Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy
Abstract
1. Introduction
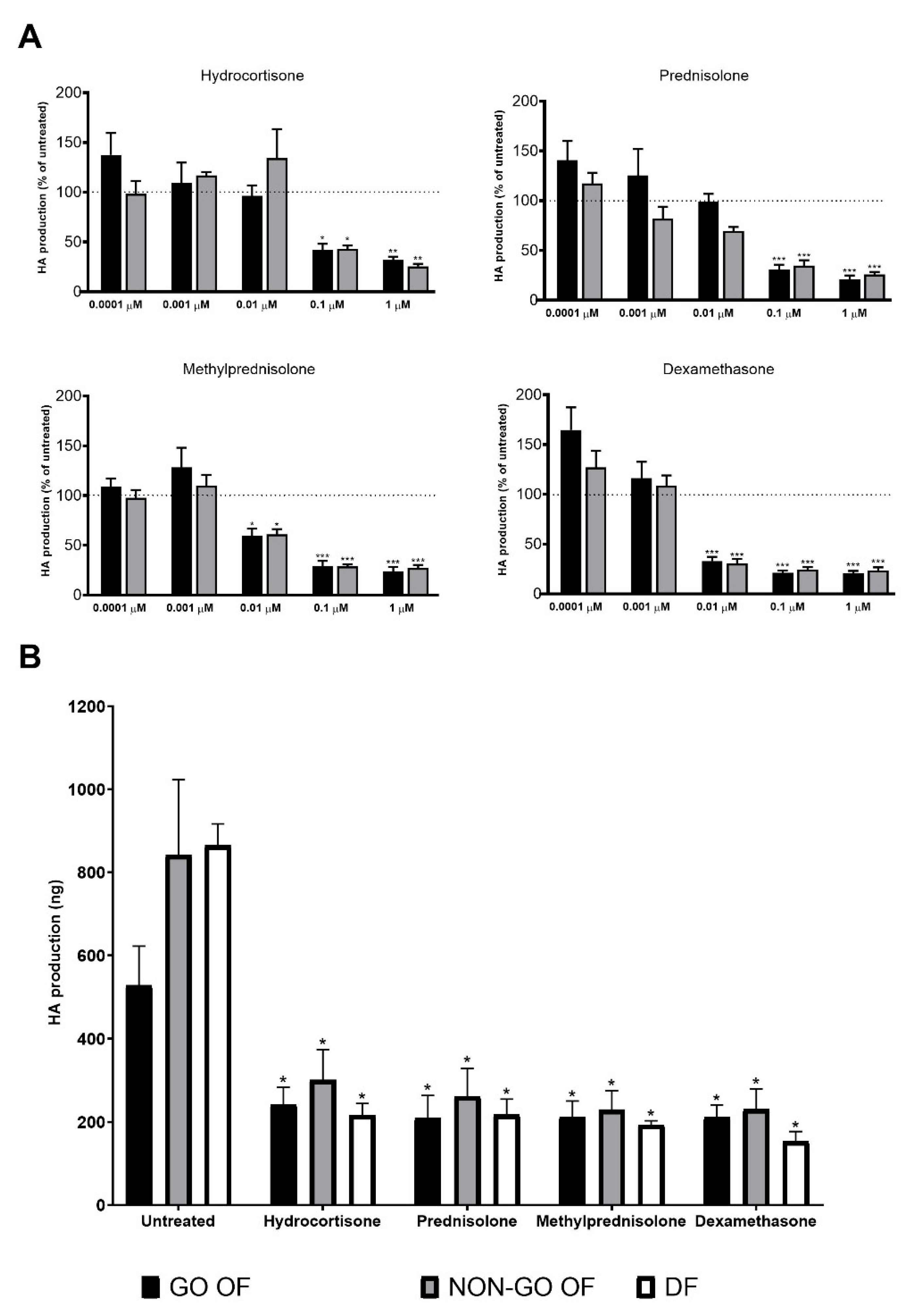
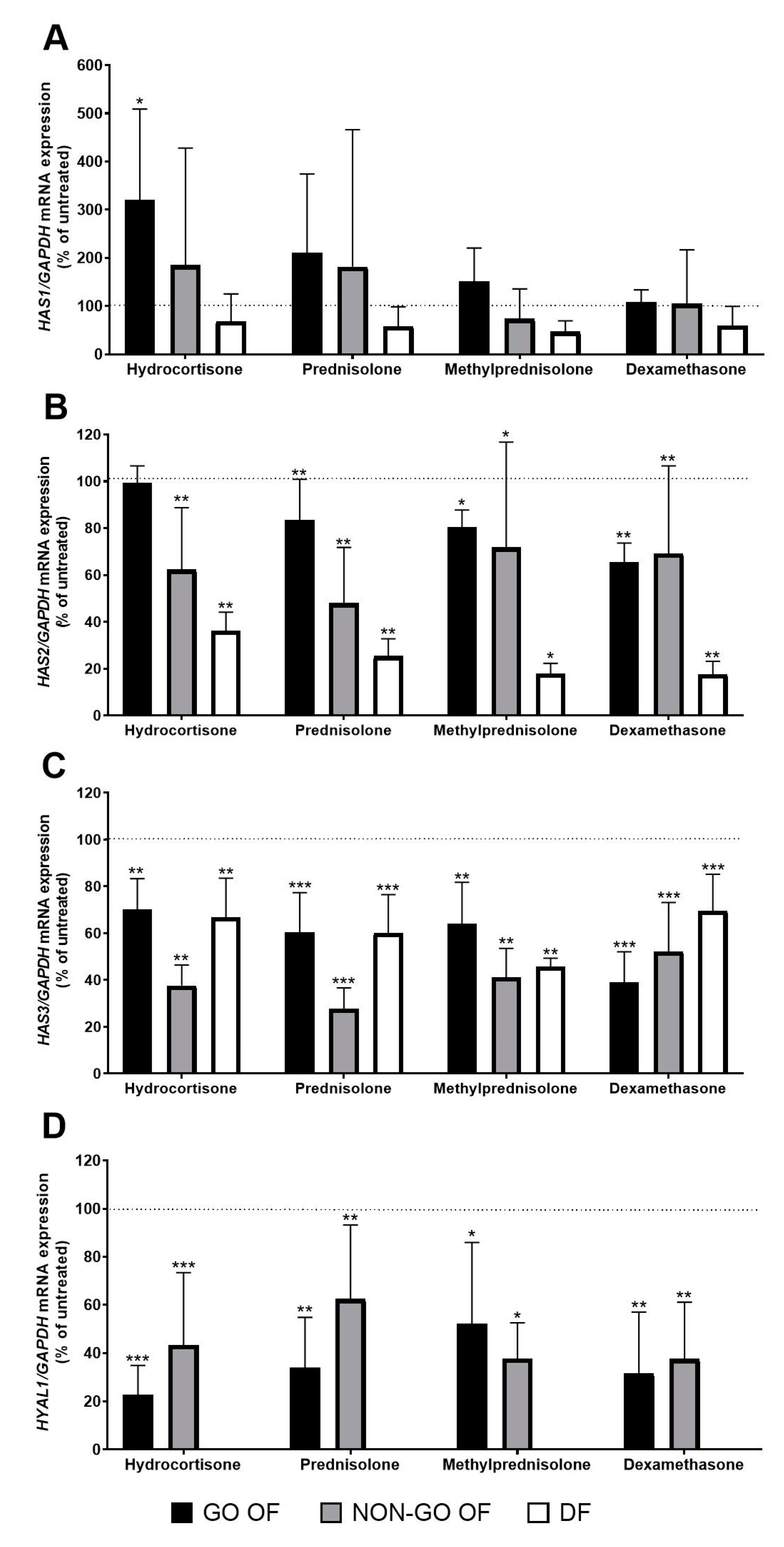
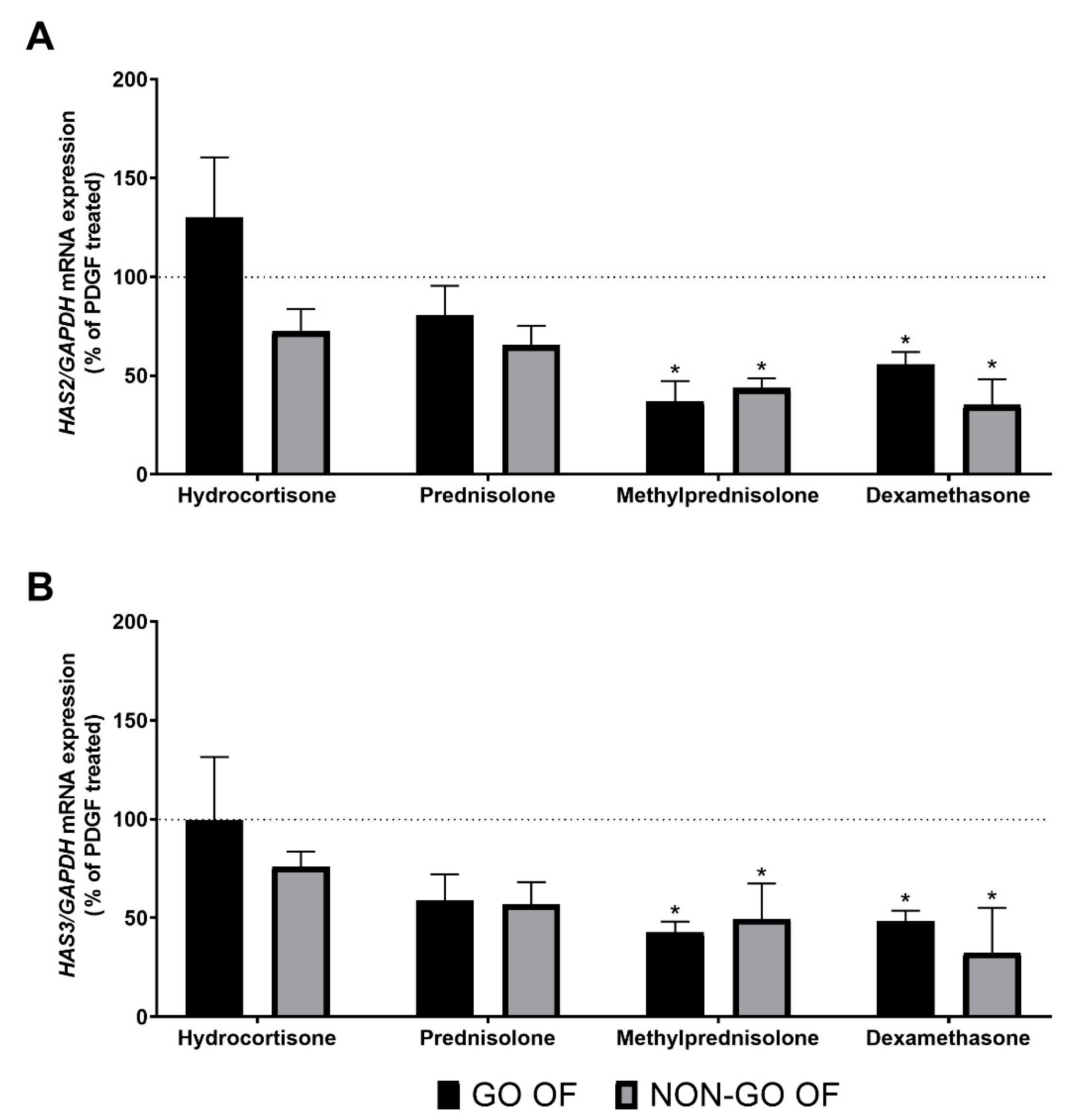
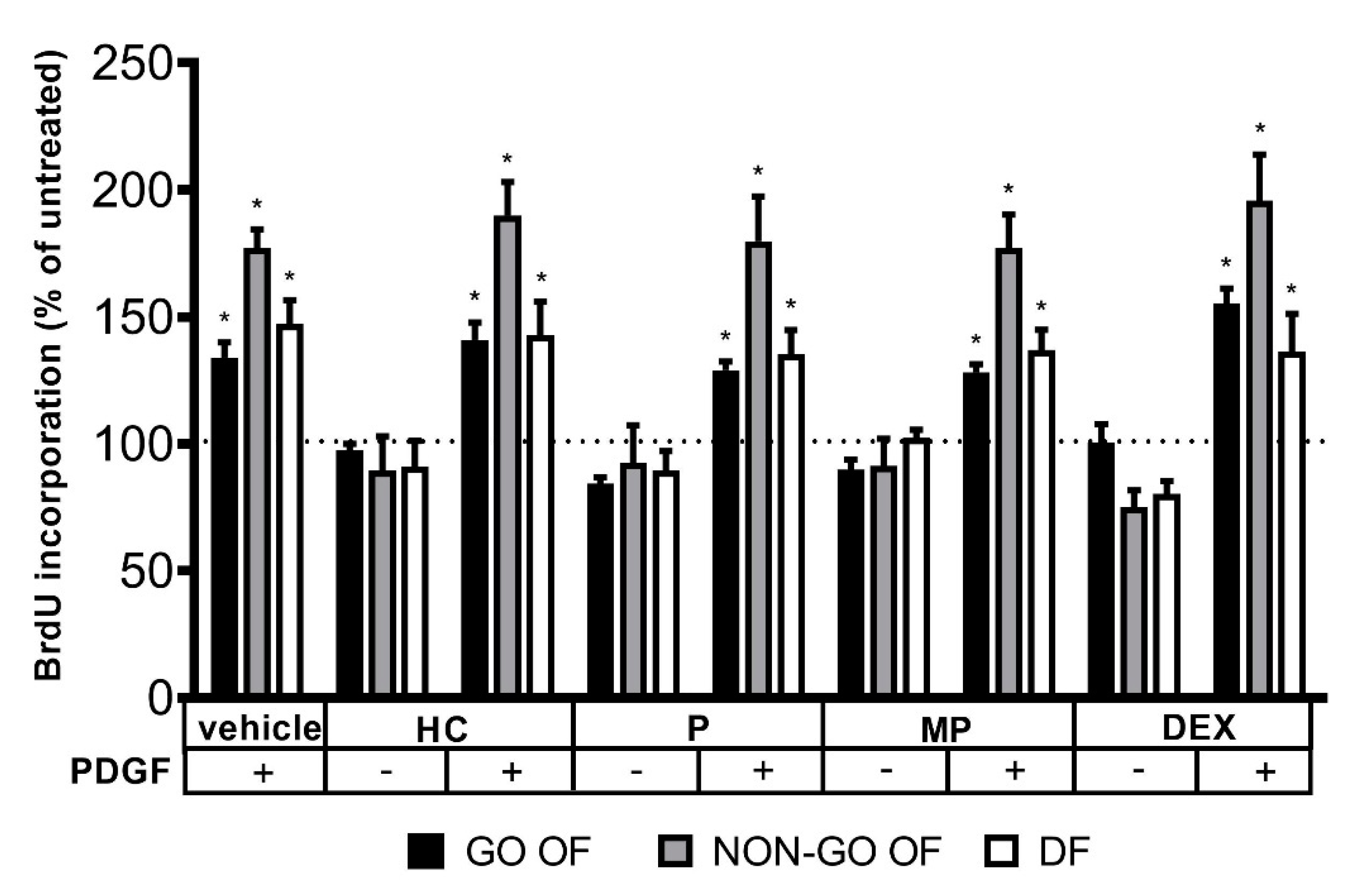
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Tissue Samples and Cell Cultures
4.3. Cell Proliferation Assay
4.4. Quantitation of HA
4.5. Real-Time Polymerase Chain Reactions (RT-PCR)
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef]
- Mishra, S.; Maurya, V.K.; Kumar, S.; Ankita; Kaur, A.; Saxena, S.K. Clinical Management and Therapeutic Strategies for the Thyroid-Associated Ophthalmopathy: Current and Future Perspectives. Curr. Eye Res. 2020, 45, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Quality of life in Graves’ ophthalmopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Längericht, J.; Krämer, I.; Kahaly, G.J. Glucocorticoids in Graves’ orbitopathy: Mechanisms of action and clinical application. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820958335. [Google Scholar] [CrossRef]
- Londzin-Olesik, M.; Kos-Kudla, B.; Karpe, J.; Nowak, A.; Nowak, M. The Effect of Immunosuppression on Selected Antioxidant Parameters in Patients with Graves’ Disease with Active Thyroid-Associated Orbitopathy. Exp. Clin. Endocrinol. Diabetes 2021, 129, 762–769. [Google Scholar] [CrossRef]
- Sackstein, R. Effects of methylprednisolone administration on lymphocyte LECAM-1, CD44, and LFA-1 expression. Implications for steroid-induced lymphopenia. Ann. N. Y. Acad. Sci. 1993, 696, 417–419. [Google Scholar] [CrossRef]
- Korducki, J.M.; Loftus, S.J.; Bahn, R.S. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest. Ophthalmol. Vis. Sci. 1992, 33, 2037–2042. [Google Scholar]
- Sisson, J.C. Stimulation of glucose utilization and glycosaminoglycans production by fibroblasts derived from retrobulbar tissue. Exp. Eye Res. 1971, 12, 285–292. [Google Scholar] [CrossRef][Green Version]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef]
- Hascall, V.C.; Wang, A.; Tammi, M.; Oikari, S.; Tammi, R.; Passi, A.; Vigetti, D.; Hanson, R.W.; Hart, G.W. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix. Biol. 2014, 35, 14–17. [Google Scholar] [CrossRef]
- van Steensel, L.; Hooijkaas, H.; Paridaens, D.; van den Bosch, W.A.; Kuijpers, R.W.; Drexhage, H.A.; van Hagen, P.M.; Dik, W.A. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J. Clin. Endocrinol. Metab. 2012, 97, E944–E953. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S. Pathophysiology of Graves’ ophthalmopathy: The cycle of disease. J. Clin. Endocrinol. Metab. 2003, 88, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuk, T.; Gopinath, B.; Ploski, R.; Wall, J.R. Susceptibility genes in Graves’ ophthalmopathy: Searching for a needle in a haystack? Clin. Endocrinol. 2007, 67, 3–19. [Google Scholar] [CrossRef]
- Gebhardt, C.; Averbeck, M.; Diedenhofen, N.; Willenberg, A.; Anderegg, U.; Sleeman, J.P.; Simon, J.C. Dermal hyaluronan is rapidly reduced by topical treatment with glucocorticoids. J. Invest. Dermatol. 2010, 130, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts. Similar effects of glucocorticoid and thyroid hormones. J. Clin. Invest. 1984, 74, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, M.; Papp, S.; Schaffer, L.; Pouyani, T. Hydrocortisone and triiodothyronine regulate hyaluronate synthesis in a tissue-engineered human dermal equivalent through independent pathways. J. Biosci. Bioeng. 2015, 119, 226–236. [Google Scholar] [CrossRef]
- Stuhlmeier, K.M.; Pollaschek, C. Glucocorticoids inhibit induced and non-induced mRNA accumulation of genes encoding hyaluronan synthases (HAS): Hydrocortisone inhibits HAS1 activation by blocking the p38 mitogen-activated protein kinase signalling pathway. Rheumatology 2004, 43, 164–169. [Google Scholar] [CrossRef]
- Gianoukakis, A.G.; Jennings, T.A.; King, C.S.; Sheehan, C.E.; Hoa, N.; Heldin, P.; Smith, T.J. Hyaluronan accumulation in thyroid tissue: Evidence for contributions from epithelial cells and fibroblasts. Endocrinology 2007, 148, 54–62. [Google Scholar] [CrossRef][Green Version]
- Smith, T.J.; Bahn, R.S.; Gorman, C.A. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: Evidence for differences between retroocular and dermal fibroblasts. J. Clin. Endocrinol Metab. 1989, 69, 1019–1023. [Google Scholar] [CrossRef]
- Tuuminen, R.; Syrjälä, S.; Krebs, R.; Arnaudova, R.; Rouvinen, E.; Nykänen, A.I.; Lemström, K.B. Combined donor simvastatin and methylprednisolone treatment prevents ischemia-reperfusion injury in rat cardiac allografts through vasculoprotection and immunomodulation. Transplantation 2013, 95, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Kaback, L.A.; Smith, T.J. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1beta in human orbital fibroblasts: Potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 1999, 84, 4079–4084. [Google Scholar] [CrossRef] [PubMed]
- Galgoczi, E.; Jeney, F.; Gazdag, A.; Erdei, A.; Katko, M.; Nagy, D.M.; Ujhelyi, B.; Steiber, Z.; Gyory, F.; Berta, E.; et al. Cell density-dependent stimulation of PAI-1 and hyaluronan synthesis by TGF-β in orbital fibroblasts. J. Endocrinol. 2016, 229, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Heldin, P.; Lin, C.Y.; Kolliopoulos, C.; Chen, Y.H.; Skandalis, S.S. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 2019, 78–79, 100–117. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Klagas, I.; Karakiulakis, G.; Hostettler, K.; S’ng, C.T.; Kotoula, V.; Savic, S.; Tamm, M.; Roth, M. Steroids and β2-agonists regulate hyaluronan metabolism in asthmatic airway smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Puissant, E.; Gilis, F.; Dogné, S.; Flamion, B.; Jadot, M.; Boonen, M. Subcellular trafficking and activity of Hyal-1 and its processed forms in murine macrophages. Traffic 2014, 15, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Tobisawa, Y.; Fujita, N.; Yamamoto, H.; Ohyama, C.; Irie, F.; Yamaguchi, Y. The cell surface hyaluronidase TMEM2 is essential for systemic hyaluronan catabolism and turnover. J. Biol. Chem. 2021, 297, 101281. [Google Scholar] [CrossRef]
- Shiozawa, J.; de Vega, S.; Cilek, M.Z.; Yoshinaga, C.; Nakamura, T.; Kasamatsu, S.; Yoshida, H.; Kaneko, H.; Ishijima, M.; Kaneko, K.; et al. Implication of HYBID (Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization) in Hyaluronan Degradation by Synovial Fibroblasts in Patients with Knee Osteoarthritis. Am. J. Pathol. 2020, 190, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Spataro, S.; Guerra, C.; Cavalli, A.; Sgrignani, J.; Sleeman, J.; Poulain, L.; Boland, A.; Scapozza, L.; Moll, S.; Prunotto, M. CEMIP (HYBID, KIAA1199): Structure, function and expression in health and disease. FEBS J. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Virakul, S.; Heutz, J.W.; Dalm, V.A.; Peeters, R.P.; Paridaens, D.; van den Bosch, W.A.; Hirankarn, N.; van Hagen, P.M.; Dik, W.A. Basic FGF and PDGF-BB synergistically stimulate hyaluronan and IL-6 production by orbital fibroblasts. Mol. Cell Endocrinol. 2016, 433, 94–104. [Google Scholar] [CrossRef]
- DeGrendele, H.C.; Estess, P.; Siegelman, M.H. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 1997, 278, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lv, S.; Li, Y.; Zhu, J.; Chen, S.; Zhen, G.; Cao, X.; Wu, S.; Crane, J.L. Glucocorticoids Disrupt Skeletal Angiogenesis Through Transrepression of NF-κB-Mediated Preosteoclast Pdgfb Transcription in Young Mice. J. Bone. Miner. Res. 2020, 35, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- van Steensel, L.; Paridaens, D.; Dingjan, G.M.; van Daele, P.L.; van Hagen, P.M.; Kuijpers, R.W.; van den Bosch, W.A.; Drexhage, H.A.; Hooijkaas, H.; Dik, W.A. Platelet-derived growth factor-BB: A stimulus for cytokine production by orbital fibroblasts in Graves’ ophthalmopathy. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1002–1007. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Li, L.; Asteriou, T.; Bernert, B.; Heldin, C.H.; Heldin, P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem. J. 2007, 404, 327–336. [Google Scholar] [CrossRef]
- Viola, M.; Karousou, E.; D’Angelo, M.L.; Caon, I.; De Luca, G.; Passi, A.; Vigetti, D. Regulated Hyaluronan Synthesis by Vascular Cells. Int. J. Cell Biol. 2015, 2015, 208303. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim Biophys. Acta. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Vigetti, D.; Clerici, M.; Deleonibus, S.; Karousou, E.; Viola, M.; Moretto, P.; Heldin, P.; Hascall, V.C.; De Luca, G.; Passi, A. Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J. Biol. Chem. 2011, 286, 7917–7924. [Google Scholar] [CrossRef]
- Puthanveetil, P.; Rodrigues, B. Glucocorticoid excess induces accumulation of cardiac glycogen and triglyceride: Suggested role for AMPK. Curr. Pharm. Des. 2013, 19, 4818–4830. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgoczi, E.; Katko, M.; Papp, F.R.; Csiki, R.; Csiha, S.; Erdei, A.; Bodor, M.; Ujhelyi, B.; Steiber, Z.; Gyory, F.; et al. Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy. Molecules 2023, 28, 15. https://doi.org/10.3390/molecules28010015
Galgoczi E, Katko M, Papp FR, Csiki R, Csiha S, Erdei A, Bodor M, Ujhelyi B, Steiber Z, Gyory F, et al. Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy. Molecules. 2023; 28(1):15. https://doi.org/10.3390/molecules28010015
Chicago/Turabian StyleGalgoczi, Erika, Monika Katko, Fruzsina Reka Papp, Robert Csiki, Sara Csiha, Annamaria Erdei, Miklos Bodor, Bernadett Ujhelyi, Zita Steiber, Ferenc Gyory, and et al. 2023. "Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy" Molecules 28, no. 1: 15. https://doi.org/10.3390/molecules28010015
APA StyleGalgoczi, E., Katko, M., Papp, F. R., Csiki, R., Csiha, S., Erdei, A., Bodor, M., Ujhelyi, B., Steiber, Z., Gyory, F., & Nagy, E. V. (2023). Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy. Molecules, 28(1), 15. https://doi.org/10.3390/molecules28010015





