pH and Salt-Assisted Macroscopic Chirality Inversion of Gadolinium Coordination Polymer
Abstract
:1. Introduction
2. Results
2.1. pH Effect on the Reaction Product
2.2. Structures of R-Block and R-Rod
2.3. Characterization of Superhelices R-M and R-P
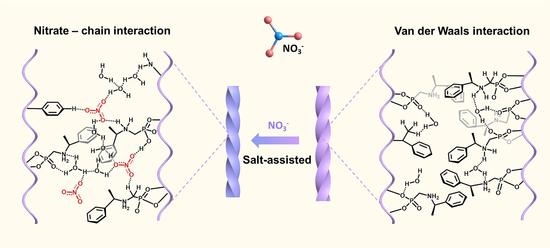
2.4. Salt Effect on the Chirality Inversion of Superhelices
3. Conclusions
4. Materials and Methods
4.1. Materials and Physical Measurements
4.2. Synthesis of (H3O)[Gd6(R-pempH2)3(R-pempH)15] (NO3)4∙9H2O (R-Block)
4.3. Synthesis of Gd[(R-pempH)2.70(R-pempH2)0.30](NO3)0.30·2H2O (R-M)
4.4. Synthesis of Gd[(R-pempH)3]·2H2O (R-P)
4.5. Synthesis of Gd[(R-pempH)2.70(R-pempH2)0.30](NO3)0.30·2H2O (R-M’)
4.6. Single Cryatal X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, R.K.; Zouani, O.F.; Labrugere, C.; Oda, R.; Durrieu, M.C. Influence of Nanohelical Shape and Periodicity on Stem Cell Fate. ACS Nano 2013, 7, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Meng, Y.; Zhang, L.; Liu, M. Self-Assembled Single-Walled Metal-Helical Nanotube (M-HN): Creation of Efficient Supramolecular Catalysts for Asymmetric Reaction. J. Am. Chem. Soc. 2016, 138, 15629–15635. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Duan, P.; Zhang, L.; Liu, M. Chirality and Energy Transfer Amplified Circularly Polarized Luminescence in Composite Nanohelix. Nat. Commun. 2017, 8, 15727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Sun, Z.; Mu, A.U.; Zeng, M.; Kalin, A.J.; Cheng, Z.; Olson, M.A.; Fang, L. Assembly and Chiral Memory Effects of Dynamic Macroscopic Supramolecular Helices. Chem. Eur. J. 2018, 24, 16553–16557. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, L.; Liu, M. Self-Assembly of 1D Helical Nanostructures into Higher Order Chiral Nanostructures in Supramolecular Systems. ChemNanoMat 2018, 4, 720–729. [Google Scholar] [CrossRef]
- Janssen, P.G.; Ruiz-Carretero, A.; Gonzalez-Rodriguez, D.; Meijer, E.W.; Schenning, A.P. pH-Switchable Helicity of DNA-Templated Assemblies. Angew. Chem. Int. Ed. 2009, 48, 8103–8106. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, W.; Li, Y.; Li, B.Z.; Yang, Y.-G. pH-Influenced Handedness Inversion of Circularly Polarized Luminescence. New J. Chem. 2021, 45, 21941–21946. [Google Scholar] [CrossRef]
- Kousar, A.; Liu, J.; Mehwish, N.; Wang, F.; Dang-i, A.Y.; Feng, C. pH-Regulated Supramolecular Chirality of Phenylalanine-Based Hydrogels. Mater. Today Chem. 2019, 11, 217–224. [Google Scholar] [CrossRef]
- Kurouski, D.; Lu, X.; Popova, L.; Wan, W.; Shanmugasundaram, M.; Stubbs, G.; Dukor, R.K.; Lednev, I.K.; Nafie, L.A. Is Supramolecular Filament Chirality the Underlying Cause of Major Morphology Differences in Amyloid Fibrils? J. Am. Chem. Soc. 2014, 136, 2302–2312. [Google Scholar] [CrossRef]
- Mason, M.L.; Lalisse, R.F.; Finnegan, T.J.; Hadad, C.M.; Modarelli, D.A.; Parquette, J.R. pH-Controlled Chiral Packing and Self-Assembly of a Coumarin Tetrapeptide. Langmuir 2019, 35, 12460–12468. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Reconfigurable Chiral Self-Assembly of Peptides through Control of Terminal Charges. Small 2017, 13, 1700999. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Seco, J.M.; Quinoa, E.; Riguera, R. Chiral Amplification and Helical-Sense Tuning by Mono- and Divalent Metals on Dynamic Helical Polymers. Angew. Chem. Int. Ed. 2011, 50, 11692–11696. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sheng, J.; Teo, W.-L.; Yang, G.; Wu, H.; Li, Y.; Zhao, Y. Control on Dimensions and Supramolecular Chirality of Self-Assemblies through Light and Metal Ions. J. Am. Chem. Soc. 2018, 140, 16275–16283. [Google Scholar] [CrossRef]
- Qin, P.; Wu, Z.; Li, P.; Niu, D.; Liu, M.; Yin, M. Triple-Modulated Chiral Inversion of Co-Assembly System Based on Alanine Amphiphile and Cyanostilbene Derivative. ACS Appl. Mater. Interfaces 2021, 13, 18047–18055. [Google Scholar] [CrossRef]
- Wang, F.; Feng, C.-L. Metal-Ion-Mediated Supramolecular Chiralityof l-Phenylalanine Based Hydrogels. Angew. Chem. Int. Ed. 2018, 57, 5655–5659. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ji, W.; Yang, P.; Feng, C.-L. Inversion of Circularly Polarized Luminescence of Nanofibrous Hydrogels through Co-assembly with Achiral Coumarin Derivatives. ACS Nano 2019, 13, 7281–7290. [Google Scholar] [CrossRef]
- Zha, X.; Chen, Y.; Fan, H.; Yang, Y.; Xiong, Y.; Xu, G.; Yan, K.; Wang, Y.; Xie, Y.; Wang, D. Handedness Inversion of Chiral 3-Aminophenol Formaldehyde Resin NanotubesMediatedbyMetal Coordination. Angew. Chem. Int. Ed. 2021, 60, 7759–7769. [Google Scholar] [CrossRef]
- Gillissen, M.A.; Koenigs, M.M.; Spiering, J.J.; Vekemans, J.A.; Palmans, A.R.; Voets, I.K.; Meijer, E.W. Triple Helix Formation in Amphiphilic Discotics: Demystifying Solvent Effects in Supramolecular Self-Assembly. J. Am. Chem. Soc. 2014, 136, 336–343. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, K.; Meng, L.; Chen, L.; Chen, L.; Yi, T. Solvent Induced Helical Aggregation in the Self-Assembly of Cholesterol Tailed Platinum Complexes. Soft Matter 2014, 10, 7615–7622. [Google Scholar] [CrossRef]
- Urushima, A.; Ousaka, N.; Yashima, E. Tug-of-War in a Dynamic Helical Peptide: Solvent-Induced Helix-Helix Transition of a Lactam-Bridged Peptide Composed of Point- and Axial Chiralities Remote from Each Other. Chem. Asian J. 2018, 13, 3150–3154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Huang, R.; Yang, X.; Wang, M.; Su, R.; He, Z. Rational Design of Chiral Nanostructures from Self-Assembly of a Ferrocene-Modified Dipeptide. J. Am. Chem. Soc. 2015, 137, 7869–7880. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, M.; Zhu, X.; Xue, C.; Wang, H.-X.; Liu, M. Solvent-Modulated Chiral Self-Assembly: Selective Formation of Helical Nanotubes, Nanotwists, and Energy Transfer. ACS Appl. Mater. Interfaces 2022, 14, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-Y.; Cao, X.-C.; Cheng, P.; Ma, J.-G. Solvent Induced Conformational Transformation of Helical Chains in New Copper(II) Coordination Polymers. Inorg. Chem. Commun. 2012, 24, 7–10. [Google Scholar] [CrossRef]
- Choi, H.; Cho, K.J.; Seo, H.; Ahn, J.; Liu, J.; Lee, S.S.; Kim, H.; Feng, C.; Jung, J.H. Transfer and Dynamic Inversion of Coassembled Supramolecular Chirality through 2D-Sheet to Rolled-Up Tubular Structure. J. Am. Chem. Soc. 2017, 139, 17711–17714. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, B.; Han, D.; Duan, P.; Liu, M.; Yin, M. Stoichiometry-controlled inversion of circularly polarized luminescence in co-assembly of chiral gelators with an achiral tetraphenylethylene derivative. Chem. Commun. 2019, 55, 2194–2197. [Google Scholar] [CrossRef]
- Liu, G.; Sheng, J.; Wu, H.; Yang, C.; Yang, G.; Li, Y.; Ganguly, R.; Zhu, L.; Zhao, Y. Controlling Supramolecular Chirality of Two-Component Hydrogels by J- and H-Aggregation of Building Blocks. J. Am. Chem. Soc. 2018, 140, 6467–6473. [Google Scholar] [CrossRef]
- Liu, G.-F.; Zhu, L.-Y.; Ji, W.; Feng, C.-L.; Wei, Z.-X. Inversion of the Supramolecular Chirality of Nanofibrous Structures through Co-Assembly with Achiral Molecules. Angew. Chem. Int. Ed. 2016, 55, 2411–2415. [Google Scholar] [CrossRef]
- Martial, B.; Lefevre, T.; Buffeteau, T.; Auger, M. Vibrational Circular Dichroism Reveals Supramolecular Chirality Inversion of α-Synuclein Peptide Assemblies upon Interactions with Anionic Membranes. ACS Nano 2019, 13, 3232–3242. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, J.; Qi, W.; You, S.; Su, R.; He, Z. Self-Templated, Enantioselective Assembly of an Amyloid-like Dipeptide into Multifunctional Hierarchical Helical Arrays. ACS Nano 2021, 15, 9827–9840. [Google Scholar] [CrossRef]
- Wu, A.; Guo, Y.; Li, X.; Xue, H.; Fei, J.; Li, J. Co-assembled Supramolecular Gel of Dipeptide and Pyridine Derivatives with Controlled Chirality. Angew. Chem. Int. Ed. 2021, 60, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lu, H.; Zhang, Q.; Chen, M.; Shan, Y.; Xu, T.-Y.; Tong, F.; Qu, D.-H. Surfactant-Induced Chirality Transfer, Amplification and Inversion in a Cucurbit[8]uril–Viologen Host–Guest Supramolecular System. J. Mater. Chem. C 2022, 10, 2763–2774. [Google Scholar] [CrossRef]
- Go, M.; Choi, H.; Kim, K.Y.; Moon, C.J.; Choi, Y.; Miyake, H.; Lee, S.S.; Jung, S.H.; Choi, M.Y.; Jung, J.H. Temperature-Controlled Helical Inversion of Asymmetric Triphenylamine-Based Supramolecular Polymers; Difference of Handedness at the Micro- and Macroscopic Levels. Org. Chem. Front. 2019, 6, 1100–1108. [Google Scholar] [CrossRef]
- Komori, H.; Inai, Y. Control of Peptide Helix Sense by Temperature Tuning of Noncovalent Chiral Domino Effect. J. Org. Chem. 2007, 72, 4012–4022. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-Directed Dynamic Chirality Inversion in Functional Self-Organized Helical Superstructures. Angew. Chem. Int. Ed. 2016, 55, 2994–3010. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.; Hifsudheen, M.; Furumi, S.; Takeuchi, M.; Ajayaghosh, A. Thermally Assisted Photonic Inversion of Supramolecular Handedness. Angew. Chem. Int. Ed. 2012, 51, 10505–10509. [Google Scholar] [CrossRef]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary Nitrate Metabolism and the Microbiome: Intersection of Microbial Metabolism, Nitric Oxide and Diet in Cardiac and Pulmonary Vascular Health. Free Radic. Biol. Med. 2017, 105, 48–67. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Pang, B.; Hu, L.; Feng, X.; Hu, L.; Wang, J.; Zhang, C.; Wang, S. Dietary Nitrate Protects Submandibular Gland from Hyposalivation in Ovariectomized Rats via Suppressing Cell Apoptosis. Biochem. Biophys. Res. Commun. 2018, 497, 272–278. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Qu, Y.; Wang, X.; An, W.; Li, Z.; Han, Z.; Qin, L. Nitrate Partially Inhibits Lipopolysaccharide-Induced Inflammation by Maintaining Mitochondrial Function. J. Int. Med. Res. 2020, 48, 300060520902605. [Google Scholar] [CrossRef] [Green Version]
- Lowenberg, C.; Julich-Gruner, K.K.; Neffe, A.T.; Behl, M.; Lendlein, A. Salt-Induced Shape-Memory Effect in Gelatin-Based Hydrogels. Biomacromolecules 2020, 21, 2024–2031. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Sun, Y.; Cai, Z.; Yang, L.; Lu, H. Salt- and pH-Triggered Helix-Coil Transition of Ionic Polypeptides under Physiology Conditions. Biomacromolecules 2018, 19, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Adamcik, J.; Mezzenga, R. Adjustable Twisting Periodic Pitch of Amyloid Fibrils. Soft Matter 2011, 7, 5437–5443. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, R.; Zhang, P.; Fern, J.; Cheetham, A.G.; Patel, K.; Schulman, R.; Kan, C.; Cui, H. Electrostatic-Driven Lamination and Untwisting of β-Sheet Assemblies. ACS Nano 2016, 10, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Drew, H.; Takano, T.; Tanaka, S.; Itakura, K.; Dickerson, R.E. High-Salt d(CpGpCpG), a Left-Handed Z’ DNA Double Helix. Nature 1980, 286, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bukharina, D.; Kim, M.; Han, M.J.; Tsukruk, V.V. Cellulose Nanocrystals’ Assembly under Ionic Strength Variation: From High Orientation Ordering to a Random Orientation. Langmuir 2022, 38, 6363–6375. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Lee, S.C. Salt-assisted Synthesis of Hollow Bi2WO6 Microspheres with Superior Photocatalytic Activity for NO Removal. Chin. J. Catal. 2017, 38, 348–356. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Kakiuchi, K.; Imai, H. Growth of Submicrometer-Scale Rectangular Parallelepiped Rutile TiO2 Films in Aqueous TiCl3 Solutions under Hydrothermal Conditions. J. Am. Chem. Soc. 2004, 126, 7790–7791. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Z.; Bu, S.; Zhao, J.; Liu, Z. Growth of ZnO Films with Controlled Morphology by Aqueous Solution Method. J. Am. Ceram. Soc. 2006, 89, 1226–1231. [Google Scholar] [CrossRef]
- Yin, B.; Zhou, W.; Long, Q.; Li, C.; Zhang, Y.; Yao, S. Salt-assisted Rapid Transformation of NaYF4:Yb3+,Er3+ Nanocrystals from Cubic to Hexagonal. CrystEngComm 2014, 16, 8348–8355. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, S.-S.; Huang, X.-D.; Zheng, L.-M. Homochiral Erbium Coordination Polymers: Salt-Assisted Conversion from Triple to Quadruple Helices. Cryst. Growth Des. 2018, 18, 4045–4053. [Google Scholar] [CrossRef]
- Huang, J.; Ding, H.-M.; Xu, Y.; Zeng, D.; Zhu, H.; Zang, D.-M.; Bao, S.-S.; Ma, Y.-Q.; Zheng, L.-M. Chiral Expression from Molecular to Macroscopic Level via pH Modulation in Terbium Coordination Polymers. Nat. Commun. 2017, 8, 2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.-Q.; Xu, Y.; Hou, T.; Jia, J.-G.; Huang, X.-D.; Weng, G.-G.; Bao, S.-S.; Zheng, L.-M. Controllable Macroscopic Chirality of Coordination Polymers through pH and Anion-Mediated Weak Interactions. Chem. Eur. J. 2021, 27, 16722–16734. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.O.; Begum, R.A.; Bowman-James, K. Amide-Based Ligands for Anion Coordination. Angew. Chem. Int. Ed. 2006, 45, 7882–7894. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, M.Y.; Zdravkova, V.R.; Ivanova, E.Z.; Aleksandrov, H.A.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. J. Catal. 2021, 394, 245–258. [Google Scholar] [CrossRef]
- Morozov, I.V.; Serezhkin, V.N.; Troyanov, S.I. Modes of Coordination and Stereochemistry of the NO3− Anions in Inorganic Nitrates. Russ. Chem. Bull. 2008, 57, 439–450. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Liu, Y.-C.; Chao, T.-C. From 1D helix to 0D Loop: Nitrite Anion Induced Structural Transformation Associated with Unexpected N-Nitrosation of Amine Ligand. Inorg. Chem. 2014, 53, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.J.; Duckworth, L.C.; Beer, P.D. Nitrate Anion Templated Assembly of a [2]Rotaxane for Selective Nitrate Recognition in Aqueous Solvent Mixtures. Chem. Commun. 2013, 49, 8608–8610. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, K.A.; Mehrotra, G.K.; Mrozinski, J.; Butcher, R.J. Anion Assisted Self-Assembly of a Ni(II) Complex into Metallo-Supramolecular Network Involving H-bonded Synthons as Nodes. J. Mol. Struct. 2010, 964, 18–26. [Google Scholar] [CrossRef]
- Xie, T.-Z.; Guo, C.; Yu, S.-Y.; Pan, Y.-J. Fine-Tuning Conformational Motion of a Self-Assembled Metal-Organic Macrocycle by Multiple C-H·Anion Hydrogen Bonds. Angew. Chem. Int. Ed. 2012, 51, 1177–1181. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Sun, Q.-F. A self-assembled Pd2L4 cage that selectively encapsulates nitrate. Chem. Commun. 2015, 51, 16767–16770. [Google Scholar] [CrossRef]
- TOPAS. Version 5.0; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Liu, X.-G.; Bao, S.-S.; Huang, J.; Otsubo, K.; Feng, J.-S.; Ren, M.; Hu, F.-C.; Sun, Z.-H.; Zheng, L.-M.; Wei, S.-Q.; et al. Homochiral metal phosphonate nanotubes. Chem. Commun. 2015, 51, 15141–15144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, G.-G.; Hong, B.-K.; Bao, S.-S.; Wen, Y.; Wu, L.-Q.; Huang, X.-D.; Jia, J.-G.; Wen, G.-H.; Li, S.-H.; Peng, L.; et al. From Helices to Superhelices: Hierarchical Assembly of Homochiral van der Waals 1D Coordination Polymers. Chem. Sci. 2021, 12, 12619–12630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Bao, S.-S.; Li, Y.-Z.; Zheng, L.-M. Polymorphism in Homochiral Zinc Phosphonates. Inorg. Chem. 2008, 47, 5525–5527. [Google Scholar] [CrossRef] [PubMed]
- SAINT. Version 8.40 A, Program for Data Extraction and Reduction; Bruker Nano Inc.: Madison, WI, USA, 2019. [Google Scholar]
- SHELXT 2014/5, Sheldrick, 2014; SHELXL 2018/3, Sheldrick, 2018. Available online: http://shelx.uni-goettingen.de/ (accessed on 1 March 2018).








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, T.; Wu, L.-Q.; Xu, Y.; Bao, S.-S.; Zheng, L.-M. pH and Salt-Assisted Macroscopic Chirality Inversion of Gadolinium Coordination Polymer. Molecules 2023, 28, 163. https://doi.org/10.3390/molecules28010163
Hou T, Wu L-Q, Xu Y, Bao S-S, Zheng L-M. pH and Salt-Assisted Macroscopic Chirality Inversion of Gadolinium Coordination Polymer. Molecules. 2023; 28(1):163. https://doi.org/10.3390/molecules28010163
Chicago/Turabian StyleHou, Ting, Lan-Qing Wu, Yan Xu, Song-Song Bao, and Li-Min Zheng. 2023. "pH and Salt-Assisted Macroscopic Chirality Inversion of Gadolinium Coordination Polymer" Molecules 28, no. 1: 163. https://doi.org/10.3390/molecules28010163
APA StyleHou, T., Wu, L.-Q., Xu, Y., Bao, S.-S., & Zheng, L.-M. (2023). pH and Salt-Assisted Macroscopic Chirality Inversion of Gadolinium Coordination Polymer. Molecules, 28(1), 163. https://doi.org/10.3390/molecules28010163