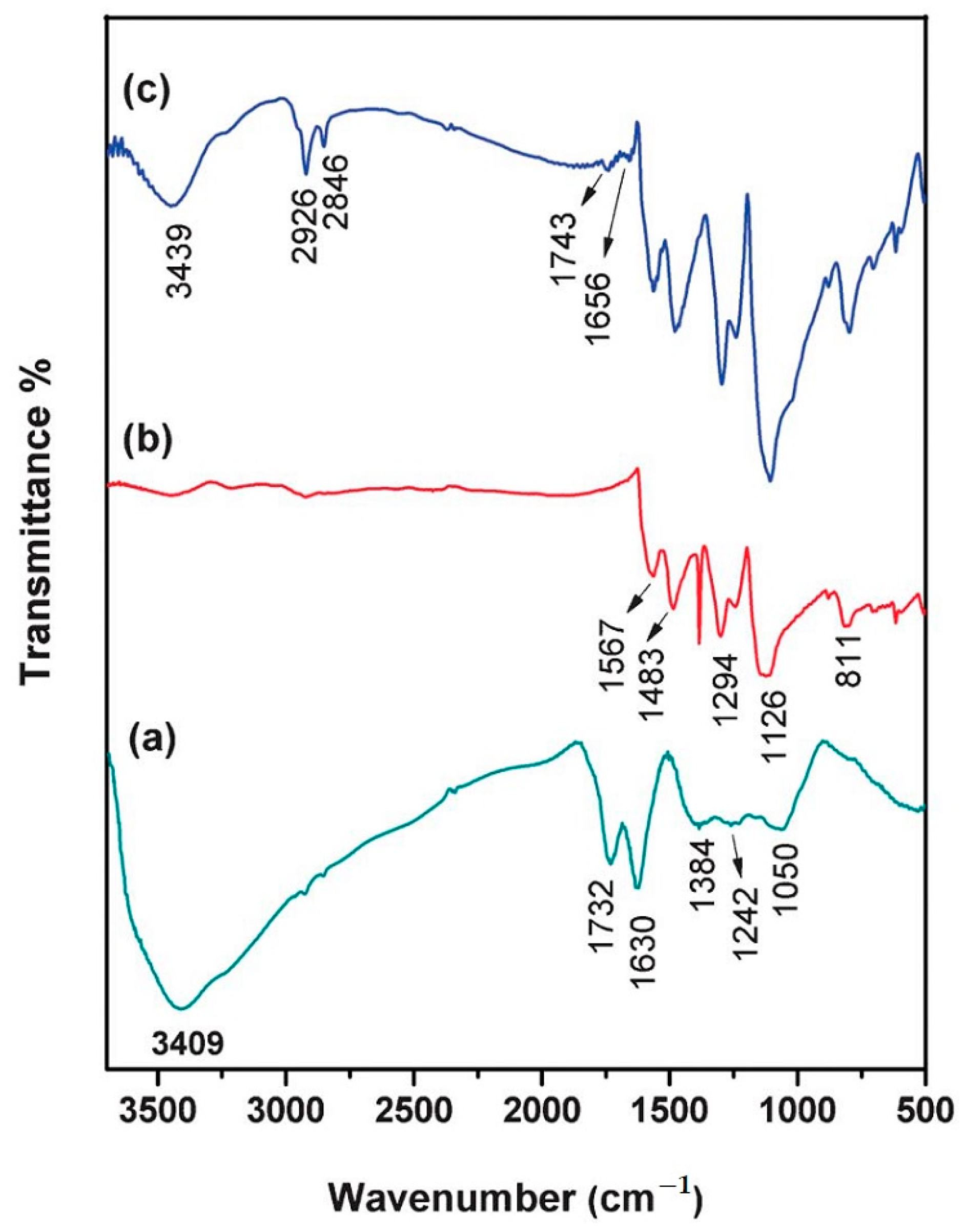
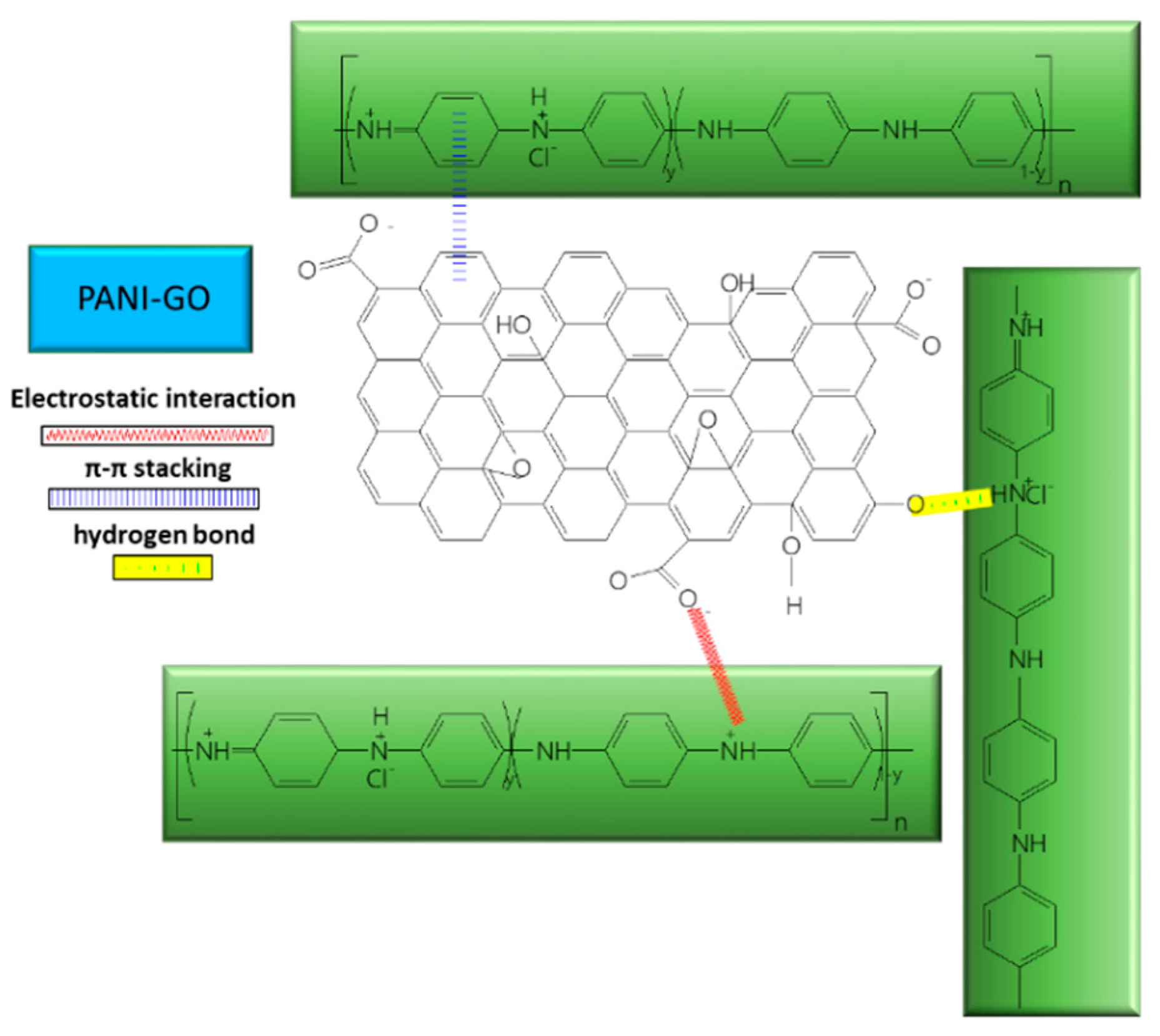
2.4. Chemical and Vibrational Properties of GO/PANI Composites
2.5. Overview of Sensorial Platforms Based on the CP/GO Composites
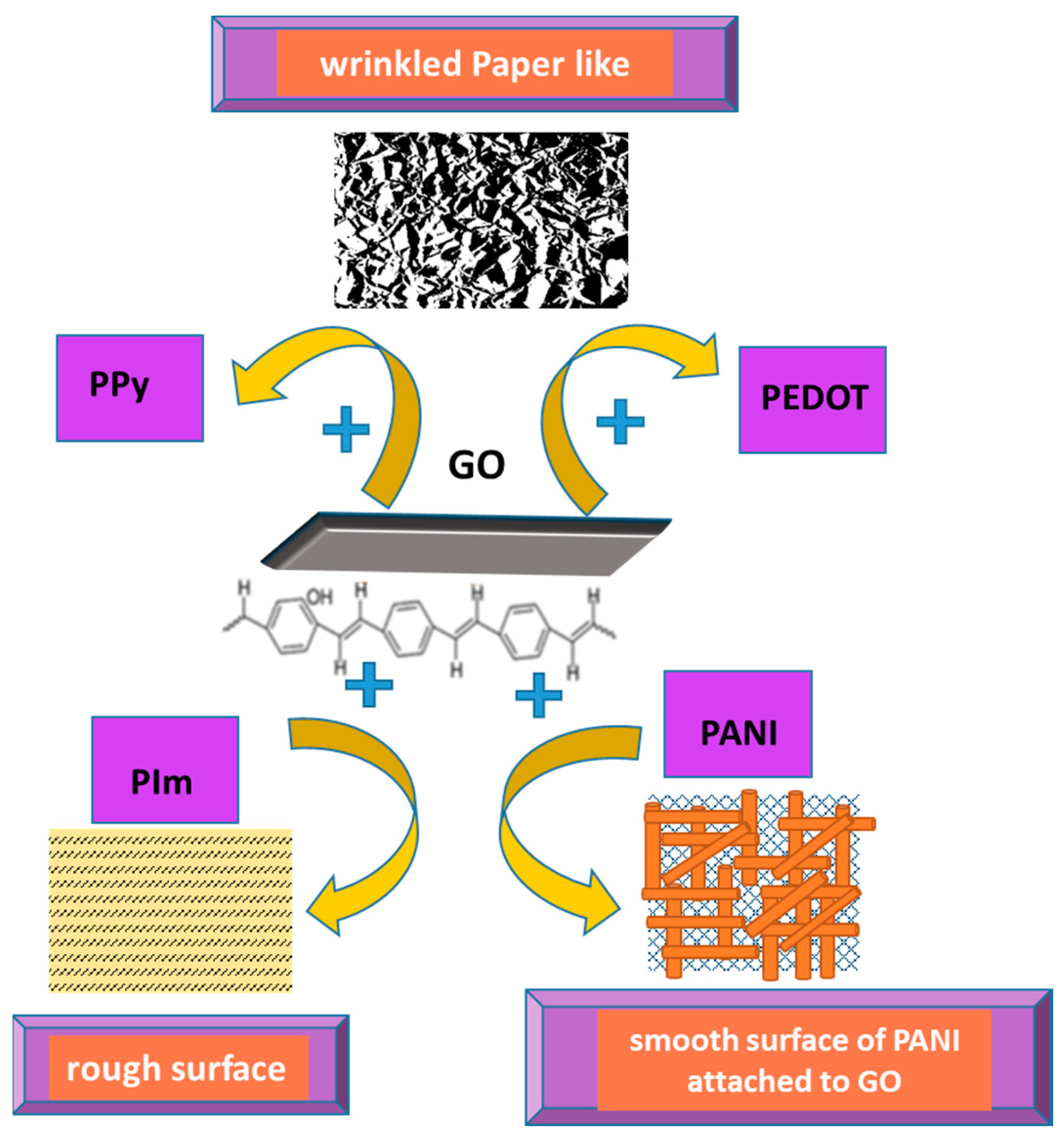
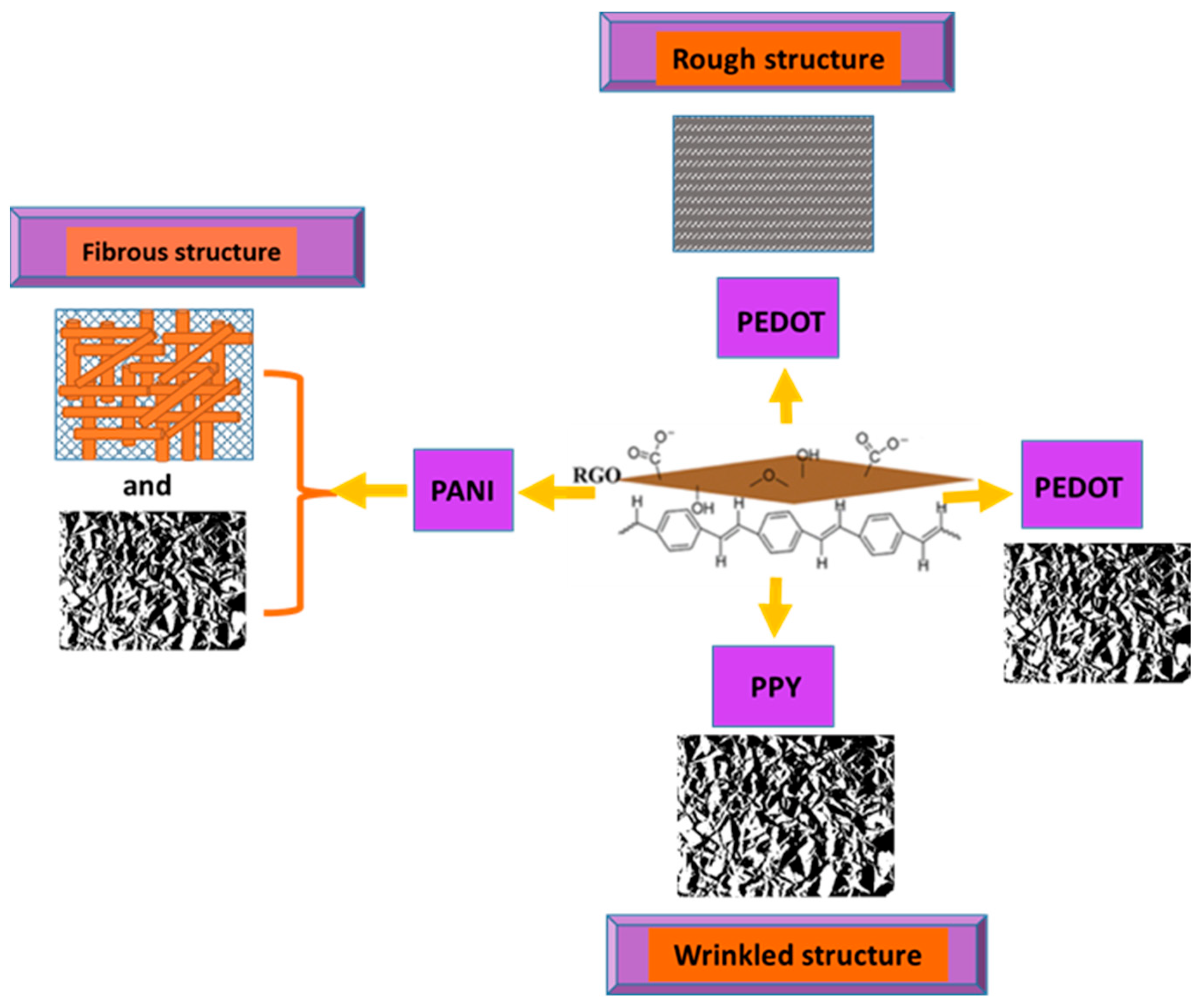
3. Chemical and Vibrational Properties of CPs/RGO
3.1. Synthesis and Interaction between RGO and PPy Inside the Composite
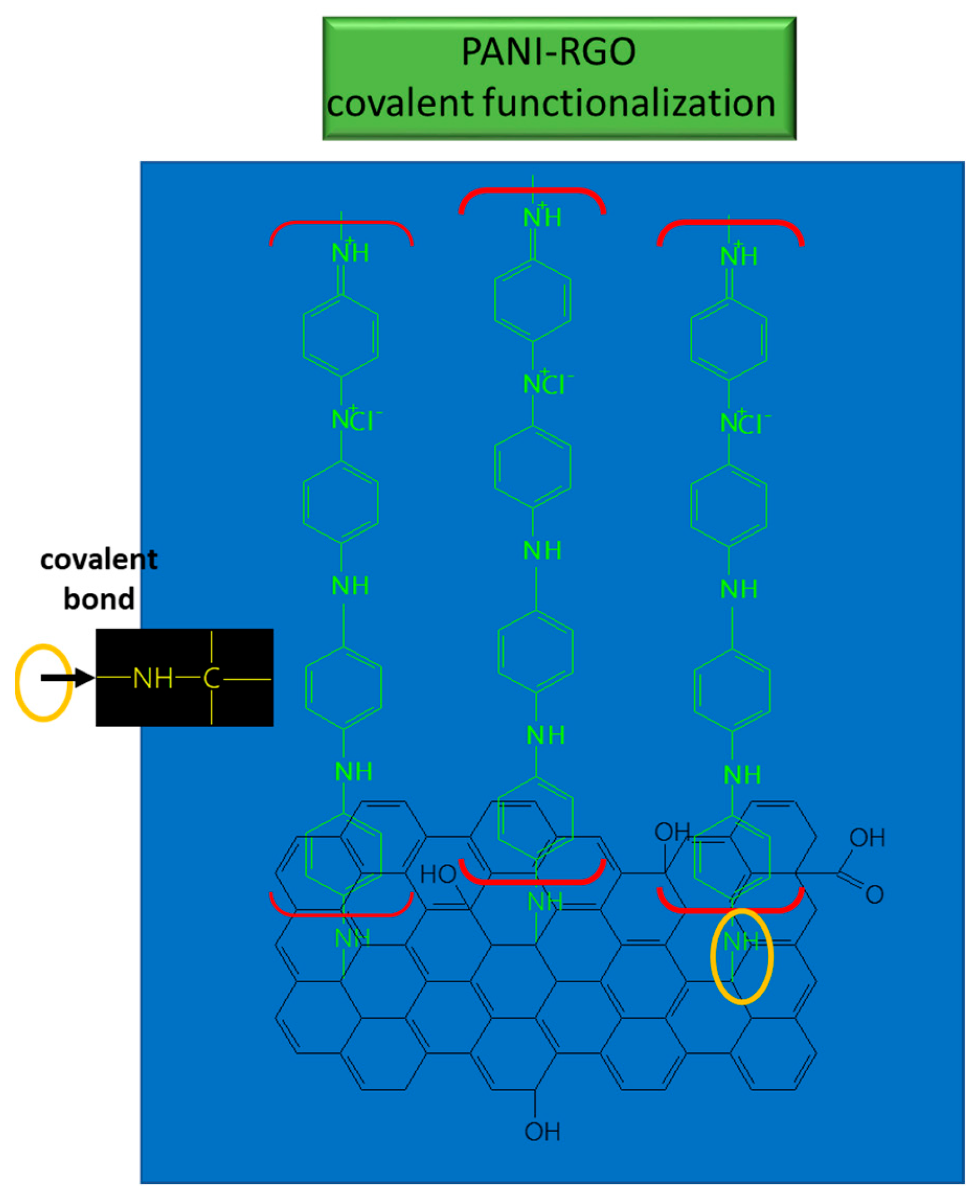
3.2. Chemical and Vibrational Properties of RGO/PANI Composites
3.3. Chemical and Vibrational Properties of RGO/PEDOT Composites
3.4. Overview concerning Sensorial Platforms Based on the CP/RGO Composites
4. Synthesis and Characteriz4. Chem4. Chemical and Vibrational Properties of CP/Graphene Quantum Dot (GQD) Composites
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shibasaki, K.; Kimura, M.; Ikarashi, R.; Yamaguchi, A.; Watanabe, T. Uric acid concentration in saliva and its changes with the patients receiving treatment for hyperuricemia. Metabolomics 2012, 8, 484–491. [Google Scholar] [CrossRef]
- Elangovan, A.; Sudha, K.; Jeevika, A.; Bhuvaneshwari, C.; Kalimuthu, P.; Balakumar, V. Construction of ternary Au@GO coupled with poly-L_ethionine nanocomposite as a robust platform for electrochemical recognition of uric acid in diabetic patients. Colloid Surf. A 2020, 602, 125050. [Google Scholar] [CrossRef]
- Kucherenko, I.; Soldatkin, O.; Kucherenko, D.; Soldatkina, O.; Dzyadevych, S. Advances in nanomaterial application in enzyme-based electrochemical biosensors: A review. Nanoscale Adv. 2019, 1, 4560–4577. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, K.; Moludi, M. Flower-like ZnO decorated polyaniline/reduced graphene oxide nanocomposites for simultaneous determination of dopamine and uric acid. Anal. Biochem. 2016, 512, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Wang, N.; Wang, D.; Zhang, H.; Ma, H.; Lin, M. Voltammetric uric acid sensor based on a glassy carbon electrode modified with a nanocomposite consisting of polytetraphenylporphyrin, polypyrrole, and graphene oxide. Microchim. Acta 2016, 183, 3053–3059. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold nanoparticles decorated polypyrrole/graphene oxide nanosheets as a modified electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. New J. Chem. 2020, 44, 4916–4926. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.; Mishra, A.; Shetti, N.; Malode, S.; Basu, S.; Naveen, S.; Raghu, A. Role of conducting polymer and metal oxide-based hybrids for applications in amperometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Huang, X.; Shi, W.; Li, J.; Bao, N.; Yu, C.; Gu, H. Determination of salivary uric acid by using poly(3,4-ethylenedioxythiophene) and graphene oxide in a disposable paper-based analytical device. Anal. Chim. Acta 2020, 1103, 75–83. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Wei, S.; Chen, S.; Ou, X.; Lu, Q. Overoxidized polyimidazole/graphene oxide copolymer modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid, guanine and adenine. Biosens. Bioelectron 2014, 57, 232–238. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Ma, W.; Chen, X.; Zhang, Y. Electrodeposited reduced graphene oxide incorporating polymerization of l-lysine on electrode surface and its application in simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Mat. Sci. Eng. C 2017, 70, 241–249. [Google Scholar] [CrossRef]
- He, S.; He, P.; Zhang, X.; Zhang, X.; Liu, K.; Jia, L.; Dong, F. Poly(glycine)/graphene oxide modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of dopamine, uric ac-id, guanine and adenine. Anal. Chim. Acta 2018, 2016, 75–82. [Google Scholar] [CrossRef]
- Arulraj, A.; Arunkumar, A.; Vijayan, M.; Viswanath, K.; Vasantha, V. A simple route to develop highly porous nanopolypyrrole/reduced graphene oxide composite film for selective determination of dopamine. Electrochim. Acta 2016, 206, 77–85. [Google Scholar] [CrossRef]
- Pihel, K.; Walker, Q.; Wightman, R. Overoxidized polypyrrole-coated carbon fiber microelectrodes for dopamine measurements with fast-scan cyclic voltammetry. Anal. Chem. 1996, 68, 2084–2089. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Lu, L.; Wu, L.; Zhang, K.; Nie, T.; Zhu, F.; Wu, Y. Overoxidized polypyrrole/graphene nanocomposite with good electrochemical performance as novel electrode material for the detection of adenine and guanine. Biosens. Bioelectron. 2014, 62, 261–267. [Google Scholar] [CrossRef]
- Beck, F.; Braun, P.; Oberst, M. Organic electrochemistry in the solid state-overoxidation of polypyrrole. Ber Bunsenges. Phys. Chem. 1987, 91, 967–974. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Development of a PrGO-modified electrode for uric acid determination in the presence of ascorbic acid by an electrochemical technique. Sensors 2017, 17, 1539. [Google Scholar] [CrossRef] [Green Version]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells–an overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Gueye, M.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure, V.; Poget, S.; Rieutord, F.; Okuno, H.; Be-nayad, A.; et al. Structure and dopant engineering in PEDOT thin films: Practical tools for a dramatic conductivity enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef]
- Vreeland, R.; Atcherley, C.; Russell, W.; Xie, J.; Lu, D.; Laude, N.; Porreca, F.; Heien, M. Biocompatible PEDOT:Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. Anal. Chem. 2015, 87, 2600–2607. [Google Scholar] [CrossRef]
- Yang, G.; Kampstra, K.; Abidian, M. High performance conducting polymer nanofiber biosensors for detection of biomolecules. Adv. Mater. 2014, 26, 4954–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.; Xiao, Q.; Liu, H.; Li, C.; Chen, S.; Wang, C.; Chiu, K.; Chen, N.; Tu, Y.; Ramakrishna, S.; et al. Bionanotube/poly(3,4-ethylenedioxythiophene) nanohybrid as an electrode for the neural interface and dopamine sensor. ACS Appl. Mater. Interfaces 2019, 11, 18254–18267. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Hamdan, K.S.; Rahim, N.A. Towards the Conducting Polymer Based Catalysts to Eliminate Pt for Dye Sensitized Solar Cell Applications. In Advances in Hybrid Conducting Polymer Technology; Engineering Materials; Shahabuddin, S., Pandey, A.K., Khalid, M., Jagadish, P., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 311–326. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, R.; Chai, Y.; Chen, S.; Hu, F.; Zhang, M. Simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan on gold nanoparticles/overoxidized-polyimidazole composite modified glassy carbon electrode. Anal. Chim. Acta 2012, 741, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.; Narayan, T.; Solanki, S.; Malhotra, B. Recent Advances of conducting polymers and their composites for electrochemical biosensing applications. J. Funct. Biomater. 2020, 11, 71. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuat. B-Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Minta, D.; Moyseowicz, A.; Gryglewicz, S.; Gryglewicz, G. A promising Electrochemical Platform for dopamine and uric acid detection based on a a polyaniline/iron oxide-tin oxide/reduced graphene oxide ternary composite. Molecules 2020, 25, 5869. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Cai, J.; Yang, T.; Chen, J.; Hou, X. Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens. Actuat. Chem-B 2019, 298, 126872. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arikan, K.; Yilmaz, M.; Savk, A.; Calimli, M.; Nas, M.; Atalar, M.; Alma, M.; et al. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef] [Green Version]
- Yola, M.L.; Atar, N. Functionalized graphene quantum dots with bi-metallic nanoparticles composite: Sensor application for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. J. Electrochem. Soc. 2016, 163, B718–B725. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An electrochemical avenue to green luminescent graphene quantum dots as potential electron acceptors for photovoltaics. Adv. Mater. 2011, 23, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Gan, T.; Hu, C.; Sun, Z.; Hu, S. Facile synthesis of water-soluble fullerene-graphene oxide composites for electrodeposition of phosphotungstic acid-based electrocatalysts. Electrochim. Acta 2013, 111, 738–745. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct Electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef]
- Smaranda, I.; Benito, A.M.; Maser, W.K.; Baltog, I.; Baibarac, M. Electrochemical grafting of reduced graphene oxide with polydiphenylamine doped with heteropolyanions and its optical properties. J. Phys. Chem. C 2014, 118, 25704. [Google Scholar] [CrossRef]
- Baibarac, M.; Ilie, M.; Baltog, I.; Lefrant, S.; Humbert, B. Infrared dichroism studies and anisotropic photoluminescence properties of poly(para-phenylene vinylene) functionalized reduced graphene oxide. RSC Adv. 2017, 7, 6931–6942. [Google Scholar] [CrossRef] [Green Version]
- Baibarac, M.; Daescu, M.; Fejer, S.N. Adsorption of 1, 4-phenylene diisocyanate onto the graphene oxide sheets functionalized with polydiphenylamine in doped state. Sci. Rep. 2019, 9, 11968. [Google Scholar] [CrossRef] [Green Version]
- Baibarac, M.; Daescu, M.; Socol, M.; Bartha, C.; Negrila, C.; Fejer, S.N. Influence of reduced graphene oxide on the electropolymerization of 5-amino-1-naphthol and the interaction of 1, 4-phenylene diisothiocyanate with the poly(5-amino-1-naphtol)/reduced graphene oxide composite. Polymer 2020, 12, 1299. [Google Scholar] [CrossRef]
- Lakshmi, D.; Whitcombe, M.; Davis, F.; Sharma, P.; Prasad, B.B. Electrochemical Detection of uric acid in mixed and clinical samples: A review. Electroanal 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Feng, X.-M.; Li, R.-M.; Ma, Y.-W.; Chen, R.-F.; Shi, N.-E.; Fan, Q.-L.; Huang, W. One-step electrochemical synthesis of graphene/polyaniline composite film and its applications. Adv. Funct. Mater. 2011, 21, 2989–2996. [Google Scholar] [CrossRef]
- Lim, C.; Chua, C.; Pumera, M. Detection of biomarkers graphene nanoplatelets and nanoribbons. Analyst 2014, 139, 1072–1080. [Google Scholar] [CrossRef]
- Zhan, H.; Li, J.; Liu, Z.; Zheng, Y.; Jing, Y. A highly sensitive electrochemical OP biosensor based on electrodeposition of Au–Pd bimetallic nanoparticles onto a functionalized graphene modified glassy carbon electrode. Anal. Methods 2015, 7, 3903–3911. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Arotiba, A.O. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid. Interf. Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanwatanakul, S. Simultaneous determination of ascorbic acid, dopamine and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuat. B-Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Tian, J.; Deng, S.; Li, L.; Shan, D.; He, W.; Zhang, J.; Shi, Y. Bioinspired polydopamine as the scaffold for the active AuNPs anchoring and the simultaneously reduced graphene oxide: Characterization and the enhanced biosensing application. Biosens. Bioelectron. 2013, 49, 466–471. [Google Scholar] [CrossRef]
- Raj, V.; Madheswari, D.; MubarakAli, M. Chemical synthesis, characterization, and properties of conducting copolymers of imidazole and pyridine. J. Appl. Polym. Sci. 2012, 124, 1649–1658. [Google Scholar] [CrossRef]
- Yang, J.; Ramaraj, B.; Yoon, K. Preparation and characterization of superparamagnetic graphene oxide nanohybrids anchored with Fe3O4 nanoparticles. J. Alloys. Compd. 2014, 583, 128–133. [Google Scholar] [CrossRef]
- Xu, C.; Sun, J.; Gao, L. Synthesis of novel hierarchical graphene/polypyrrole nanosheet composites and their superior electrochemical performance. J. Mater. Chem. 2011, 21, 11253–11258. [Google Scholar] [CrossRef]
- Kumar, G.; Kirubaharan, C.; Udhayakumar, S.; Ramachandran, K.; Karthikeyan, C.; Renganathan, R.; Nahm, K. Synthesis, structural, and morphological characterizations of reduced graphene oxide-supported polypyrrole anode catalysts for improved microbial fuel cell performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. [Google Scholar] [CrossRef]
- Harpale, K.; Bansode, S.; More, M. One-pot synthesis, characterization, and field emission investigations of composites of polypyrrole with graphene oxide, reduced graphene oxide, and graphene nanoribbons. J. Appl. Polym. Sci. 2017, 134, 1–8. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Paulmony, T. Evaluation of a new biosensor based on in situ synthesized pPy-Ag-PVP nanohybrid for selective detection of dopamine. J. Phys. Chem. B 2017, 121, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Shao, D.; Wang, X. Graphene oxide/polypyrrole composites for highly selective enrichment of U (VI) from aqueous solutions. Polym. Chem. 2014, 5, 6207–6215. [Google Scholar] [CrossRef]
- Bora, C.; Dolui, S. Fabrication of polypyrrole/graphene oxide nanocomposites by liqui/liqui interfacial polymerization and evaluation of their optical, electrical and electrochemical properties. Polymer 2012, 53, 923–932. [Google Scholar] [CrossRef]
- Han, H.; Lee, H.; You, J.; Jeong, H.; Jeon, S. Electrochemical biosensor for simultaneous determination of dopamine and serotonin based on electrochemically reduced GO-porphyrin. Sens. Actuat. B-Chem. 2014, 190, 886–895. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Guo, B.; Cheng, S.; Wang, F. Electrochemical investigation of a metalloporphyrin-graphene composite modified electrode and its electrocatalysis on ascorbic acid. J. Electroanal. Chem. 2016, 760, 105–112. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Silke, M.; Qiu, W.; Xu, M.; Borghs, G.; Chen, H. Electrochemical deposition of polypyrrole/graphene oxide composite on microelectrodes towards tuning the electrochemical properties of neural probes. Sens. Actuat. B-Chem. 2011, 158, 176–184. [Google Scholar] [CrossRef]
- Lu, G.; Zhou, M.; Lu, J.; Li, Z. Raman spectrum of mesosubstituted tetraphenylporphyrins. J. Jilin Univ. 2008, 2, 358–360. Available online: http://xuebao.jlu.edu.cn/lxb/EN/Y2008/V46/I02/358 (accessed on 1 November 2022).
- Li, D.; Liu, M.; Zhan, Y.; Su, Q.; Zhang, Y.; Zhang, D. Electrodeposited poly(3,4-ethylenedioxythiophene) doped with graphene oxide for the simultaneous voltametric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2020, 187, 94. [Google Scholar] [CrossRef]
- Azman, N.; Lim, H.; Sulaiman, Y. Effect of electropolymerization potential on the preparation of PEDOT/graphene oxide hybrid material for supercapacitor application. Electrochim. Acta 2016, 188, 785–792. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, O.; Xu, J.; Wen, T.; Duan, X.; Yu, H.; Wu, L.; Nie, T. A facile one-step redox route for the synthesis of graphene/poly (3,4-ethylenedioxythiophene) nanocomposite and their applications in biosensing. Sens. Actuat. Chem-B 2013, 181, 567–574. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef] [PubMed]
- Manivel, P.; Dhakshnamoorthy, M.; Balamurugan, A.; Ponpandian, N.; Mangalaraja, D.; Viswanathan, C. Conducting polyaniline-graphene oxide fibrous nanocomposites: Preparation, characterization and simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2013, 3, 14428–14437. [Google Scholar] [CrossRef]
- Chen, G.; Shau, S.; Juang, T.; Lee, R.; Chen, C.; Suen, S.; Jeng, R. Single-layered graphene oxide nanosheet/polyaniline hybrids fabricated through direct molecular exfoliation. Langmuir 2011, 27, 14563–14569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl. Mater. Interfaces 2010, 2, 821–828. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, S.; Ding, Y.; Zhou, L.; Zhang, Z.; Li, L. Electrooxidation and determination of cefotaxime on Au nanoparticles/poly(L-arginine) modified carbon paste electrode. J. Electroanal. Chem. 2013, 698, 25–30. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited‖. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Prasert, K.; Sutthibutpong, T. Unveiling the fundamental mechanisms of graphene oxide selectivity on the ascorbic acid, dopamine, and uric acid by Density Functional Theory Calculations and Charge Population Analysis. Sensors 2021, 21, 2773. [Google Scholar] [CrossRef]
- Domancich, N.; Fernandez, A.; Meier, L.; Fuente, S.; Castellani, N. DFT study of graphene oxide reduction by a dopamine species. Mol. Phys. 2020, 118, e1637029. [Google Scholar] [CrossRef]
- Pandey, R.; Lakshminarayanan, V. Electro-oxidation of formic acid, methanol, and ethanol on electrodeposited Pd-polyaniline nanofiber films in acidic and alkaline medium. J. Phys. Chem. C 2009, 113, 21596–21603. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Ma, W.; Yang, T.; Zhang, Y.; Zhang, D. Preparation of a glassy carbon electrode modified with reduced graphene oxide and overoxidized electropolymerized polypyrrole, and its application to the determination of dopamine in the presence of ascorbic acid and uric acid. Microchim. Acta 2019, 186, 407. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Electrodeposition of poly(3,4-ethylenedioxytiophene)/reduced graphene oxide/manganese dioxide for simultaneous detection of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2018, 820, 74–81. [Google Scholar] [CrossRef]
- Tiwari, I.; Gupta, M.; Pandey, C.; Mishra, V. Gold nanoparticle decorated graphene sheet-polypyrrole based nanocomposite: Its synthesis, characterization and genosensing application. Dalton Trans. 2015, 44, 15557–15566. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cai, X.; Wang, X.; Gao, C.; Liu, S.; Gao, F.; Wang, Q. Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode. Sens. Actuat B-Chem. 2013, 186, 380–387. [Google Scholar] [CrossRef]
- Li, F.; Chai, J.; Yang, H.; Han, D.; Niu, L. Synthesis of Pt/ionic liquid/graphene nanocomposite and its simultaneous determination of ascorbic acid and dopamine. Talanta 2010, 81, 1063–1068. [Google Scholar] [CrossRef]
- Rahman, M.; Lopa, N.; Kim, K.; Lee, J.-J. Selective detection of L-tyrosine in the presence of ascorbic acid, dopamine, and uric acid at poly (thionine)-modified glassy carbon electrode. J. Electroanal. Chem. 2015, 754, 87–93. [Google Scholar] [CrossRef]
- Li, X.-B.; Rahman, M.; Xu, G.-R.; Lee, J.-J. Highly sensitive and selective detection of dopamine at poly (chromotrope 2B)-modified glassy carbon electrode in the presence of uric acid and ascorbic acid. Electrochim. Acta 2015, 173, 440–447. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.; Li, F.; Xu, B.; Yang, Y.; Liu, S.; Sun, Z. Chitosan-assisted fabrication and electrocatalytic activity of the composite film electrode of heteropolytungstate/carbon nanotubes. Electrochim. Acta 2010, 55, 1523–1527. [Google Scholar] [CrossRef]
- Juan, L.; Xiaoli, Z. Fabrication of poly(aspartic acid)-Nanogold modified electrode and its application for simultaneous determination of dopamine, ascorbic acid, and uric acid. Am. J. Anal. Chem. 2012, 3, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Silva, M.; Corona-Avendano, S.; Alarcon-Angeles, G.; Palomar-Parave, M.; Romero-Romo, M.; Rojas-Hernandez, A. Construction of Supramolecular Systems for the selective and quantitative determination of dopamine in the presence of ascorbic acid. Procedia Chem. 2014, 12, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Gu, S.; Ding, Y.; Zhang, Z.; Li, L. A novel sensor based on electropolymerization of beta-cyclodextrin and L-arginine on carbon paste electrode for determination of fluoroquinolones. Anal. Chim. Acta 2013, 770, 53–61. [Google Scholar] [CrossRef]
- Qin, Q.; Bai, X.; Hua, Z. Electropolymerization of a conductive β-cyclodextrine polymer on reduced graphene oxide modified screen-printed electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2016, 782, 50–58. [Google Scholar] [CrossRef]
- Wan, M.; Li, M.; Li, J.; Liu, Z. Structure and electrical properties of the oriented polyaniline films. J. Appl. Polym. Sci. 1994, 53, 131–139. [Google Scholar] [CrossRef]
- Andrés-Vergés, M.; Serna, C. Morphological characterization of ZnO powders by X-ray and IR spectroscopy. J. Mater. Sci. Lett. 1988, 7, 970–972. [Google Scholar] [CrossRef]
- Domingues, H.; Salvatierr, R.V.; Oliveira, M.; Zarbin, A. Transparent and conductive thin films of graphene/polyaniline nanocomposites prepared through interfacial polymerization. Chem. Commun. 2011, 47, 2592–2594. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, A.; Botas, C.; ÁIvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndezb, R.; Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Roy, P.; Okajima, T.; Ohsaka, T. Simultaneous electrochemical detection of uric acid and ascorbic acid at a poly (N,N-dimethylaniline) film-coated GC electrode. J. Electroanal. Chem. 2004, 561, 75–82. [Google Scholar] [CrossRef]
- Sheng, Z.; Zheng, X.; Xu, J.; Bao, W.J.; Wang, F.; Xia, X. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef]
- Tang, D.; Liu, J.; Yan, X.; Kang, L. Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Adv. 2016, 6, 50609–50617. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamo, M.; Galvagno, S.; Romeo, R.; Giofre, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Dong, P.; Jiang, B.P.; Liang, W.Q.; Huang, Y.; Shi, Z.; Shen, X.C. Synthesis of white-light-emitting graphene quantum dots via a one step reduction and their interfacial characteristics-dependent luminescence properties. Inorg. Chem. Front 2017, 4, 712–718. [Google Scholar] [CrossRef]
- Vazquez-Nakagawa, M.; Rodriguez-Perez, L.; Herranz, M.A.; Martin, N. Chirality transfer from graphene quantum dots. Chem. Commun. 2016, 52, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, S.P.; Marković, Z.M.; Syrgiannis, Z.; Dramićanin, M.D.; Arcudi, F.; Parola, V.L.; Budimir, M.D.; Marković, B.M.T. Enhancing photoluminescence of graphene quantum dots by thermal annealing of the graphite precursor. Mater. Res. Bull. 2017, 93, 183–193. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Du, P.; Feng, X.; Wu, P.; Cai, C. Electrolyzing synthesis of boron-doped graphene quantum dots for fluorescence determination of Fe3+ ions in water samples. Talanta 2017, 164, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Wang, Y.; Wang, J.; Liu, H.; Liu, X.; Wang, L.; Liu, X.; Xue, W.; Ma, N. Electrochemical synthesis of phosphorus-doped graphene quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. [Google Scholar] [CrossRef]
- Jovanovic, S.P.; Syrgiannis, Z.; Markovic, Z.M.; Bonasera, A.; Kepic, D.P.; Budimir, M.D.; Milivojevic, D.D.; Spasojevic, V.D.; Dramicanin, M.D.; Pavlovic, V.B.; et al. Modification of structural and luminescence properties of graphene quantum dots by gamma irradiation and their application in a photodynamic therapy. ACS. Appl. Mater. Interf. 2015, 7, 25865–25874. [Google Scholar] [CrossRef]
- Prasad, B.B.; Kumar, A.; Singh, R. Synthesis of novel monomeric graphene quantum dots and corresponding nanocomposite with molecularly imprinted polymer for electrochemical detection of an anticancerous ifosfamide drug. Biosens. Bioelectron. 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Gao, H.; Xue, C.; Hu, G.; Zhu, K. Production of graphene quantum dots by ultrasound-assisted exfoliation in supercritical CO2/H2O medium. Ultrason. Sonochem. 2017, 37, 120–127. [Google Scholar] [CrossRef]
- Zuo, W.; Tang, L.; Xiang, J.; Ji, R.; Luo, L.; Rogée, L.; Lau, P.S. Functionalization of graphene quantum dots by fluorine: Preparation, properties, application, and their mechanisms. Appl. Phys. Lett. 2017, 110, 221901. [Google Scholar] [CrossRef]
- Hassan, M.; Haque, E.; Reddy, K.R.; Minett, A.I.; Chen, J.; Gomes, V.G. Edge-enriched graphene quantum dots for enhanced photo-luminescence and supercapacitance. Nanoscale 2014, 6, 11988–11994. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Y.; Shi, C.; Pei, Y.T. Large-scale synthesis of defect selective graphene quantum dots by ultrasonic-assisted liquid-phase exfoliation. Carbon 2016, 109, 373–383. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; et al. Green, Rapid, and Universal Preparation Approach of Graphene Quantum Dots under Ultraviolet Irradiation. ACS. Appl. Mater. Interf. 2017, 9, 14470–14477. [Google Scholar] [CrossRef]
- Park, B.; Kim, S.J.; Sohn, J.S.; Nam, M.S.; Kang, S.; Jun, S.C. Surface plasmon enhancement of photoluminescence in photochemically synthesized graphene quantum dot and Au nanosphere. Nano. Res. 2016, 9, 1866–1875. [Google Scholar] [CrossRef]
- Sapkota, B.; Benabbas, A.; Lin, H.G.; Liang, W.; Champion, P.; Wanunu, M. Peptide-Decorated Tunable-Fluorescence Graphene Quantum Dots. ACS. Appl. Mater. Interf. 2017, 9, 9378–9387. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Khandelwal, P.; Das, R.; Salunke, G.; Alam, A.; Ghorai, S.; Chattopadhyay, S.; Poddar, P. Oxidant mediated one step complete conversion of multi-walled carbon nanotubes to graphene quantum dots and their bioactivity against mammalian and bacterial cells. J. Mater. Chem. B. 2017, 5, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Khatun, Z.; Nafiujjaman, M.; Lee, D.G.; Lee, Y.K. Surface coating of graphene quantum dots using mussel-inspired polydopamine for biomedical optical imaging. ACS. Appl. Mater. Interf. 2013, 5, 8246–8253. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; SafardoustHojaghan, H. Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J. Mol. Liq. 2017, 242, 447–455. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Ahmed, B.; Kumar, A.; Das, J.; Materny, A. Tunable (violet to green) emission by high-yield graphene quantum dots and exploiting its unique properties towards sun-light driven photocatalysis and supercapacitor electrode materials. Mater. Today. Comm. 2017, 11, 7. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Analytical methodology for the electrocatalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 2017, 174, 243–255. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Zhao, C. Microwave-assisted synthesis of nitrogen-doped multi-layer graphene quantum dots with oxygen- rich functional groups. Aust. J. Chem. 2016, 69, 357. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, P.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Liu, F.; Sun, Y.; Zheng, Y.; Tang, N.; Li, M.; Zhong, W.; Du, Y. Gram-scale synthesis of high-purity graphene quantum dots with multicolor photoluminescence. RSC Adv. 2015, 5, 103428. [Google Scholar] [CrossRef]
- Kalita, H.; Palaparthy, V.S.; Baghini, M.S.; Aslam, M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon 2020, 165, 9–17. [Google Scholar] [CrossRef]
- Hu, H.; Ma, X.Y.; Tian, J.; Huang, Z. Rapid and facile synthesis of graphene quantum dots with high antioxidant activity. Inorg. Chem. Commun. 2020, 122, 108288. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Via, B.K. Facile synthesis of soluble graphene quantum dots and its improved property in detecting heavy metal ions. Colloid Surf. B 2014, 118, 72. [Google Scholar] [CrossRef]
- Hsu, W.F.; Wu, T.M. Electrochemical sensor based on conductive polyaniline coated hollow tin oxide nanoparticles and nitrogen doped graphene quantum dots for sensitively detecting dopamine. J. Mater. Sci. Mater Electron. 2019, 30, 8449–8456. [Google Scholar] [CrossRef]
- Lim, H.C.; Jang, S.J.; Cho, Y.; Cho, H.; Venkataprasad, G.; Vinothkumar, V.; Shin, I.S.; Kim, T.H. Graphene quantum dot-doped PEDOT for simultaneous determination of ascorbic acid, dopamine and uric acid. Chem. Electro. Chem. 2022, 9, e20220557. [Google Scholar] [CrossRef]
- Baltog, I.; Baibarac, M.; Lefrant, S.; Mevellec, J.Y. Electrochemical functionalisation of SWNTs with poly(3,4-ethylenedioxythiophene) evidenced by anti-Stokes/Stokes Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 303–312. [Google Scholar] [CrossRef]
- Culebras, M.; Gómez, C.M.; Cantarero, A. Enhanced thermoelectric performance of PEDOT with different counter-ions optimized by chemical reduction. J. Mater. Chem. A 2014, 2, 10109–10115. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, T.; Xi, J.; Wang, D.; Shuai, Z. Unravelling Doping Effects on PEDOT at the Molecular Level: From Geometry to Thermoelectric Transport Properties. J. Am. Chem. Soc. 2015, 137, 12929–12938. [Google Scholar] [CrossRef]
- Cai, J.; Sun, B.; Gou, X.; Gou, Y.; Li, W.; Hu, F. A novel way for analysis of calycosin via polyaniline functionalized graphene quantum dots fabricated electrochemical sensor. J. Electroanal. Chem. 2018, 816, 123–131. [Google Scholar] [CrossRef]
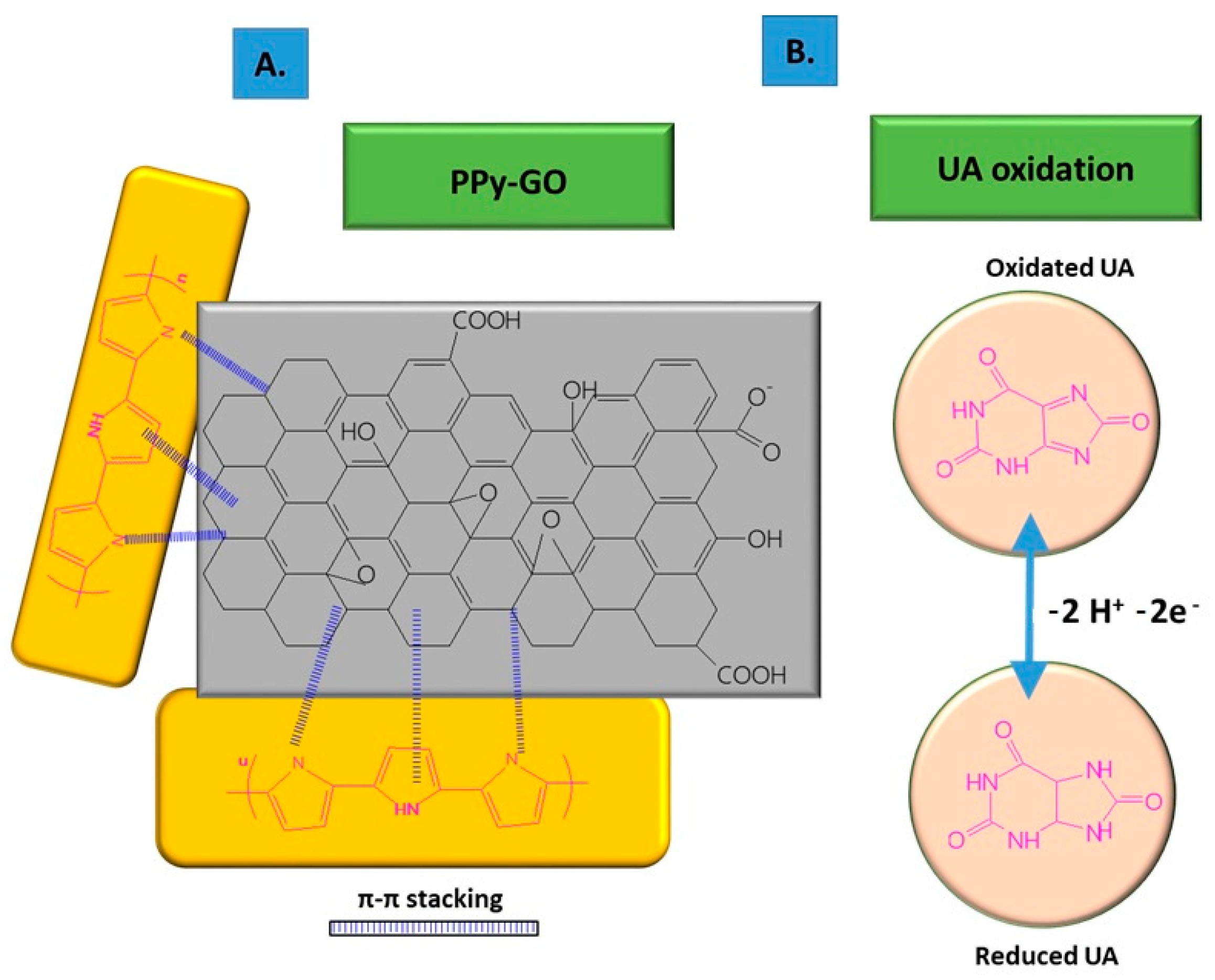
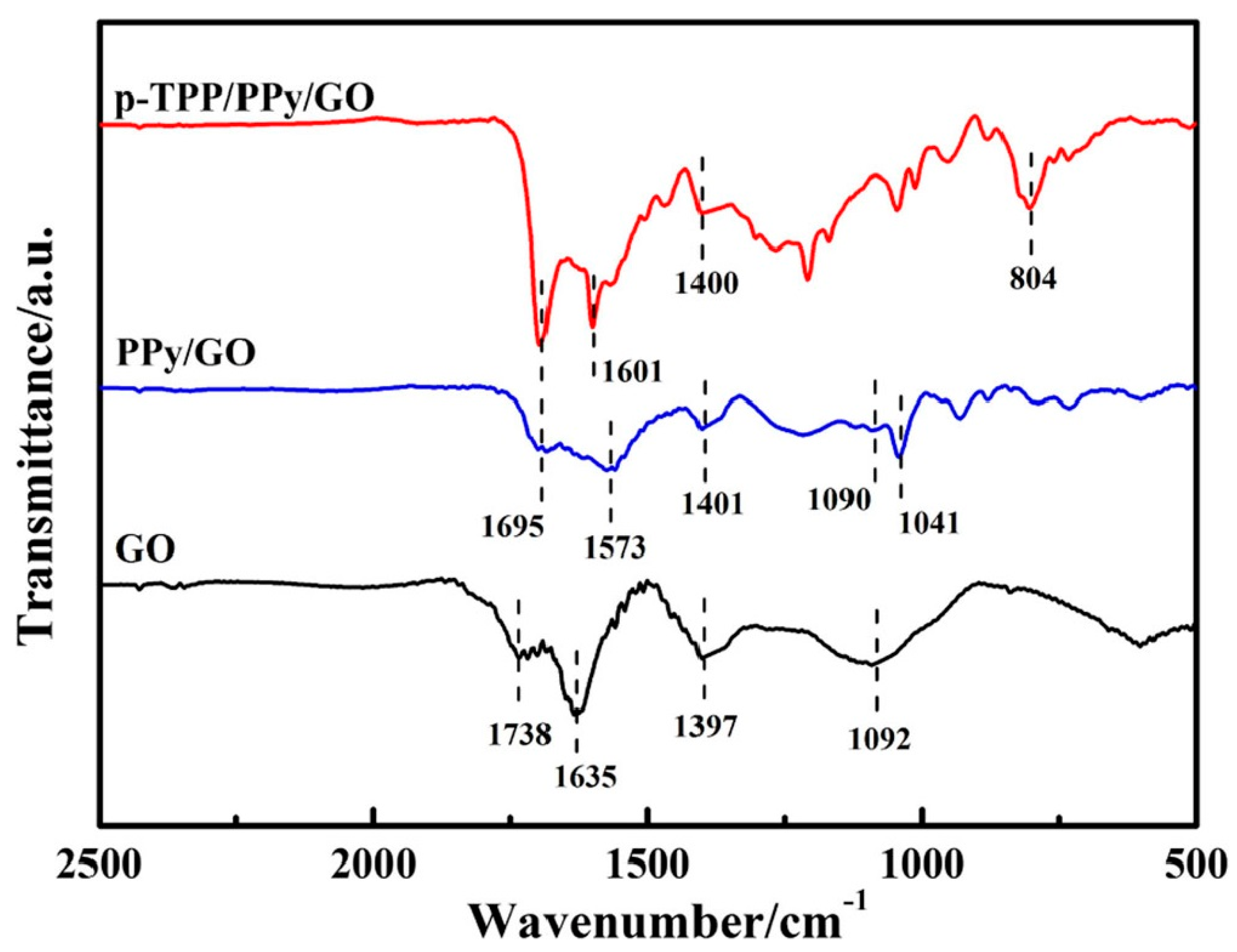
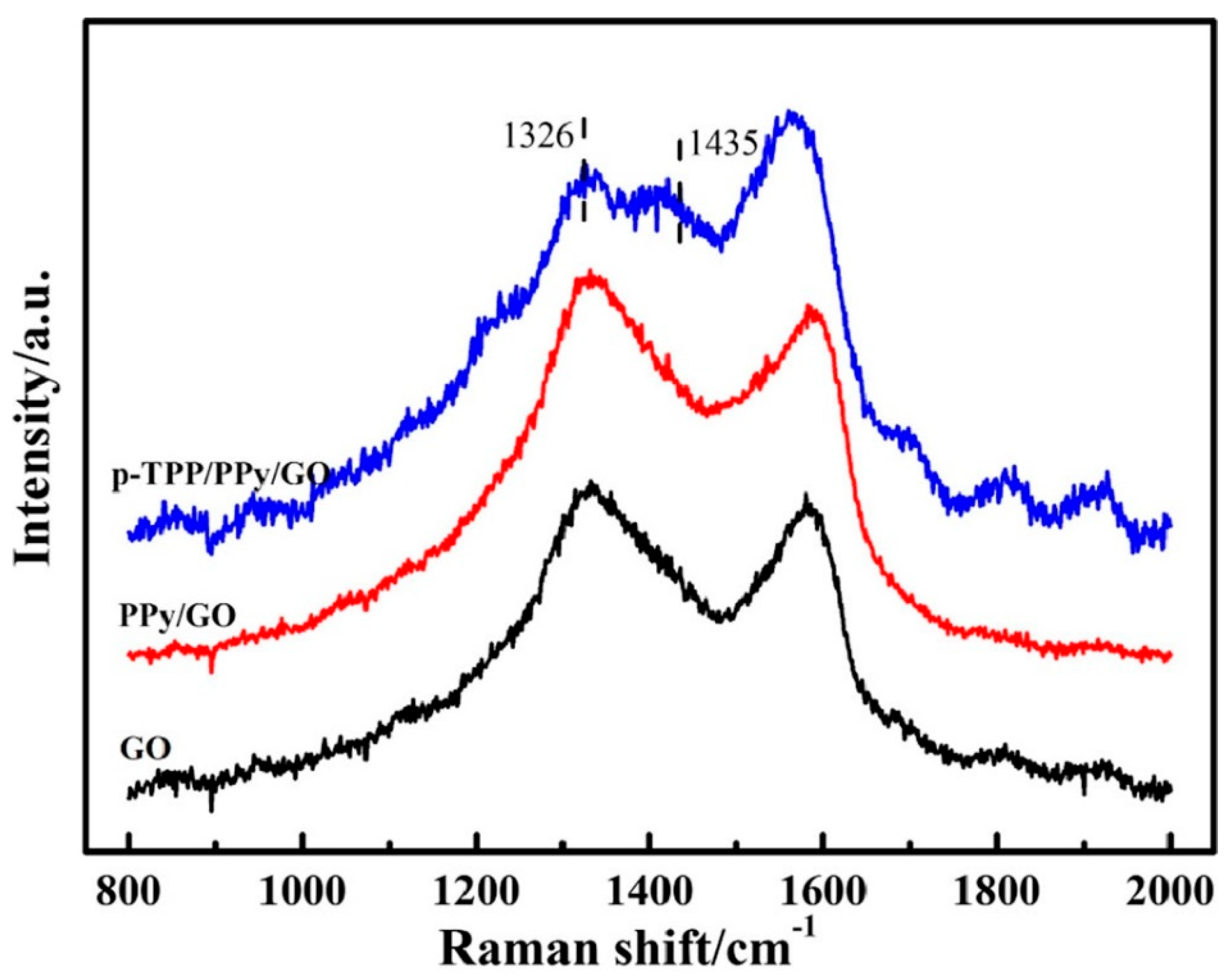
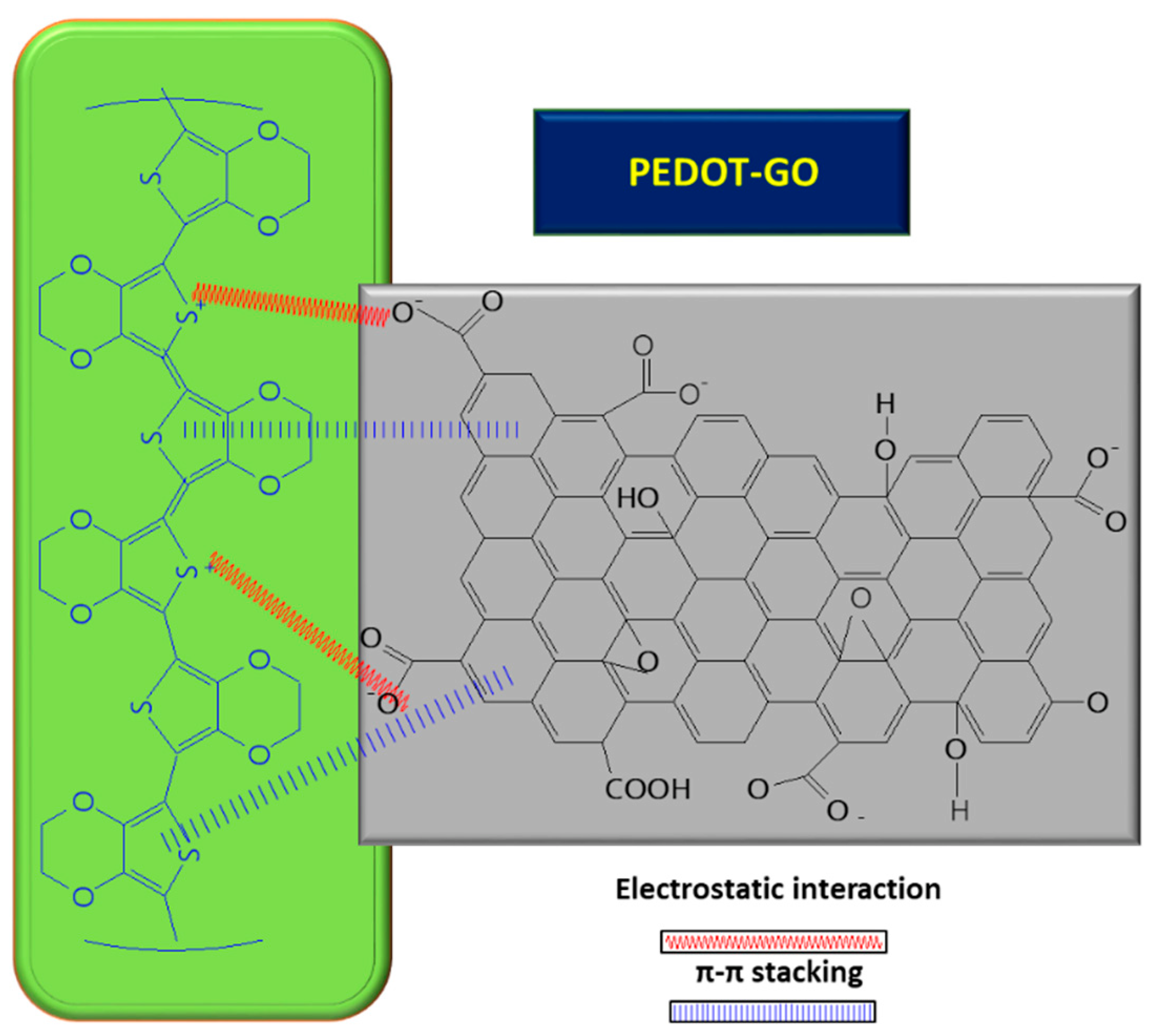

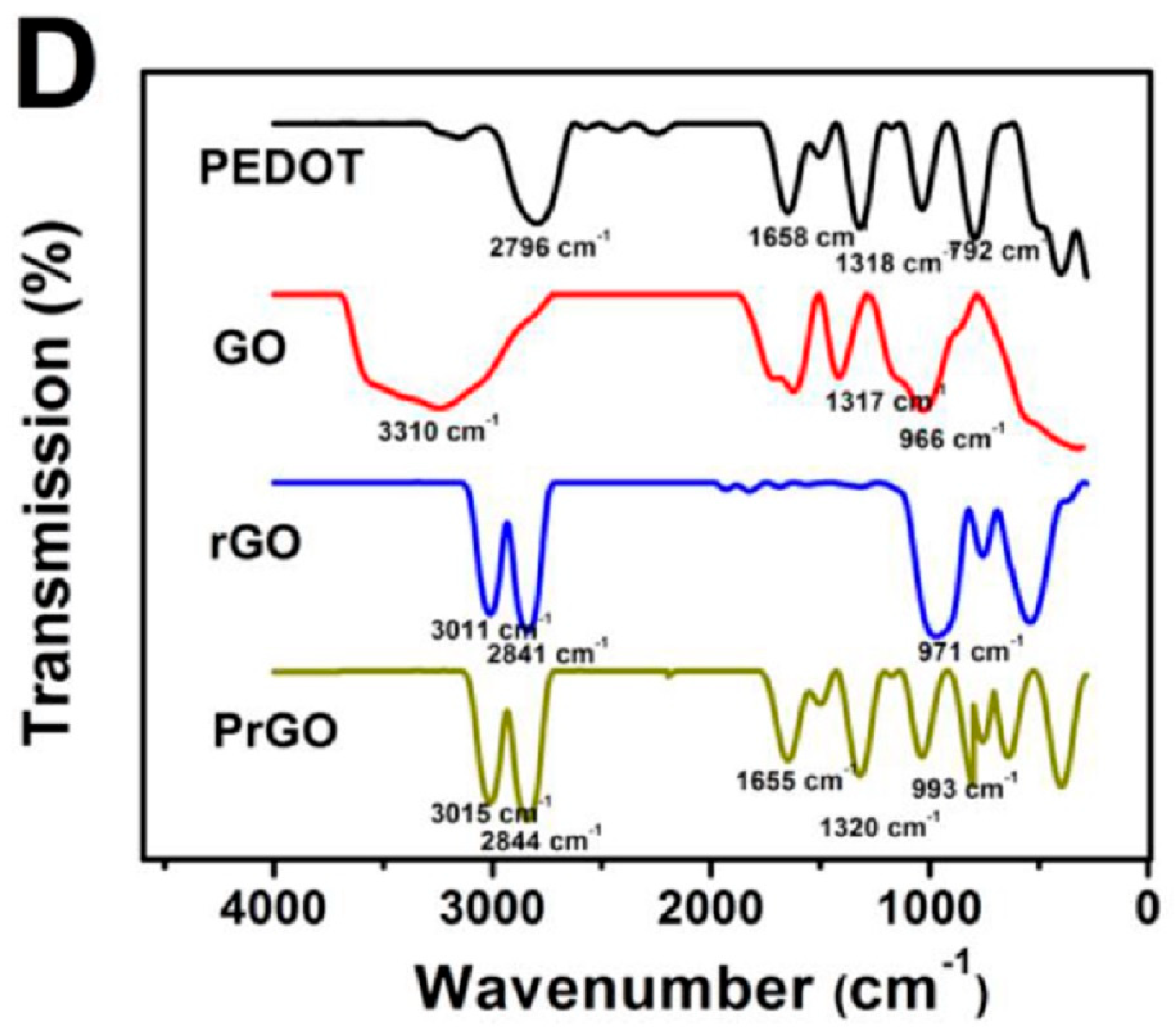

| Electrode | Surface Area (cm2) | Rct (Ω) |
|---|---|---|
| PEDOT-GO/GCE | 0.1385 | 0.2 |
| PEDOT/GCE | 0.0787 | 77 |
| GO/PEDOT/GCE | 0.1027 | 300 |
| Sensor (Composite/Electrode) | Method of Synthesis | Linear Range (µM) | LOD (µM) | Ref. | ||
|---|---|---|---|---|---|---|
| AuNPs/PPy–GO/CFP | Co-deposition through electro-polymerization | 2–437 | 2–360-SD | 1.39 | 1.68-SD | [6] |
| p-TPP/PPy–GO/GCE | Oxidative polymerization | 5–200 | - | 1.15 | - | [5] |
| PANI–GO | In situ chemical polymerization | 2–18 | - | 0.2 | - | [66] |
| PEDOT–GO/ITO PADS | Co-deposition | 2–1000 | - | 0.75 | - | [8] |
| PImox–GO/GCE | Polymerization | 3.6–249.6-SD | - | 0.59-SD | [9] | |
| PEDOT/GO/GCE | Co-electrochemical deposition | 40–240-SD | - | 10-SD | [59] | |
| Composite Material | Method of Synthesis | LR of Concentration (µM) | LOD (µM) | References | ||
|---|---|---|---|---|---|---|
| PPy/RGO (rGO/Pd@PPy NPs) | Co-polymerization | 1.4–219 | - | 0.047 | - | [29] |
| OPPy/RGO | Electrodeposition followed by over-oxidation of electropolymerized PPy | Used mainly for DA detection, the parameters for UA signal were also investigated | - | LR and LOD for UA were not reported in this paper, but the oxidation peak of UA was recorded at: OPPy/ERGO/GCE 15 µA, ERGO/GCE 3.1 µA, OPPY/GCE 1.1 µA, and GCE 0.8 µA | - | [71] |
| PANI/RGO (ZnO/PANI/RGO/GCE) | Electrochemical deposition | 0.1–1000 100–1000 | 0.5–90 µM-SD | 0.042 | 0.12 µM-SD | [4] |
| PANI/Fe2O3-SnO2/rGO (PFSG) ternary nanocomposite | Two-step hydrothermal | 5–300-SD | - | 1.6-SD | - | [27] |
| PEDOT/RGO | Electrodeposition | 1–300 | - | 0.19 (S/N = 3) | - | [16] |
| MnO2/PEDOT/RGO (PrGO/MnO2) | Co-deposition through electropolymerization | 0.3–80 | - | 0.05 | - | [72] |
| Electrode | Analytes | LOD | Reference |
|---|---|---|---|
| Au-PtNPs/GQD/GCE | AA, DA, UA, RT | 1 × 10−9 M | [30] |
| GQDs/IL-SPCE | UA | 3 × 10−8 M | [45] |
| GQDs/IL-SPCE | AA, DA, UA | 2 × 10−8 M | [45] |
| SnO2/PANI/N-GQD | DA mixed with AA and UA | 2.2 × 10−7 M | [116] |
| GQDs-doped PEDOT/GCE | AA, DA, UA | 4.1 × 10−6 M | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Văduva, M.; Baibarac, M.; Cramariuc, O. Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection. Molecules 2023, 28, 135. https://doi.org/10.3390/molecules28010135
Văduva M, Baibarac M, Cramariuc O. Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection. Molecules. 2023; 28(1):135. https://doi.org/10.3390/molecules28010135
Chicago/Turabian StyleVăduva, Mirela, Mihaela Baibarac, and Oana Cramariuc. 2023. "Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection" Molecules 28, no. 1: 135. https://doi.org/10.3390/molecules28010135
APA StyleVăduva, M., Baibarac, M., & Cramariuc, O. (2023). Functionalization of Graphene Derivatives with Conducting Polymers and Their Applications in Uric Acid Detection. Molecules, 28(1), 135. https://doi.org/10.3390/molecules28010135






