Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds
Abstract
:1. Introduction
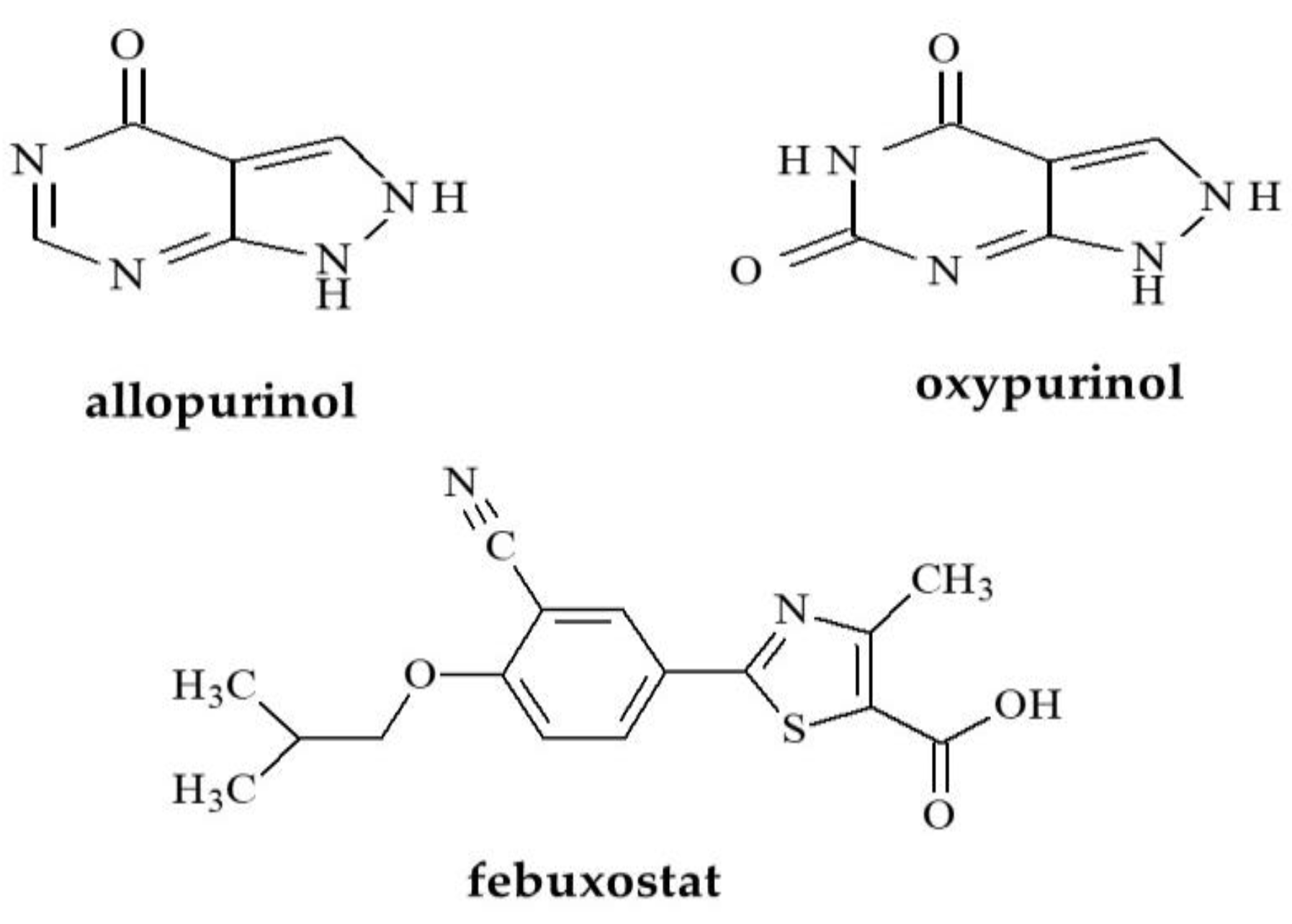
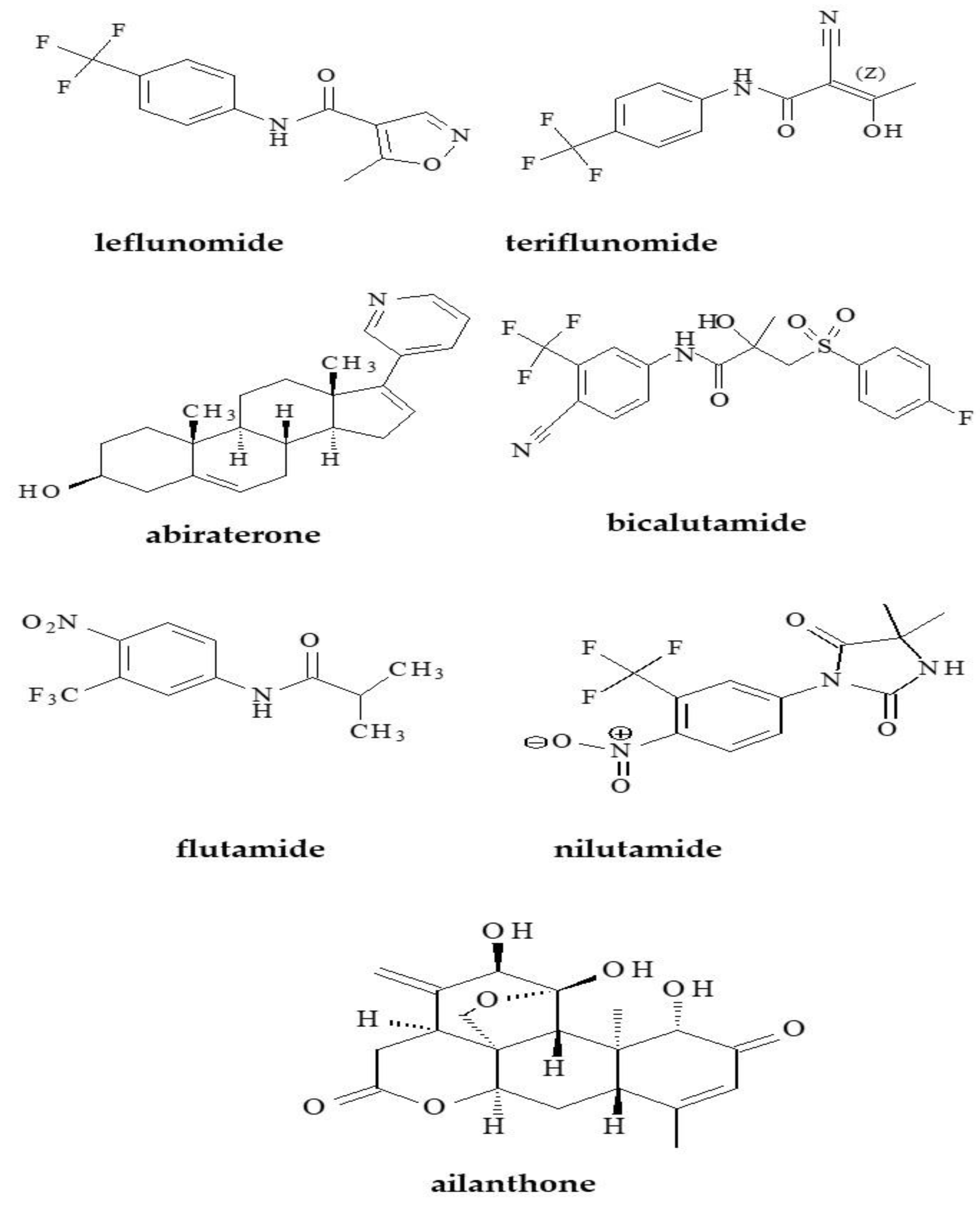
1.1. Uric Acid Lowering Compounds
1.2. Anti-Androgens
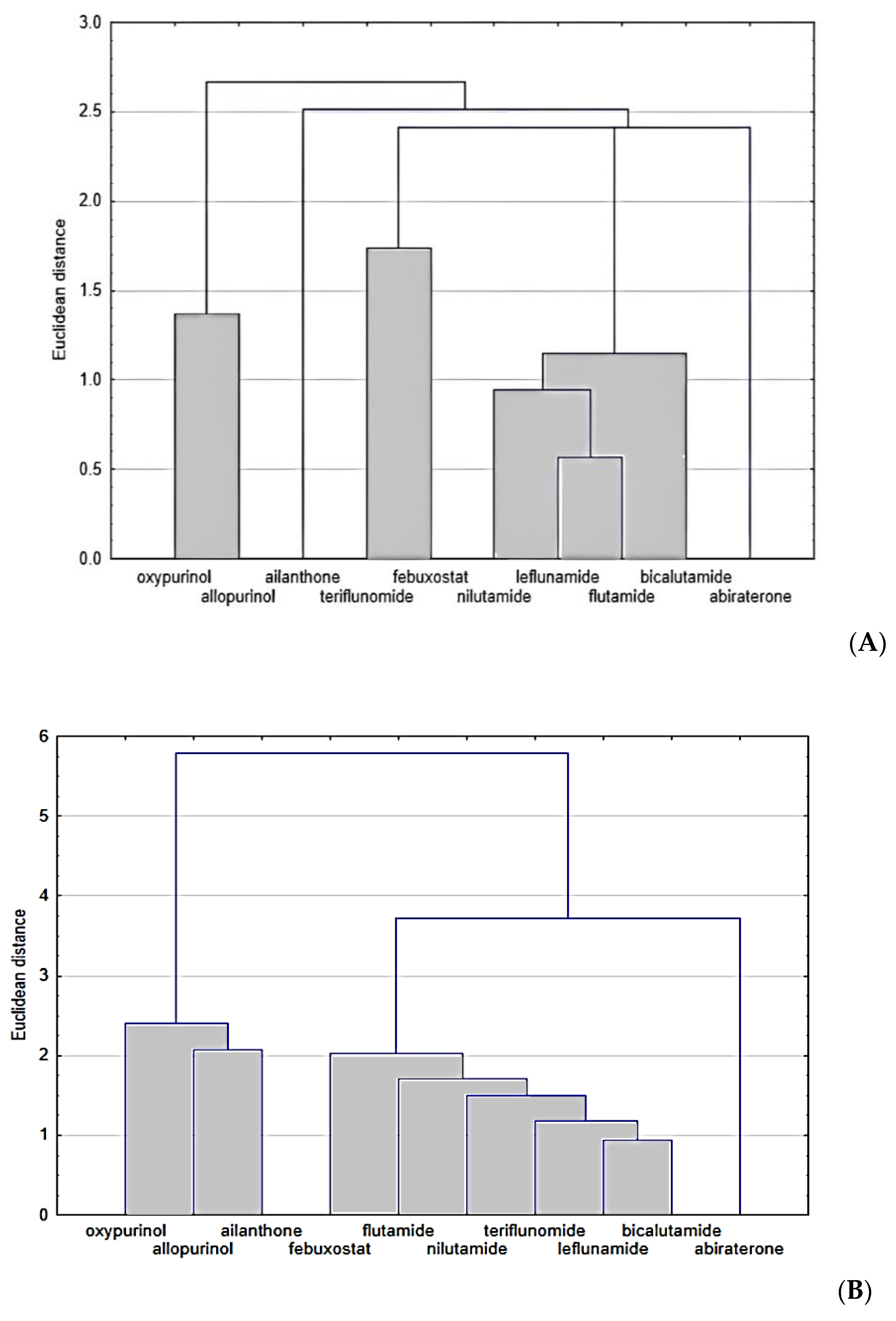
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Analytes
3.3. Materials
3.4. Chromatographic Analysis
3.5. In Silico Study
3.6. Cluster Analysis (CA)
3.7. Principal Component Analysis (PCA)
3.8. Sum of Ranking Differences (SRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jóźwiak, K.; Szumilo, H.; Soczewinski, E. Lipophilicity, methods of determination and its role in biological effect of chemical substances. Wiad. Chem. 2001, 55, 1047–1074. [Google Scholar]
- Miller, R.R.; Madeira, M.; Wood, H.B.; Geissler, W.M.; Raab, C.E.; Martin, I.J. Integrating the impact of lipophilicity on potency and pharmacokinetic parameters enables the use of diverse chemical space during small molecule drug optimization. J. Med. Chem. 2020, 63, 12156–12170. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S. Is there enough focus on lipophilicity in drug discovery? Expert Opin. Drug Discov. 2019, 15, 261–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dołowy, M.; Jampilek, J.; Bober-Majnusz, K. A Comparative study of the lipophilicity of metformin and phenformin. Molecules 2021, 26, 6613. [Google Scholar] [CrossRef] [PubMed]
- Dołowy, M.; Pyka, A. Lipophilicity assessment of spironolactone by means of reversed phase liquid chromatography and by newly developed calculation procedures. Acta Pol. Pharm. 2015, 72, 235–244. [Google Scholar] [PubMed]
- Dołowy, M.; Pyka, A. Evaluation of lipophilic properties of betamethasone and related compounds. Acta Pol. Pharm. 2015, 72, 671–681. [Google Scholar]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Evaluation of the lipophilicity of new anticancer 1,2,3-triazole-dipyridothiazine hybrids using RP-TLC and different computational methods. Processes 2020, 8, 858. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Boryczka, S. Chromatographic and computational screening of lipophilicity and pharmacokinetics of newly synthesized betulin-1,4-quinone hybrids. Processes 2021, 9, 376. [Google Scholar] [CrossRef]
- Bębenek, E.; Bober-Majnusz, K.; Siudak, S.; Chrobak, E.; Kadela-Tomanek, M.; Wietrzyk, J.; Boryczka, S. Application of TLC to evaluate the lipophilicity of newly synthesized betulin derivatives. J. Chromatogr. Sci. 2020, 58, 323–333. [Google Scholar] [CrossRef]
- Marciniec, K.; Bafeltowska, J.; Maślankiewicz, M.; Buszman, E.; Boryczka, S. Determination of the lipophilicity of quinoline sulfonamides by reversed-phase HPLC and theoretical calculations. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 702–709. [Google Scholar] [CrossRef]
- Strzemecka, L.; Hawrył, A.; Świeboda, R.; Hawrył, M.; Jagiełło-Wójtowicz, E.; Piątkowska-Chmiel, I.; Herbet, M.; Chodkowska, A. Determination of lipophilicity of allyl thiosemicarbazide, N-1-thiocarbamylamidrazone derivatives, and their cyclic products by RP-HPLC, RP-TLC, and theoretical methods: Effects of selected compounds on the CNS of mice. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1452–1465. [Google Scholar] [CrossRef]
- Szymański, P.; Skibiński, R.; Liszka, M.; Jargieło, J.; Mikiciuk-Olasik, E.; Komsta, Ł. A TLC study of the lipophilicity of thirty-two acetylcholinesterase inhibitors—1,2,3,4-tetrahydroacridine and 2,3-dihydro- 1H-cyclopenta[b] quinoline derivatives. Cent. Eur. J. Chem. 2013, 11, 927–934. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Komsta, Ł.; Krzek, J.; Kokoszka, K. Lipophilicity study of eight cephalosporins by reversed-phase thin-layer chromatographic method. Biomed. Chromatogr. 2015, 29, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a central component of drug-like properties of chalchones and flavonoid derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowska, I.; Studziński, M.; Malinoski, H. Change of 1,2,4-triazole retention and lipophilicity descriptor values in RP-TLC and MLC-TLC systems in the presence of an external magnetic field. J. Plan. Chromatogr. 2017, 30, 106–112. [Google Scholar] [CrossRef]
- Rageh, A.H.; Atia, N.N.; Abdel-Rahman, A.M. Lipophilicity estimation of statins as a decisive physicochemical parameter for their hepato-selectivity using reversed-phase thin layer chromatography. J. Pharm. Biomed. Anal. 2017, 142, 7–14. [Google Scholar] [CrossRef]
- Karadžić, M.; Lončar, D.; Benedeković, G.; Kovačević, I.; Popsavin, V.; Kocačević, S.; Jevrić, L.; Podunavac-Kuzmanavić, S. A comparative study of chromatographic behavior and lipophilicity of selected natural styryl lactones, their derivatives and analogues. Eur. J. Pharm. Sci. 2017, 105, 99–107. [Google Scholar] [CrossRef]
- Mc Bride, E.; Kretsch, A.; Garibay, L.; Brigance, K.; Frey, B.; Buss, B.; Verbeck, G. Rapid experimental and computational determination of phenethylamine drug analogue lipophilicity. Forensic Chem. 2016, 1, 58–65. [Google Scholar] [CrossRef]
- Marciniec, K.; Boryczka, S. Chromatographic and computational assessment of lipophilicity of new anticancer acetylenequinoline derivatives. J. Chromatogr. Sci. 2017, 55, 934–939. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowska, M.; Starek, M.; Chłoń-Rzepa, G.; Zagórska, A.; Komsta, Ł.; Jankowska, A.; Ślusarczyk, M.; Pawłowski, M. Estimation of the lipophilicity of purine-2,6-dione-based TRPA1 antagonists and PDE4/7 inhibitors with analgesic activity. Bioorg. Med. Chem. 2021, 49, 128318. [Google Scholar] [CrossRef]
- Ciura, K.; Fedorowicz, J.; Andrić, F.; Greber, K.E.; Gurgielewicz, A.; Sawicki, W.; Sączewski, J. Lipophilicity determination of quaternary (fluoro)quinolones by chromatographic and theoretical approaches. Int. J. Mol. Sci. 2019, 20, 5288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Lian, H.-Z. Recent advances in lipophilicity measurement by reversed-phase high-performance liquid chromatography. Trends Analyt. Chem. 2015, 68, 28–36. [Google Scholar] [CrossRef]
- Kempińska, D.; Chmiel, T.; Kot-Wasik, A.; Mróz, A.; Mazerska, Z.; Namieśnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. Trends Analyt. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Peng, S.; Yi, Z.; Liu, M. Ailanthone: A new potential drug for castration-resistant prostate cancer. Chin. J. Cancer 2017, 36, 25. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, C.; Hwang, J.H.; Kopp, J.B.; Winkler, C.A.; Cho, S.K.C. Review of urate-lowering therapeutics: From the past to the future. Front. Pharmacol. 2022, 13, 925219. [Google Scholar] [CrossRef]
- Leland, W.K.; Chung, I.W.B.; Simons, J.W. Prostate Cancer: Biology, Genetics, and the New Therapeutics, 2nd ed.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Feldman, B.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef]
- Mainwaring, W.I. A soluble androgen receptor in the cytoplasm of rat prostate. J. Endocrinol. 1969, 45, 531–541. [Google Scholar] [CrossRef]
- Crawford, E.D.; Schellhammer, P.F.; McLeod, D.G.; Moul, J.W.; Higano, C.S.; Shore, N.; Denis, L.; Iversen, P.; Eisenberger, M.A.; Labrie, F. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J. Urol. 2018, 200, 956–966. [Google Scholar] [CrossRef]
- Maughan, B.L.; Antonarakis, E.S. Androgen pathway resistance in prostate cancer and therapeutic implications. Expert Opin. Pharmacother. 2015, 16, 1521–1537. [Google Scholar] [CrossRef]
- Tang, S.; Ma, X.; Lu, J.; Zhang, Y.; Liu, M.; Wang, X. Preclinical toxicology and toxicokinetic evaluation of ailanthone, a natural product against castration-resistant prostate cancer, in mice. Fitoterapia 2019, 136, 104161. [Google Scholar] [CrossRef]
- Numsen, H.J.; Chen, P.; Bushman, L.R. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: Evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia 2010, 12, 464–475. [Google Scholar] [CrossRef] [Green Version]
- Wysocki, P. Abiraterone acetate in the treatment of patients with metastatic prostate cancer. Oncol. Clin. Pr. 2017, 13, 220–224. [Google Scholar] [CrossRef]
- Andrić, F.; Héberger, K. Towards better understanding of lipophilicity: Assessment of in silico and chromatographic logP measures for pharmaceutically important compounds by nonparametric rankings. J. Pharm. Biomed. Anal. 2015, 115, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Andrić, F.; Héberger, K. Chromatographic and computational assessment of lipophilicity using sum of ranking differences and generalized pair-correlation. J. Chromatogr. A 2015, 1380, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Andrić, F.; Bajusz, D.; Rácz, A.; Šegan, S.; Héberger, K. Multivariate assessment of lipophilicity scales—Computational and reversed phase thin-layer chromatographic indices. J. Pharm. Biomed. Anal. 2016, 127, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Stanisz, A. Przystępny Kurs Statystyki z Zastosowaniem STATISTICA PL na Przykładach Medycyny; Analizy Wielowymiarowe; Stat Soft Polska: Krakow, Poland, 2007; Volume 3. [Google Scholar]
- Jolliffe, J.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. 2016, 374, 20150202. [Google Scholar] [CrossRef]

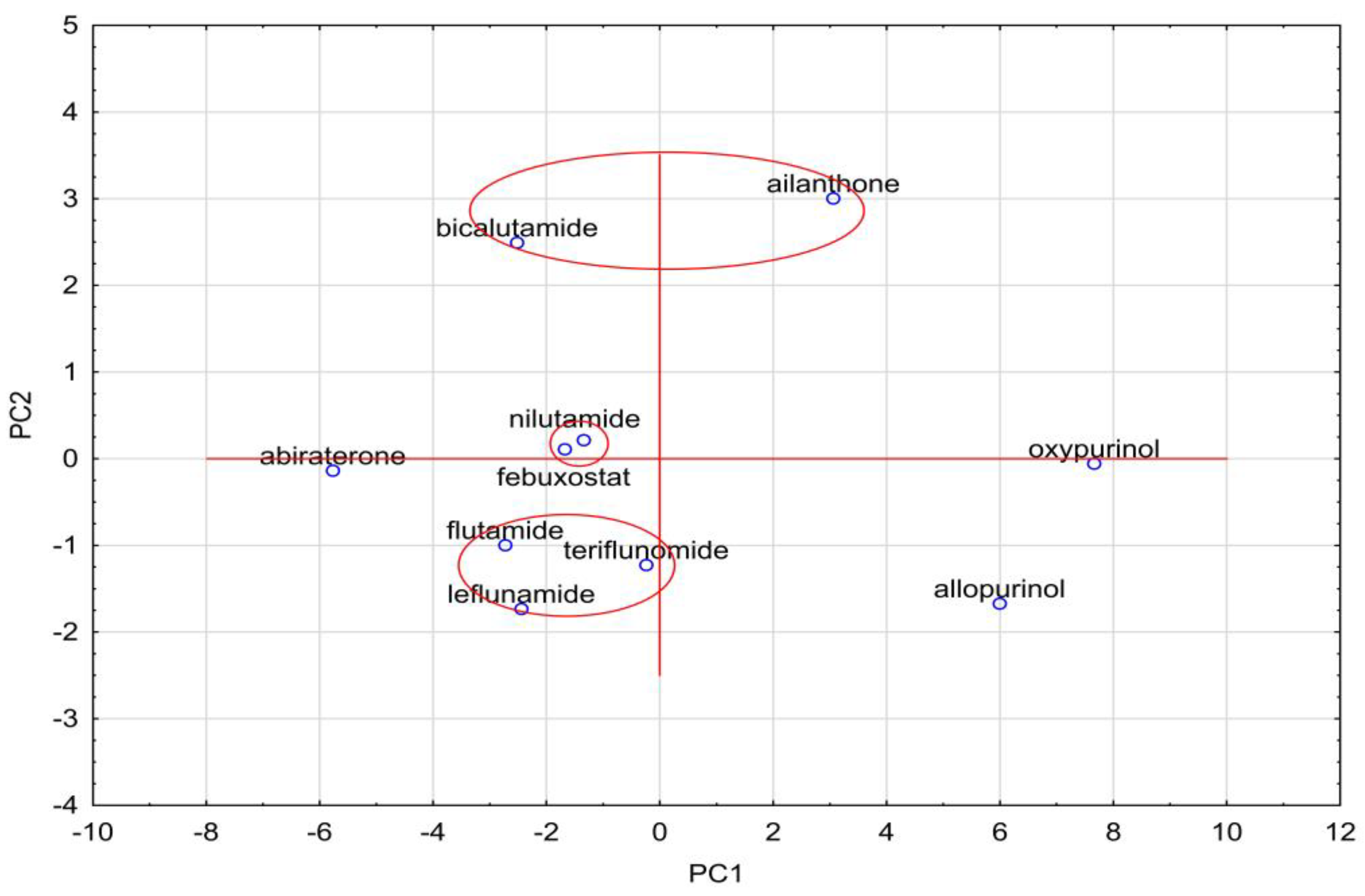
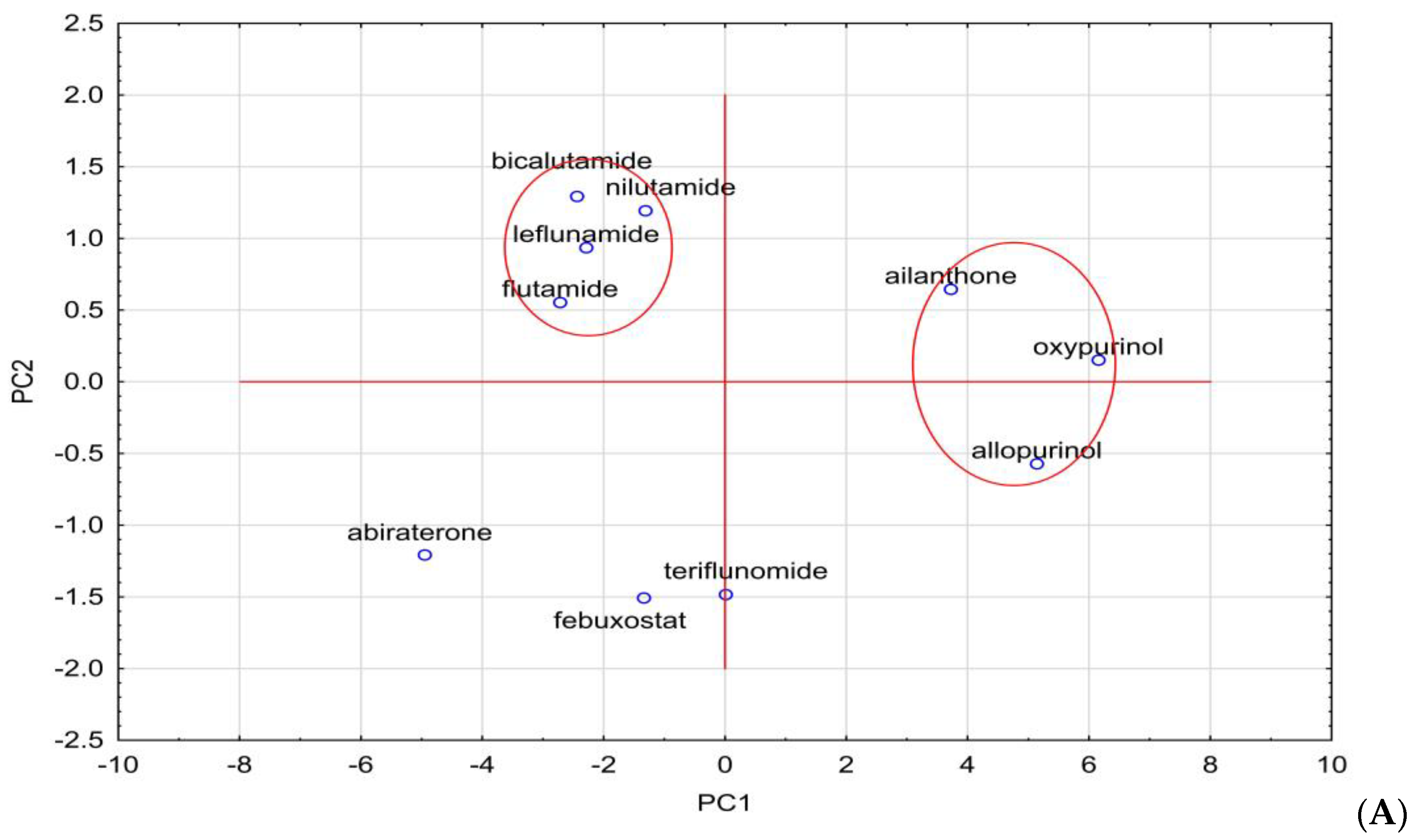

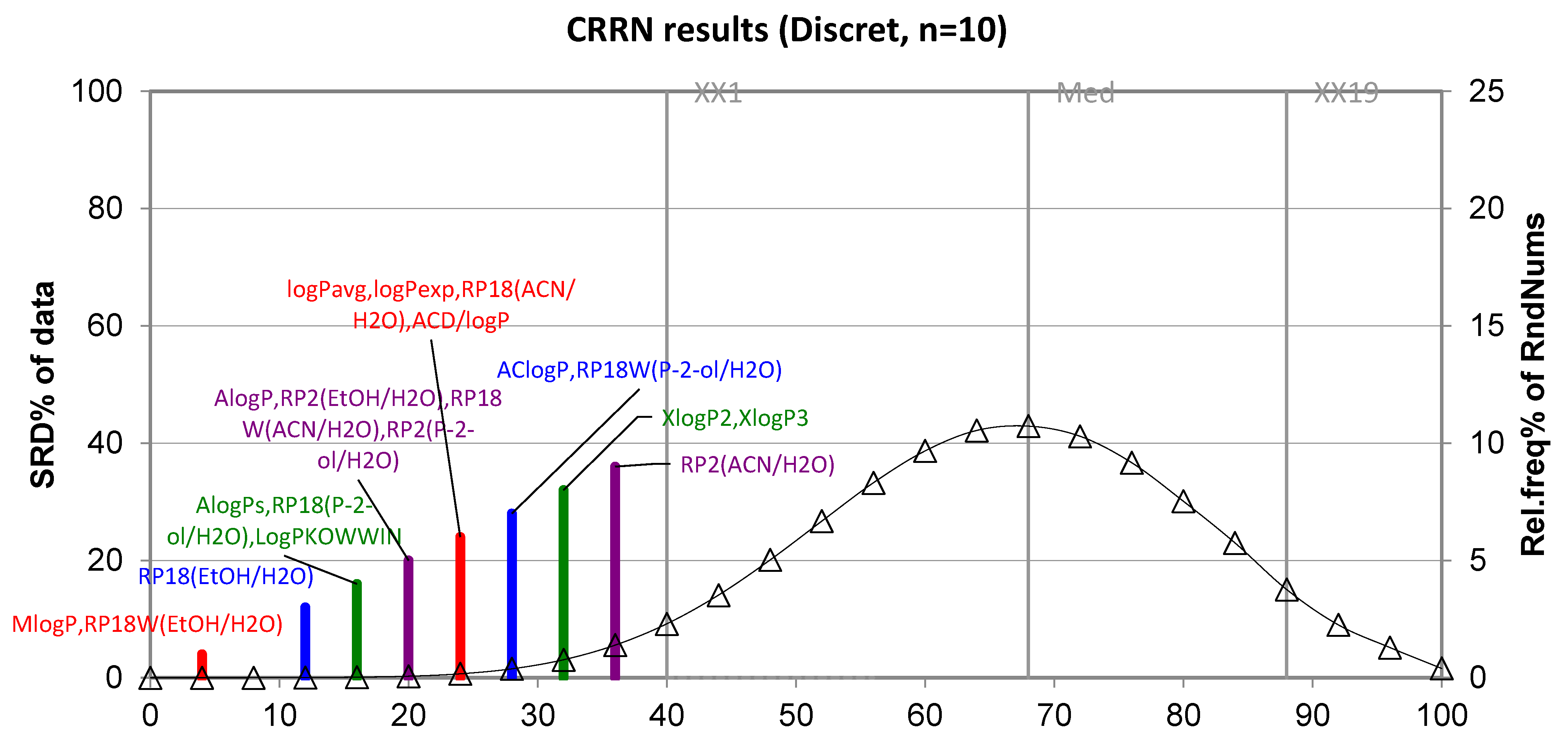
| Compound | AlogPs | AC Logp | AlogP | MlogP | XlogP2 | XlogP3 | logPavg | logPexp | logP KOWWIN | ACD/logP |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood Uric Acid Lowering Compounds | ||||||||||
| Allopurinol | −0.41 | −0.20 | 0.03 | 0.58 | −0.90 | 0.15 | 0.17 | −1.80 | −1.14 | −1.46 |
| Oxypurinol | −0.65 | −0.44 | −0.87 | −0.73 | −0.34 | −0.93 | −0.66 | - | −2.17 | −1.35 |
| Febuxostat | 3.80 | 4.09 | 3.44 | 2.00 | 2.60 | 3.90 | 3.31 | - | 4.21 | - |
| Anti-Androgens | ||||||||||
| Abiraterone | 5.10 | 4.17 | 4.22 | 4.52 | 4.02 | 4.63 | 4.44 | - | 6.40 | 5.70 |
| Bicalutamide | 2.70 | 2.00 | 2.93 | 2.74 | 2.53 | 2.31 | 2.53 | 2.50 | 2.30 | 4.94 |
| Flutamide | 2.55 | 3.02 | 2.92 | 3.16 | 2.64 | 3.35 | 2.94 | 3.35 | 3.51 | 3.72 |
| Nilutamide | 1.74 | 2.08 | 2.26 | 2.23 | 1.84 | 2.00 | 2.02 | 1.80 | 1.92 | 2.23 |
| Leflunomide | 2.52 | 2.45 | 2.16 | 2.37 | 2.79 | 2.49 | 2.46 | 2.80 | 2.43 | - |
| Teriflunomide | 2.30 | 3.06 | 2.07 | 1.68 | - | 3.27 | 2.48 | - | 2.25 | 2.51 |
| Ailanthone | 0.01 | 0.26 | −0.32 | 0.99 | −0.47 | −1.12 | −0.11 | - | 0.25 | −0.76 |
| Compound | Density [g/cm3] | Boiling Point [°C] | Index of Refraction | Molar Refractivity | Polar Surface Area [A°] | Polarizability [cm3] | Surface Tension [dyne/cm] | Molar Volume [cm3] |
|---|---|---|---|---|---|---|---|---|
| Blood Uric Acid Lowering Compounds | ||||||||
| Allopurinol | 1.7 | 423.2 | 1.816 | 34.7 | 75 | 13.8 | 126.4 | 80.0 |
| Oxypurinol | 1.9 | 662.9 | 1.903 | 36.6 | 95 | 14.5 | 170.2 | 78.4 |
| Febuxostat | 1.3 | 488.2 | 1.605 | 83.1 | 83 | 32.9 | 63.7 | 240.9 |
| Anti-Androgens | ||||||||
| Abiraterone | 1.1 | 500.2 | 1.606 | 105.2 | 33 | 91.7 | 50.1 | 305.2 |
| Bicalutamide | 1.5 | 650.3 | 1.578 | 93.8 | 116 | 37.2 | 58.2 | 282.8 |
| Flutamide | 1.4 | 400.3 | 1.521 | 61.3 | 75 | 24.3 | 38.3 | 201.3 |
| Nilutamide | 1.5 | 477.3 | 1.524 | 66.3 | 95 | 26.3 | 42.9 | 216.8 |
| Leflunomide | 1.4 | 289.3 | 1.541 | 61.0 | 55 | 24.2 | 40.6 | 194.1 |
| Teriflunomide | 1.4 | 410.8 | 1.552 | 60.6 | 73 | 24.0 | 45.4 | 189.7 |
| Ailanthone | 1.0 | 641.0 | 1.640 | 91.9 | 113 | 36.4 | 68.0 | 254.9 |
| Mobile Phase | Chromatographic Plates | ||
|---|---|---|---|
| RP2F254 | RP18F254 | RP18WF254 | |
| Blood Uric Acid Lowering Compounds | |||
| Allopurinol | |||
| Ethanol–water | 0.4000 | 0.2164 | 0.2321 |
| Propan-2-ol-water | −0.0849 | 0.0399 | −0.0722 |
| Acetonitrile–water | 0.2783 | −0.0737 | −0.1744 |
| Oxypurinol | |||
| Ethanol–water | −0.5431 | 0.1808 | −0.0346 |
| Propan-2-ol-water | −0.1945 | −0.3749 | −0.5789 |
| Acetonitrile–water | 0.7764 | 0.3765 | −0.2654 |
| Febuxostat | |||
| Ethanol–water | 1.9788 | 2.7106 | 2.0687 |
| Propan-2-ol-water | 1.2524 | 1.9112 | 1.2259 |
| Acetonitrile–water | 1.5648 | 1.9061 | 1.5489 |
| Anti-Androgens | |||
| Abiraterone | |||
| Ethanol–water | 4.3012 | 4.5660 | 3.8765 |
| Propan-2-ol-water | 2.5179 | 3.0060 | 2.0141 |
| Acetonitrile–water | 2.7366 | 1.8373 | 2.4400 |
| Bicalutamide | |||
| Ethanol–water | 2.8044 | 3.1861 | 2.8711 |
| Propan-2-ol-water | 1.9488 | 2.7550 | 1.4439 |
| Acetonitrile–water | 3.0843 | 4.0113 | 2.8946 |
| Flutamide | |||
| Ethanol–water | 2.9756 | 3.1037 | 2.9650 |
| Propan-2-ol-water | 2.4137 | 2.2432 | 1.7854 |
| Acetonitrile–water | 2.6436 | 3.3395 | 2.7053 |
| Nilutamide | |||
| Ethanol–water | 3.0454 | 2.8873 | 2.3245 |
| Propan-2-ol-water | 2.1987 | 1.9313 | 1.4803 |
| Acetonitrile–water | 2.7675 | 3.1543 | 2.3260 |
| Leflunomide | |||
| Ethanol–water | 3.0335 | 3.5015 | 2.8223 |
| Propan-2-ol-water | 2.5391 | 2.3386 | 1.8840 |
| Acetonitrile–water | 2.7559 | 3.0430 | 2.6675 |
| Teriflunomide | |||
| Ethanol–water | 1.3228 | 1.9800 | 1.2736 |
| Propan-2-ol-water | 1.0884 | 2.0438 | 1.8615 |
| Acetonitrile–water | 0.9696 | 1.3186 | 1.0250 |
| Ailanthone | |||
| Ethanol–water | 0.8299 | 0.6137 | 0.3212 |
| Propan-2-ol-water | 0.7606 | 1.7092 | 0.2488 |
| Acetonitrile–water | 0.4203 | 0.9749 | 1.2568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardecki, D.; Dołowy, M.; Bober-Majnusz, K.; Jampilek, J. Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds. Molecules 2023, 28, 166. https://doi.org/10.3390/molecules28010166
Wardecki D, Dołowy M, Bober-Majnusz K, Jampilek J. Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds. Molecules. 2023; 28(1):166. https://doi.org/10.3390/molecules28010166
Chicago/Turabian StyleWardecki, Dawid, Małgorzata Dołowy, Katarzyna Bober-Majnusz, and Josef Jampilek. 2023. "Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds" Molecules 28, no. 1: 166. https://doi.org/10.3390/molecules28010166
APA StyleWardecki, D., Dołowy, M., Bober-Majnusz, K., & Jampilek, J. (2023). Comparative Study of the Lipophilicity of Selected Anti-Androgenic and Blood Uric Acid Lowering Compounds. Molecules, 28(1), 166. https://doi.org/10.3390/molecules28010166