A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives
Abstract
:1. Introduction
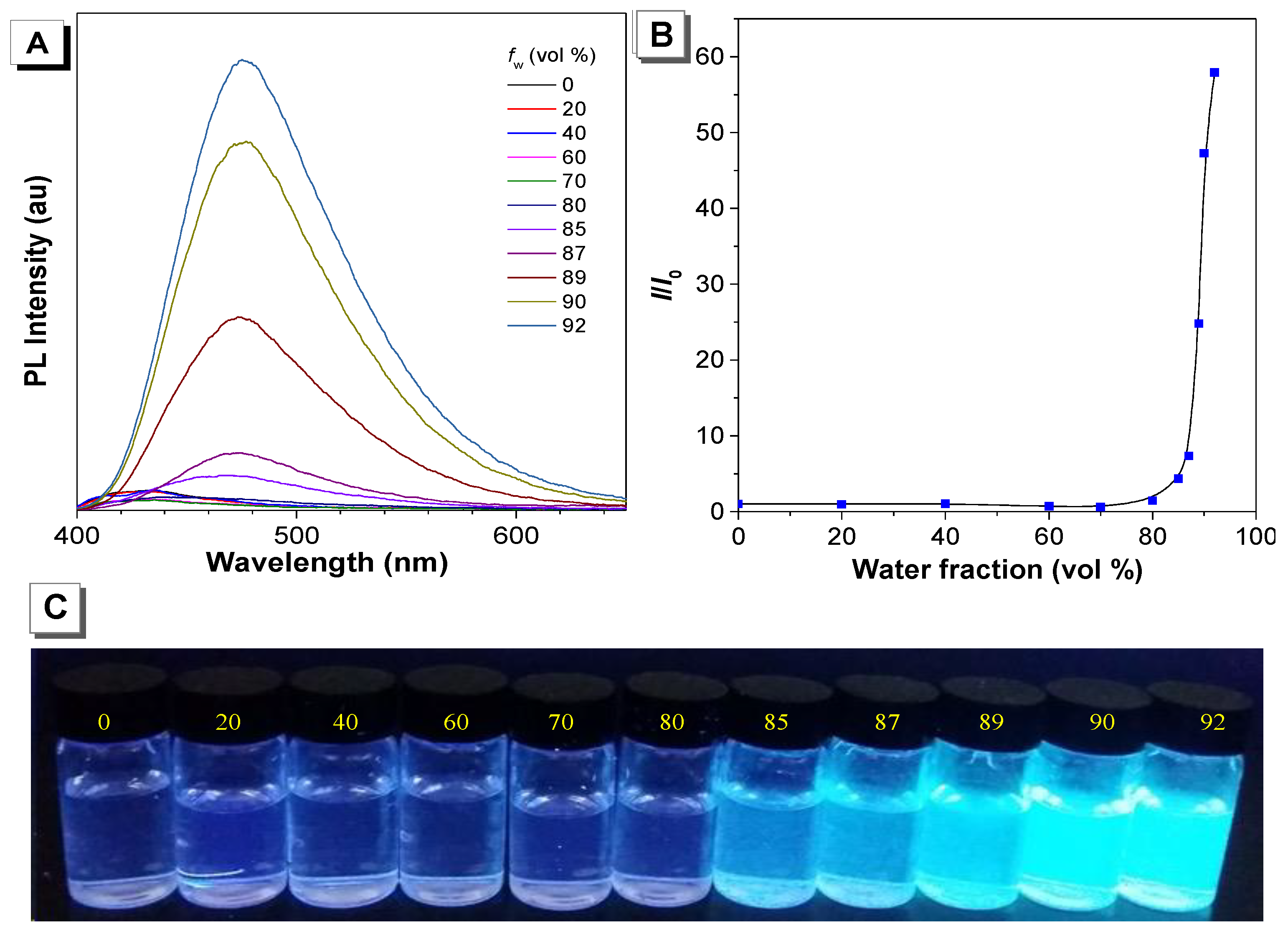
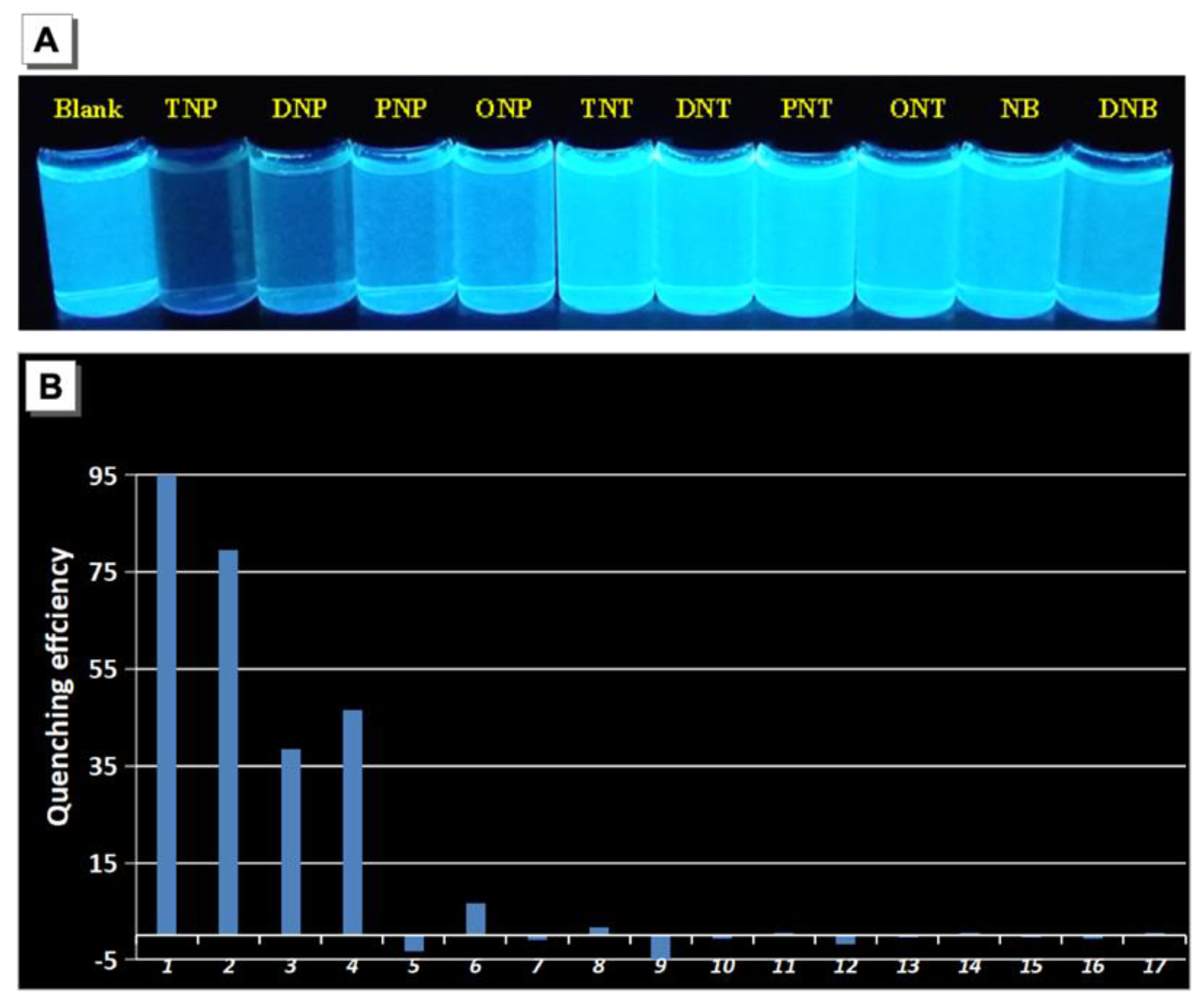
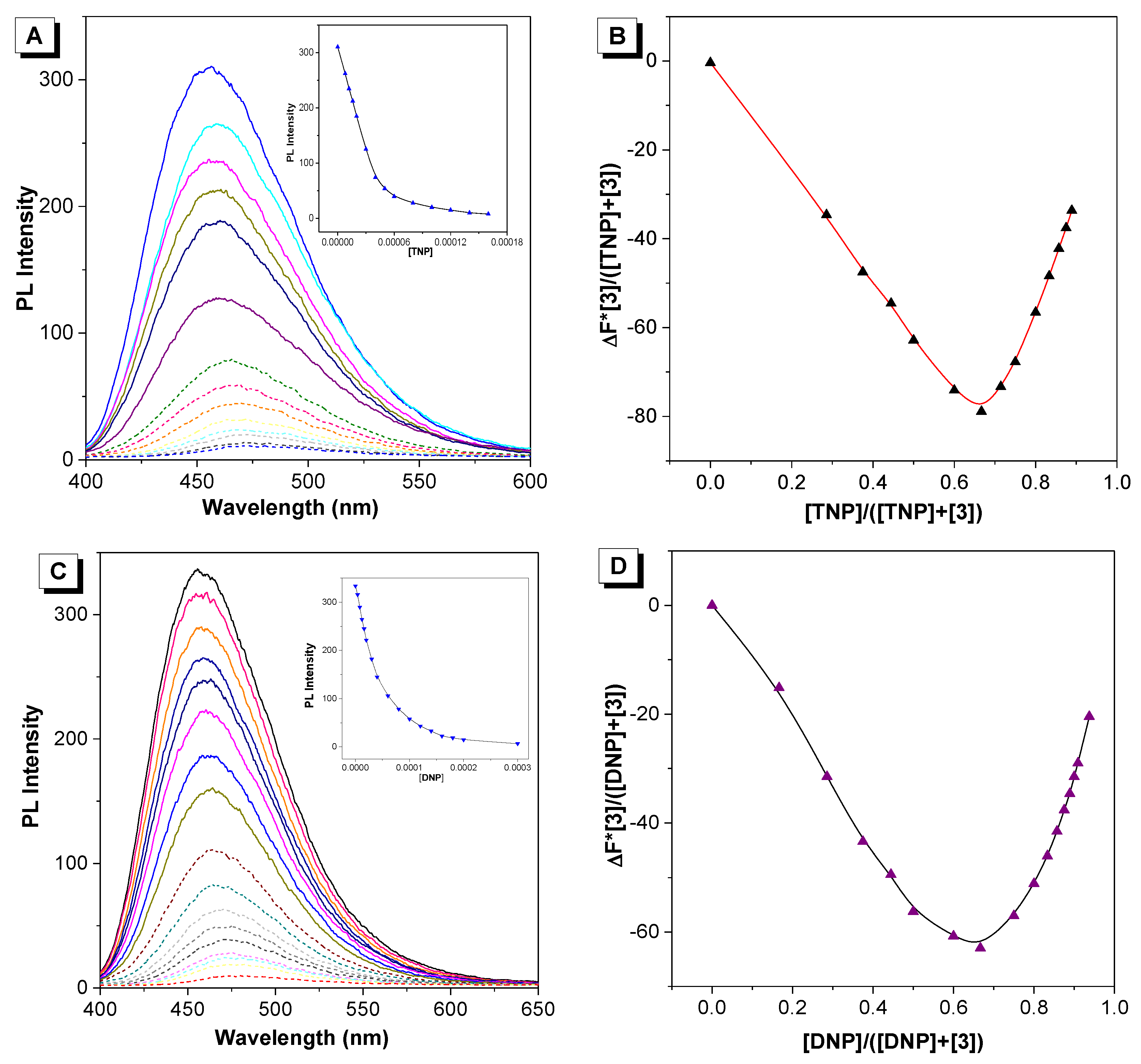
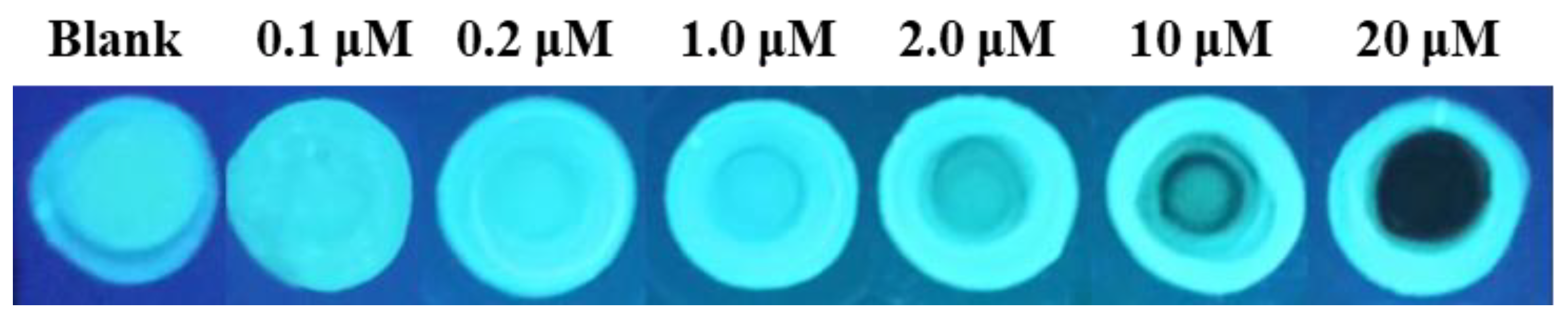
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of 2
3.2. Synthesis of 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Patil, G.; Dongre, S.-D.; Das, T. Sukumaran. S.-B. Dual Mode Selective Detection and Differentiation of TNT from Other Nitroaromatic Compounds. J. Mater. Chem. A 2020, 8, 10767–10771. [Google Scholar]
- Shanmugaraju, S.; Dabadie, C.; Byrne, K.; Savyasachi, A.-J.; Umadevi, D.; Schmitt, W.; Kitchen, J.-A.; Gunnlaugsson, T. A supramolecular Tröger’s base derived coordination zinc polymer for fluorescent sensing of phenolic-nitroaromatic explosives in water. Chem. Sci. 2017, 8, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danquah, M.-K.; Wang, S.; Wang, Q.-Y.; Wang, B.; Wilson, L.-D. A porous b-cyclodextrin-based terpolymer fluorescence sensor for in situ trinitrophenol detection. RSC. Adv. 2019, 9, 8073–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, T.-P.; Sisco, E. Recent advances in ambient mass spectrometry of trace explosives. Analyst 2018, 143, 1948–1969. [Google Scholar] [CrossRef]
- Abhiram, P.; Basanta, P.-S.; Sonam, M.; Debasis, N.; Santanab, G.; Tridib, K.-S. AIE active fluorescent organic nanoaggregates for selective detection of phenolic-nitroaromatic explosives and cell imaging. J. Photoch. Photobio. A 2019, 374, 194–205. [Google Scholar]
- Marder, D.; Tzanani, N.; Prihed, H.; Gura, S. Trace detection of explosives with a unique large volume injection gas chromatography-mass spectrometry (LVI-GC-MS) method. Anal. Methods 2018, 10, 2712–2721. [Google Scholar] [CrossRef]
- Mu, R.; Shi, H.; Yuan, Y.; Karnjanapiboonwong, A.; Burken, J.-G.; Ma, Y. Fast separation and quantification method for nitroguanidine and 2,4-dinitroanisole by ultrafast liquid chromatography–tandem mass spectrometry. Anal. Chem. 2012, 84, 3427–3432. [Google Scholar] [CrossRef]
- Najarro, M.; Dávila Morris, M.-E.; Staymates, M.-E.; Fletcher, R.; Gillen, G. Optimized thermal desorption for improved sensitivity in trace explosives detection by ion mobility spectrometry. Analyst 2012, 137, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, V.-K.; Mizaikoff, B.; Singh, S.-P.; Suri, C.-R. Electrochemical sensing of nitro-aromatic explosive compounds using silver nanoparticles modified electrochips. Anal. Methods 2016, 8, 7158–7169. [Google Scholar] [CrossRef]
- Babaee, S.; Beiraghi, A. Micellar extraction and high performance liquid chromatography-ultra violet determination of some explosives in water samples. Anal. Chim. Acta 2010, 662, 9–13. [Google Scholar] [CrossRef]
- Jha, S.-K.; Ekinci, Y.; Agio, M.; Löffler, J.-F. Towards deep-UV surface-enhanced resonance Raman spectroscopy of explosives: Ultrasensitive, real-time and reproducible detection of TNT. Analyst 2015, 140, 5671–5677. [Google Scholar] [CrossRef]
- Salinas, Y.; Martinez-Manez, R.; Marcos, M.-D.; Sancenon, F.; Costero, A.-M.; Parra, M.; Gil, S. Optical chemosensors and reagents to detect explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraju, S.; Mukherjee, P.-S. π-Electron rich small molecule sensors for the recognition of nitroaromatics. Chem. Commun. 2015, 51, 16014–16032. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.-X.; Fu, H.-R.; Han, M.-L.; Zhou, Z.; Ma, L.-F. Tetraphenylethylene immobilized metal-organic frameworks: Highly sensitive fluorescent sensor for the detection of Cr2O72− and nitroaromatic explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Dang, R.; Ma, X.-R.; Zhao, Y.-P.; Ren, M.; Guo, W.; Kang, Y.-H.; Gao, Y.; Bi, S.; Gao, W.; Hao, H.-R.; et al. Construction of 3D Ni2+-Al3+-LDH/γ-Fe2O3-Cd2+-Ni2+-Fe3+ -LDH structures and multistage recycling treatment of dye and fluoride-containing wastewater. Chem. Eng. J. 2023, 451, 138499. [Google Scholar] [CrossRef]
- Nabeel, F.; Rasheed, T.; Mahmood, M.-F.; Khan, S.-U.-D. Hyperbranched copolymer based photoluminescent vesicular probe conjugated with tetraphenylethene: Synthesis, aggregation-induced emission and explosive detection. J. Mol. Liq. 2020, 308, 113034–113042. [Google Scholar] [CrossRef]
- Liu, S.-G.; Luo, D.; Li, N.; Zhang, W.; Lei, J.-L.; Li, N.-B.; Luo, H.-Q. Water-Soluble Nonconjugated Polymer Nanoparticles with Strong Fluorescence Emission for Selective and Sensitive Detection of Nitro-Explosive Picric Acid in Aqueous Medium. ACS Appl. Mater. Interfaces 2016, 8, 21700–21709. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.-J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ma, Y.; Li, H.; Wang, L. Composite QDS@MIP nanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal. Chem. 2012, 84, 386–395. [Google Scholar] [CrossRef]
- Liu, B.; Tong, C.; Feng, L.; Wang, C.; He, Y.; Lü, C. Water-soluble polymer functionalized CdTe/ZnS quantum dots: A facile ratiometric fluorescent probe for sensitive and selective detection of nitroaromatic explosives. Chem. Eur. J. 2014, 20, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.-Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.-S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.-G.; Chen, Y.-L.; Chen, S.-J.; Deng, H.-Q.; Gui, C.; Leung, C.-W.-T.; Hong, Y.-N.; Lam, J.-W.-Y.; Tang, B.-Z. A luminogen with aggregation-induced emission characteristics for wash-free bacterial imaging, high-throughput antibiotics screening and bacterial susceptibility evaluation. Adv. Mater. 2015, 27, 4931–4937. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.-L.; Zheng, X.-L.; Zhao, Z.; Zhang, H.-K.; Wang, J.-G.; He, X.-W.; Chen, Y.-C.; Shi, X.-J.; Ma, C.; Kwok, R.-T.-K.; et al. Functionalized acrylonitriles with aggregation-induced emission: Structure tuning by simple reaction-condition variation, efficient red emission, and two-photon bioimaging. J. Am. Chem. Soc. 2019, 141, 15111–15120. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Tang, B.-Z. Fluorescent sensors based on aggregation-induced emission: Recent advances and perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.-M.; Wu, W.-J.; Peng, J.-H.; Zhang, H.-K.; Song, F.-Y.; He, B.-Z.; Wang, X.-Y.; Sung, H.H.-Y.; Chen, M.; et al. Real-time monitoring of hierarchical self-assembly and induction of circularly polarized luminescence from achiral luminogens. ACS Nano 2019, 13, 3618–3628. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation- induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Wang, J.-H.; Feng, H.-T.; Zheng, Y.-S. Synthesis of tetraphenylethylene pillar[6]arenes and the selective fast quenching of their AIE fluorescence by TNT. Chem. Commun. 2014, 50, 11407–11410. [Google Scholar] [CrossRef]
- Che, W.; Li, G.; Liu, X.; Shao, K.; Zhu, D.; Su, Z.; Bryce, M.-R. Selective sensing of 2,4,6-trinitrophenol (TNP) in aqueous media with “aggregation-induced emission enhancement” (AIEE)-active iridium(iii) complexes. Chem. Commun. 2018, 54, 1730–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chua, M.-H.; Tang, B.-Z.; Xu, J. Aggregation-induced emission (AIE)-active polymers for explosive detection. Polym. Chem. 2019, 10, 3822–3840. [Google Scholar] [CrossRef]
- Delente, J.M.; Umadevi, D.; Shanmugaraju, S.; Kotova, O.; Watson, G.W.; Gunnlaugsson, T. Aggregation induced emission (AIE) active 4-amino-1,8-naphthalimide-Tröger’s base for the selective sensing of chemical explosives in competitive aqueous media. Chem. Commun. 2020, 56, 2562–2565. [Google Scholar] [CrossRef] [PubMed]
- Prusti, B.; Chakravarty, M. An electron-rich small AIEgen as a solid platform for the selective and ultrasensitive on-site visual detection of TNT in the solid, solution and vapor states. Analyst 2020, 145, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-Z.; Zhao, H.; Shen, X.-Y.; Mahtab, F.; Lam, J.-W.-Y.; Sun, J.-Z.; Tang, B.-Z. Luminogenic Polyacetylenes and Conjugated Polyelectrolytes: Synthesis, Hybridization with Carbon Nanotubes, Aggregation-Induced Emission, Superamplification in Emission Quenching by Explosives, and Fluorescent Assay for Protein Quantitation. Macromolecules 2009, 42, 9400–9411. [Google Scholar] [CrossRef]
- Hu, R.; Maldonado, J.-L.; Rodriguez, M.; Deng, C.; Jim, C.-K.-W.; Lam, J.-W.-Y.; Yuen, M.-M.-F.; Ramos-Ortiz, G.; Tang, B.-Z. Luminogenic materials constructed from tetraphenylethene building blocks: Synthesis, aggregation-induced emission, two-photon absorption, light refraction, and explosive detection. J. Mater. Chem. 2012, 22, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Qin, A.; Lam, J.-W.-Y.; Tang, L.; Jim, C.-K.-W.; Zhao, H.; Sun, J.; Tang, B.-Z. Polytriazoles with Aggregation-Induced Emission Characteristics: Synthesis by Click Polymerization and Application as Explosive Chemosensors. Macromolecules 2009, 42, 1421–1424. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Y.; Lam, J.-W.-Y.; Lu, P.; Hong, Y.; Yu, Y.; Yue, Y.; Faisal, M.; Sung, H.-H.-Y.; Williams, I.-D.; et al. Hyperbranched Conjugated Polysiloles: Synthesis, Structure, Aggregation-Enhanced Emission, Multicolor Fluorescent Photopatterning, and Superamplified Detection of Explosives. Macromolecules 2010, 43, 4921–4936. [Google Scholar] [CrossRef]
- Feng, H.-T.; Zheng, Y.-S. Highly Sensitive and Selective Detection of Nitrophenolic Explosives by Using Nanospheres of a Tetraphenylethylene Macrocycle Displaying Aggregation-Induced Emission. Chem. Eur. J. 2014, 20, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.-T.; Wang, J.-H.; Zheng, Y.-S. CH3−π Interaction of Explosives with Cavity of a TPE Macrocycle: The Key Cause for Highly Selective Detection of TNT. ACS Appl. Mater. Interfaces 2014, 6, 20067–20074. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Wang, J.-H.; Li, B.; Zhang, C.; Tan, B.; Zheng, Y.-S. Porous Interdigitation Molecular Cage from Tetraphenylethylene Trimeric Macrocycles That Showed Highly Selective Adsorption of CO2 and TNT Vapor in Air. Org. Lett. 2018, 20, 321–324. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Ji, B. Influences of pH, urea and metal ions on the interaction of sinomenine with Lysozyme by steady state fluorescence spectroscopy. Spectrochim. Acta Part A 2014, 130, 440–446. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Cao, X.; Xu, C.; Ji, B. Investigation on the pH-dependent binding of vitamin B12 and lysozyme by fluorescence and absorbance. J. Mol. Struct. 2012, 1007, 102–112. [Google Scholar] [CrossRef]
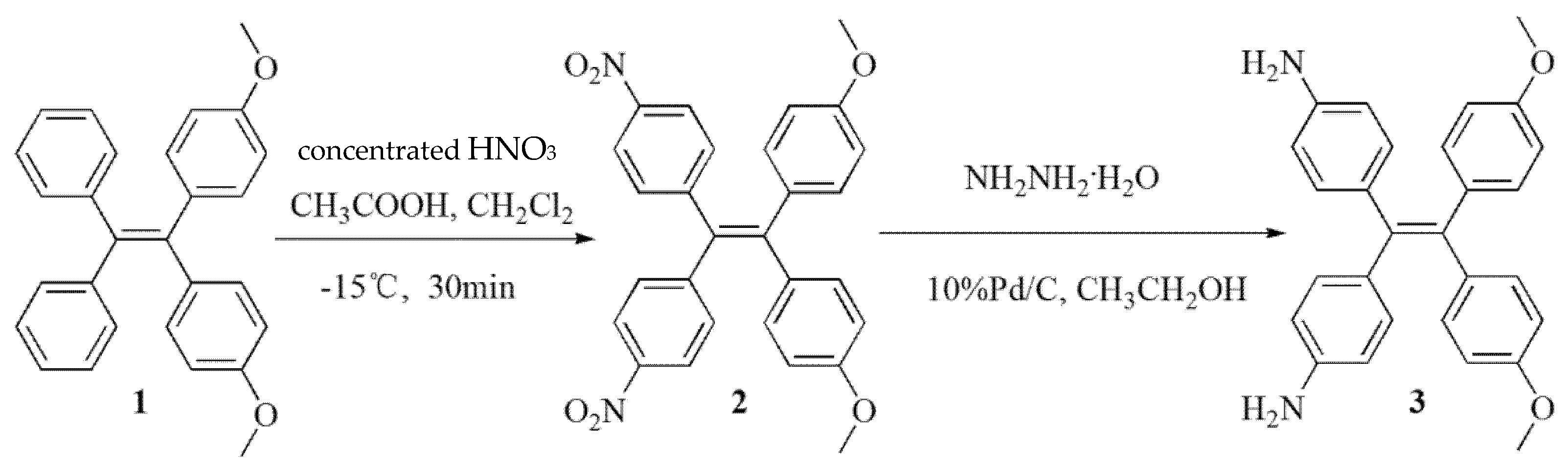

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lv, P.; Han, X.-W.; Jia, Z.; Zheng, M.; Feng, H.-T. A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives. Molecules 2023, 28, 181. https://doi.org/10.3390/molecules28010181
Li D, Lv P, Han X-W, Jia Z, Zheng M, Feng H-T. A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives. Molecules. 2023; 28(1):181. https://doi.org/10.3390/molecules28010181
Chicago/Turabian StyleLi, Dongmi, Panpan Lv, Xiao-Wen Han, Zhilei Jia, Min Zheng, and Hai-Tao Feng. 2023. "A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives" Molecules 28, no. 1: 181. https://doi.org/10.3390/molecules28010181
APA StyleLi, D., Lv, P., Han, X.-W., Jia, Z., Zheng, M., & Feng, H.-T. (2023). A Highly Efficient Fluorescent Sensor Based on AIEgen for Detection of Nitrophenolic Explosives. Molecules, 28(1), 181. https://doi.org/10.3390/molecules28010181






