Variable Temperature Behaviour of the Hybrid Double Perovskite MA2KBiCl6
Abstract
1. Introduction
2. Results and Discussions
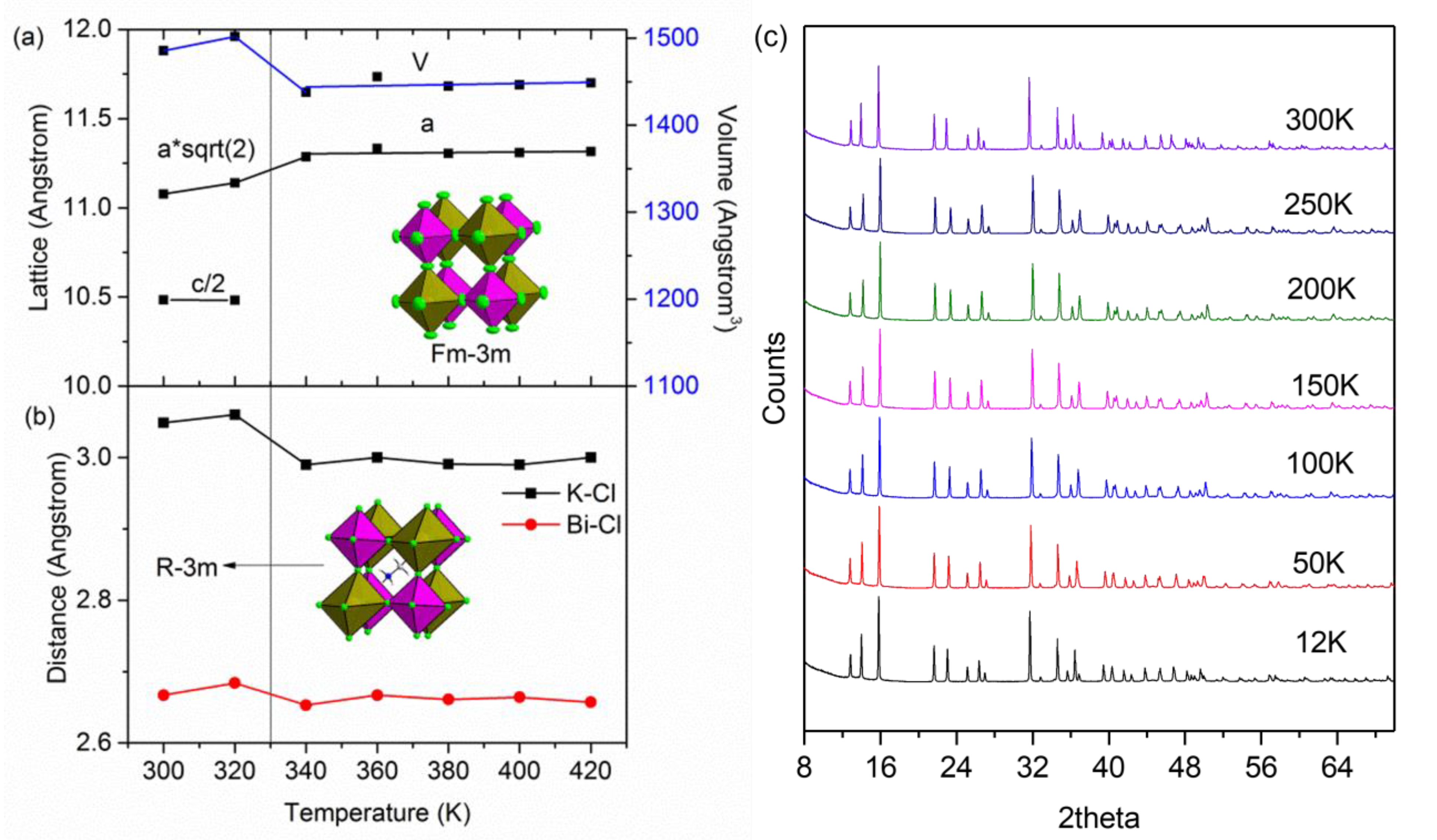
2.1. High Temperature Phase Transition
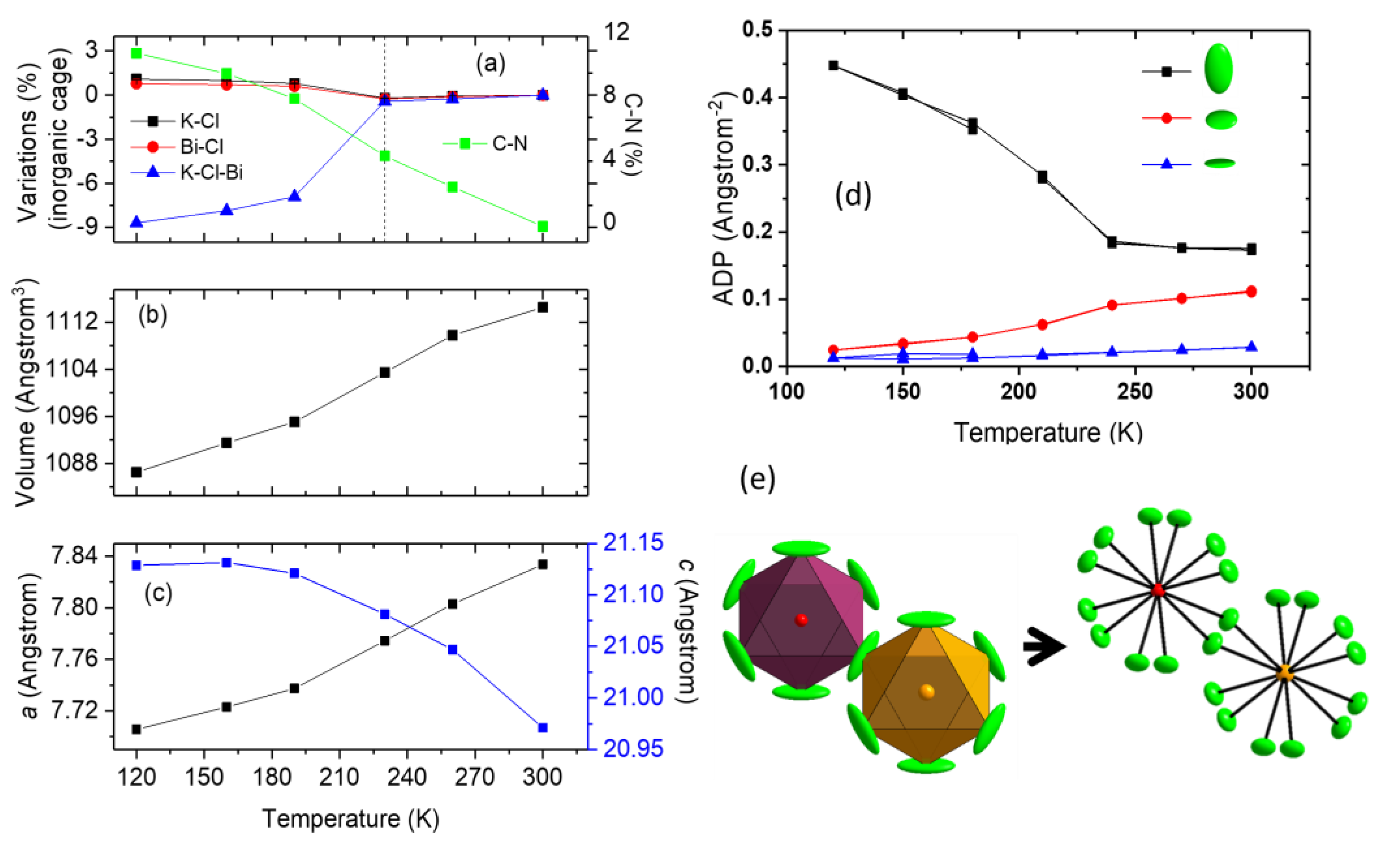
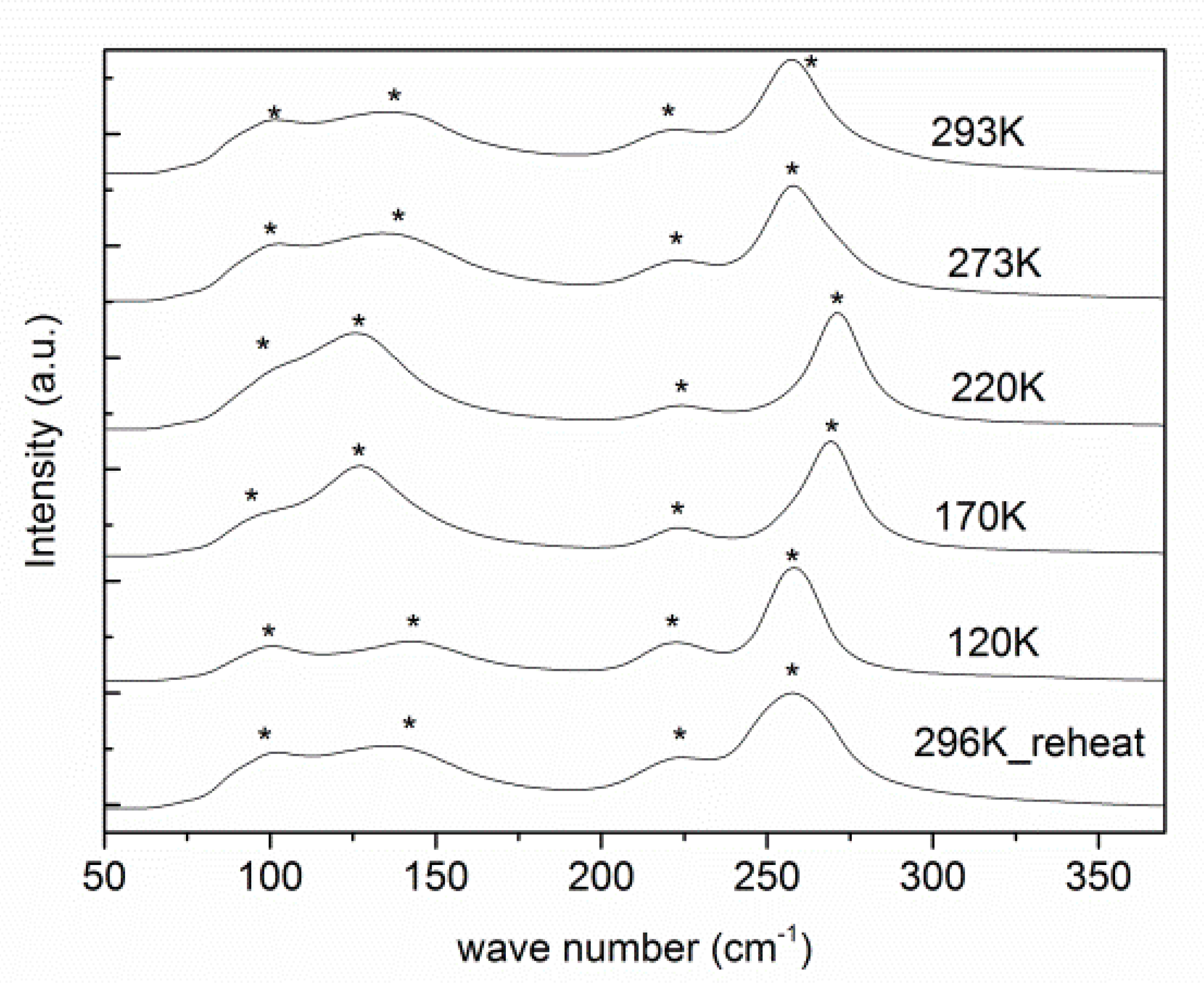
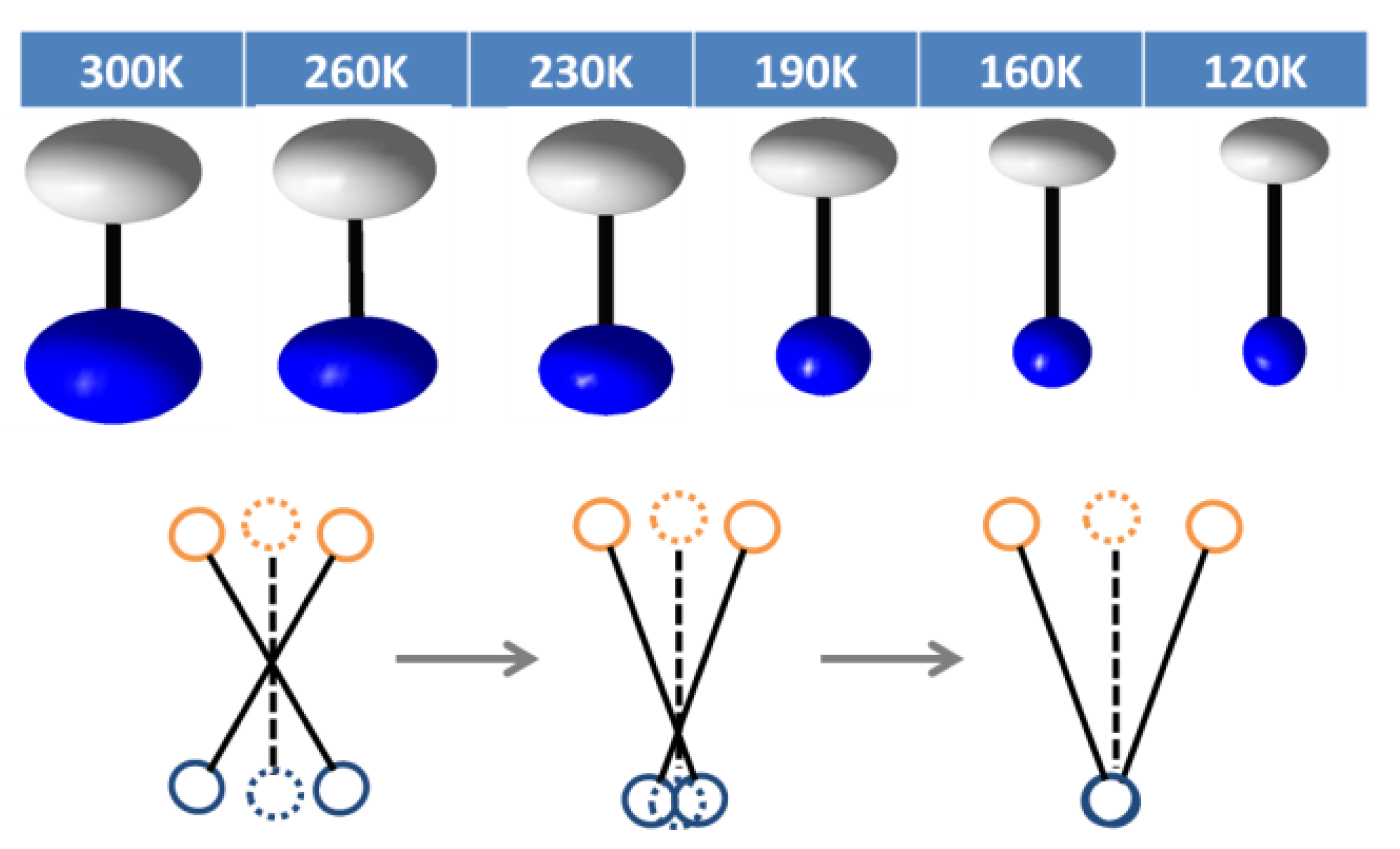
2.2. Low Temperature Behaviour
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Labs (NREL) Efficiency Chart. Available online: http://www.nrel.gov/ncpv/images/efficiency_chart.jpg (accessed on 25 July 2016).
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 Single-Crystal X-Ray Detectors with a Low Detection Limit. Nat. Photon. 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Morss, L.R.; Siegal, M.; Stenger, L.; Edelstein, N. Preparation of Cubic Chloro Complex Compounds of Trivalent Metals: Cs2NaMCl6. Inorg. Chem. 1970, 9, 1771–1775. [Google Scholar] [CrossRef]
- Flerov, I.N.; Gorev, M.V.; Aleksandrov, K.S.; Tressaud, A.; Grannec, J.; Couzi, M. Phase Transitions in Elpasolites (Ordered Perovskites). Mater. Sci. Eng. R Rep. 1998, 24, 81–151. [Google Scholar] [CrossRef]
- Couzi, M.; Khaïroun, S.; Tressaud, A. Structural Phase Transitions in Rb2KMIIIF6 Elpasolites. II. Raman Scattering and Group-Theoretical Studies of Rb2KFeF6 and Rb2KYF6. Phys. Status Solidi (a) 1986, 98, 423–434. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Xie, F.; Kieslich, G.; Evans, D.M.; Carpenter, M.A.; Bristowe, P.D.; Cheetham, A.K. The Synthesis, Structure and Electronic Properties of a Lead-Free Hybrid Inorganic-Organic Double Perovskite (MA)2KBiCl6 (MA = Methylammonium). Mater. Horiz. 2016, 3, 328–332. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, F.; Sun, S.; Kieslich, G.; Cheetham, A.K.; Bristowe, P.D. Exploring the Properties of Lead-Free Hybrid Double Perovskites Using a Combined Computational-Experimental Approach. J. Mater. Chem. A Mater. 2016, 4, 12025–12029. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Zhang, F.; Evans, D.M.; Kieslich, G.; Tominaka, S.; Carpenter, M.A.; Zhang, J.; Bristowe, P.D.; et al. Synthesis and Properties of a Lead-Free Hybrid Double Perovskite: (CH3NH3)2AgBiBr6. Chem. Mater. 2017, 29, 1089–1094. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6(X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-Free Halide Double Perovskites via Heterovalent Substitution of Noble Metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs 2 InAgCl 6: A New Lead-Free Halide Double Perovskite with Direct Band Gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Deng, Z.; Sun, S.; Hartono, N.T.P.; Seng, H.L.; Buonassisi, T.; Bristowe, P.D.; Cheetham, A.K. Enhanced Visible Light Absorption for Lead-Free Double Perovskite Cs2AgSbBr6. Chem. Communicat. 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- Bennett, T.D.; Tan, J.-C.; Moggach, S.A.; Galvelis, R.; Mellot-Draznieks, C.; Reisner, B.A.; Thirumurugan, A.; Allan, D.R.; Cheetham, A.K. Mechanical Properties of Dense Zeolitic Imidazolate Frameworks (ZIFs): A High-Pressure X-Ray Diffraction, Nanoindentation and Computational Study of the Zinc Framework Zn(Im)2, and Its Lithium—Boron Analogue, LiB(Im)4. Chem. Eur. J. 2010, 16, 10684–10690. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Song, W.D.; He, K.; Chai, J.; Sun, C.; Chow, G.; Chen, J. The role of octahedral tilting in the structural phase transition and magnetic anisotropy in SrRuO3 thin film. J. Appl. Phys. 2013, 113, 063901. [Google Scholar] [CrossRef]
- Smit, W.M.A.; Dirksen, G.J.; Stufkens, D.J. Infrared and Raman Spectra of the Elpasolites Cs2NaSbCl6 and Cs2NaBiCl6: Evidence for a Pseudo Jahn-Teller Distorted Ground State. J. Phys. Chem. Solids 1990, 51, 189–196. [Google Scholar] [CrossRef]
- Pistor, P.; Meyns, M.; Guc, M.; Wang, H.; Marques, M.; Alcobé, X.; Cabot, A.; Izquierdo-Roca, V. Advanced Raman spectroscopy of Cs2AgBiBr6 double perovskites and identification of Cs3Bi2Br9 secondary phases. Scr. Mater. 2020, 184, 24–29. [Google Scholar] [CrossRef]
- Deng, Z.; Wei, F.; Brivio, F.; Wu, Y.; Sun, S.; Bristowe, P.D.; Cheetham, A.K. Synthesis and Characterization of the Rare-Earth Hybrid Double Perovskites: (CH3NH3)2KGdCl6 and (CH3NH3)2KYCl6. J. Phys. Chem. Lett. 2017, 8, 5015–5020. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXS97, Program for the Solution of Crystal Structures. A short Hist. SHELX 2008, 64, 112–122. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Wu, Y.; Sun, S.; Deng, Z.; Chew, L.T.; Cheng, B.; Tan, C.C.; White, T.J.; Cheetham, A.K. Variable Temperature Behaviour of the Hybrid Double Perovskite MA2KBiCl6. Molecules 2023, 28, 174. https://doi.org/10.3390/molecules28010174
Wei F, Wu Y, Sun S, Deng Z, Chew LT, Cheng B, Tan CC, White TJ, Cheetham AK. Variable Temperature Behaviour of the Hybrid Double Perovskite MA2KBiCl6. Molecules. 2023; 28(1):174. https://doi.org/10.3390/molecules28010174
Chicago/Turabian StyleWei, Fengxia, Yue Wu, Shijing Sun, Zeyu Deng, Li Tian Chew, Baisong Cheng, Cheng Cheh Tan, Timothy J. White, and Anthony K. Cheetham. 2023. "Variable Temperature Behaviour of the Hybrid Double Perovskite MA2KBiCl6" Molecules 28, no. 1: 174. https://doi.org/10.3390/molecules28010174
APA StyleWei, F., Wu, Y., Sun, S., Deng, Z., Chew, L. T., Cheng, B., Tan, C. C., White, T. J., & Cheetham, A. K. (2023). Variable Temperature Behaviour of the Hybrid Double Perovskite MA2KBiCl6. Molecules, 28(1), 174. https://doi.org/10.3390/molecules28010174






