Abstract
A topological index as a graph parameter was obtained mathematically from the graph’s topological structure. These indices are useful for measuring the various chemical characteristics of chemical compounds in the chemical graph theory. The number of atoms that surround an atom in the molecular structure of a chemical compound determines its valency. A significant number of valency-based molecular invariants have been proposed, which connect various physicochemical aspects of chemical compounds, such as vapour pressure, stability, elastic energy, and numerous others. Molecules are linked with numerical values in a molecular network, and topological indices are a term for these values. In theoretical chemistry, topological indices are frequently used to simulate the physicochemical characteristics of chemical molecules. Zagreb indices are commonly employed by mathematicians to determine the strain energy, melting point, boiling temperature, distortion, and stability of a chemical compound. The purpose of this study is to look at valency-based molecular invariants for embedded in a silicate chain under various conditions. To obtain the outcomes, the approach of atom–bond partitioning according to atom valences was applied by using the application of spectral graph theory, and we obtained different tables of atom—bond partitions of . We obtained exact values of valency-based molecular invariants, notably the first Zagreb, the second Zagreb, the hyper-Zagreb, the modified Zagreb, the enhanced Zagreb, and the redefined Zagreb (first, second, and third). We also provide a graphical depiction of the results that explains the reliance of topological indices on the specified polynomial structure parameters.
1. Introduction
A molecular structure is defined as a simple and linked network G, where is the set of atoms (nodes) and is the set of atom–bonds (links between atoms) [1]. If two atoms and form an atom–bond in G, we write ; similarly, if two atoms do not form an atom–bond in G, we write . The topological index of a chemical composition is a numerical value or a continuation of a given structure under discussion, which indicates chemical, physical, and biological properties of a chemical molecule, see for details [2,3,4]. Topological indices and polynomials capture molecular structural symmetries and provide mathematical vocabulary for predicting features, such as boiling temperatures, viscosity, radius of gyrations, and so on [5,6].
Mathematical chemistry describes how to use polynomials and functions to offer instructions concealed in the symmetry of molecular graphs, and the graph theory has many applications in modern chemistry, particularly organic chemistry. In chemical graph theory, the atoms and bonds of a molecular structure are represented by vertices and edges, respectively [7]. Many applications of topological indices are employed in theoretical chemistry, particularly in research pertaining to quantitative structure–property relationships (QSPRs) and quantitative structure–activity relationships (QSARs) [8,9,10]. Many famous researchers have studied topological indices to obtain information about different families of graphs [11,12]. In (QSPR) and (QSAR), topological indices are utilized directly as simple numerical descriptors in comparison with physical, biological, and chemical characteristics of molecules, which are benefits. Many researchers have worked on various chemical compounds and computed topological descriptors of various molecular graphs during the last few decades [13,14].
The molecular graph is a simple connected graph in a chemical graph theory that contains chemical atoms and bonds, which are often referred to as vertices and edges, respectively, and there must be a linkage between the vertex set and edge set . The valency of each atom of G is actually the total number of atoms connected to v of G and is denoted by , [15].
In 1972, Gutman and Trinajstic initiated the idea of computing the branching of the carbon–atom skeleton, which was, later on, known as the first Zagreb index [16]. In 2004, Gutman and Das, adulated characteristics of the first and second Zagreb polynomials for chemical graphs of a chemical compound, which we studied in the research articles [17]. The first Zagreb polynomial corresponding to the first Zagreb index is defined as
The second Zagreb polynomial, which corresponds to the second Zagreb index [17], is written as
In 2013, Shirdel et al. initiated the concept of the hyper-Zagreb index [18]. The hyper-Zagreb polynomial and index are defined as follows:
The modified Zagreb polynomial and index [19] are defined as
In 2010, Furtula et al. introduced the augmented Zagreb index [20]. The augmented Zagreb polynomial and index are defined as
In 2013, Ranjini, Lokesha, and Usha presented [21] a redesigned version of the Zagreb indices , , and . The indices and redefined form of the Zagreb polynomial are as follows:
In this article, the above-defined eight Zagreb polynomials and Zagreb indices were constructed by the atom–bond set of silicates, partitioned according to the valencies of the and atoms, [22]. We also investigate silicon tetrahedron in a compound structure and derived the precise formulas of certain essential valency-based Zagreb indices using the approach of the atom–bond partitioning of the molecular structure of silicates; for details, see [23,24].
2. Chain of Silicates
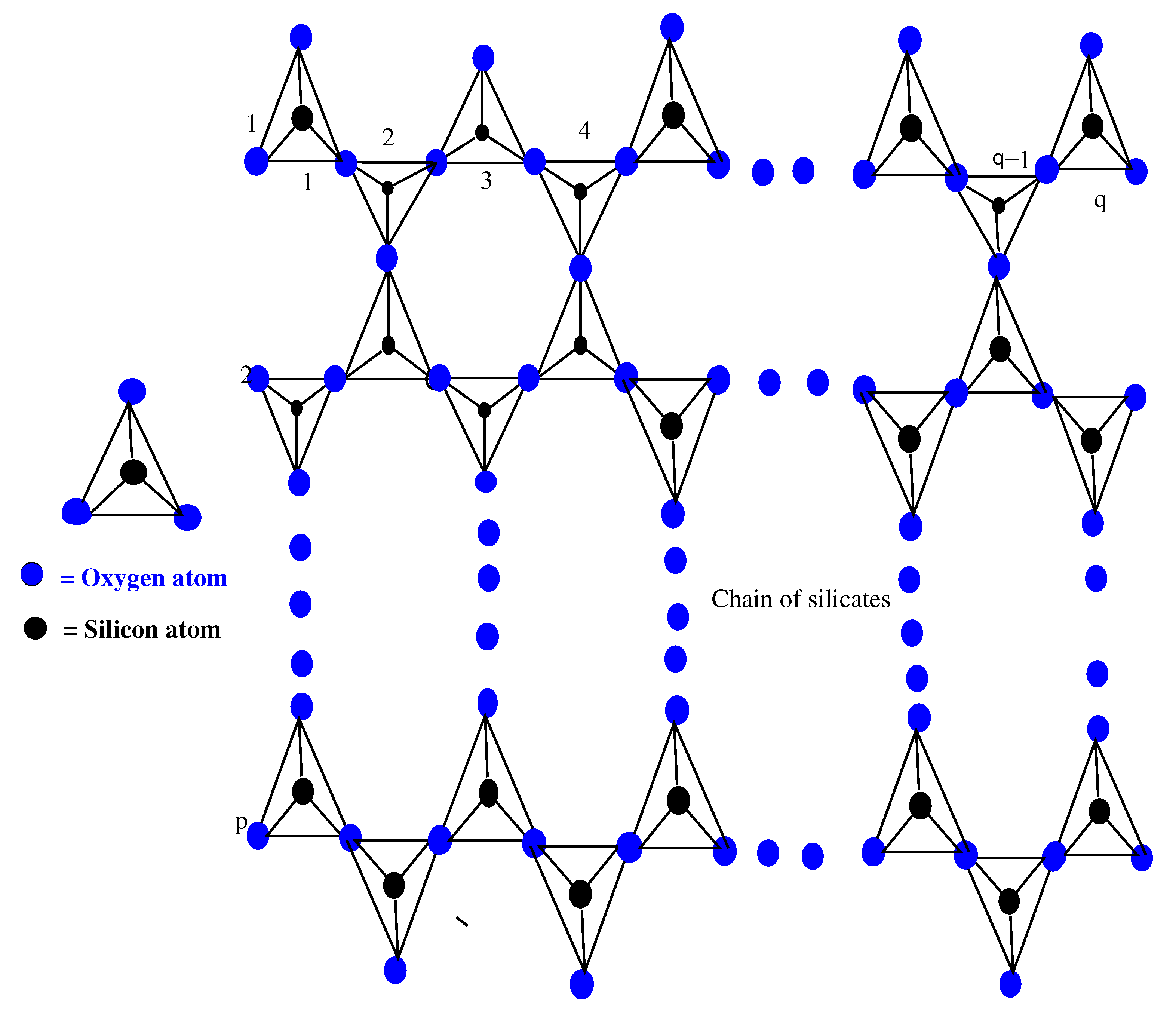
The basic unit of silicates is a tetrahedron, which is obtained by metal carbonates with sand or fusing metal oxides [25]. Almost all of the silicates contain tetrahedron. From a chemical point of view, for a tetrahedron , we consider a pyramid with a triangular base (single tetrahedron ), as shown in Figure 1, containing oxygen atoms at the four corners of the tetrahedron, and the silicon atom is bonded with equally spaced atoms of . From the resulting , a silicate tetrahedron joins with other horizontally, and a single chain of silicates is obtained. Similarly, when two molecules of join corner-to-corner, then each shares its atom with the other molecule, as seen in Figure 1. After completing this process of sharing, these two molecules of can be joined with two other molecules. Now, we obtain a chain of silicates , where p and q are the silicate chain numbers formed and the total number of in one silicate chain, respectively. Here, in the chain of silicates , is the number of tetrahedron used, see Figure 1.
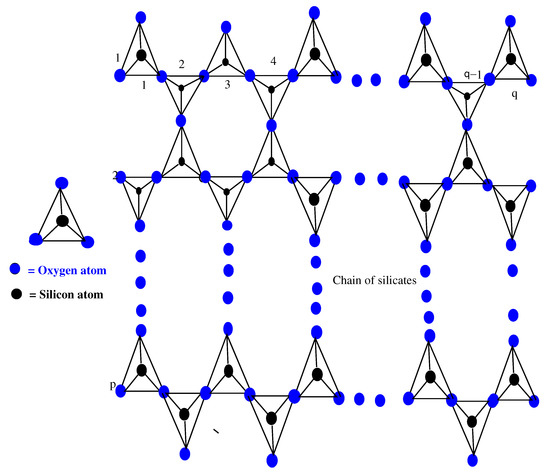
Figure 1.
Chain of .
Here, in the chain of silicates , there are three types of atom–bonds on the basis of valency of every atom of . Therefore, there are two types of atoms, and , such that and , where and mean the valencies of atoms ∀. According to valencies 3 and 6 of the atoms, there are three types of atom–bonds, which are , , and in . On the basis of valency, Table 1 provides the partition of the set of atom–bonds.

Table 1.
Atom–bond partition of for .
3. Zagreb Polynomials and Indices for 2,
Theorem 1.
For , the first Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the first Zagreb polynomial (1), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 1 at , we obtain the first Zagreb index of the silicate network as follows: For , the first Zagreb index of is .
Theorem 2.
For , the second Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the second Zagreb polynomial (2), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 2 at , we obtain the second Zagreb index of the chain of silicates as follows: For , the second Zagreb index of is .
Theorem 3.
For , the hyper-Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the hyper-Zagreb polynomial (3), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 3 at , we obtain the hyper-Zagreb index of the chain of silicates as follows: For , the hyper-Zagreb index of is .
Theorem 4.
For , the modified Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the modified Zagreb polynomial (4), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 4 at , we obtain the modified Zagreb index of the chain of silicates as follows: For , the modified Zagreb index of is .
Theorem 5.
For , the augmented Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the augmented Zagreb polynomial (5), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 5 at , we obtain the augmented Zagreb index of the chain of silicates as follows: For , the augmented Zagreb index of is .
Theorem 6.
For , the first redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the first redefined Zagreb polynomial (6), we have
This gives
□
By taking the first derivative of the polynomial in Theorem 6 at , we obtain the first redefined Zagreb index of the chain of silicates as follows: For , the first redefined Zagreb index of is .
Theorem 7.
For , the second redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the second redefined Zagreb polynomial (7), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 7 at , we obtain the second redefined Zagreb index of the chain of silicates as follows: For , the second redefined Zagreb index of is .
Theorem 8.
For , the third redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 1, in the formula of the third redefined Zagreb polynomial (8), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 8 at , we obtain the third redefined Zagreb index of the chain of silicates as follows: For , the third redefined Zagreb index of is .
Comparison
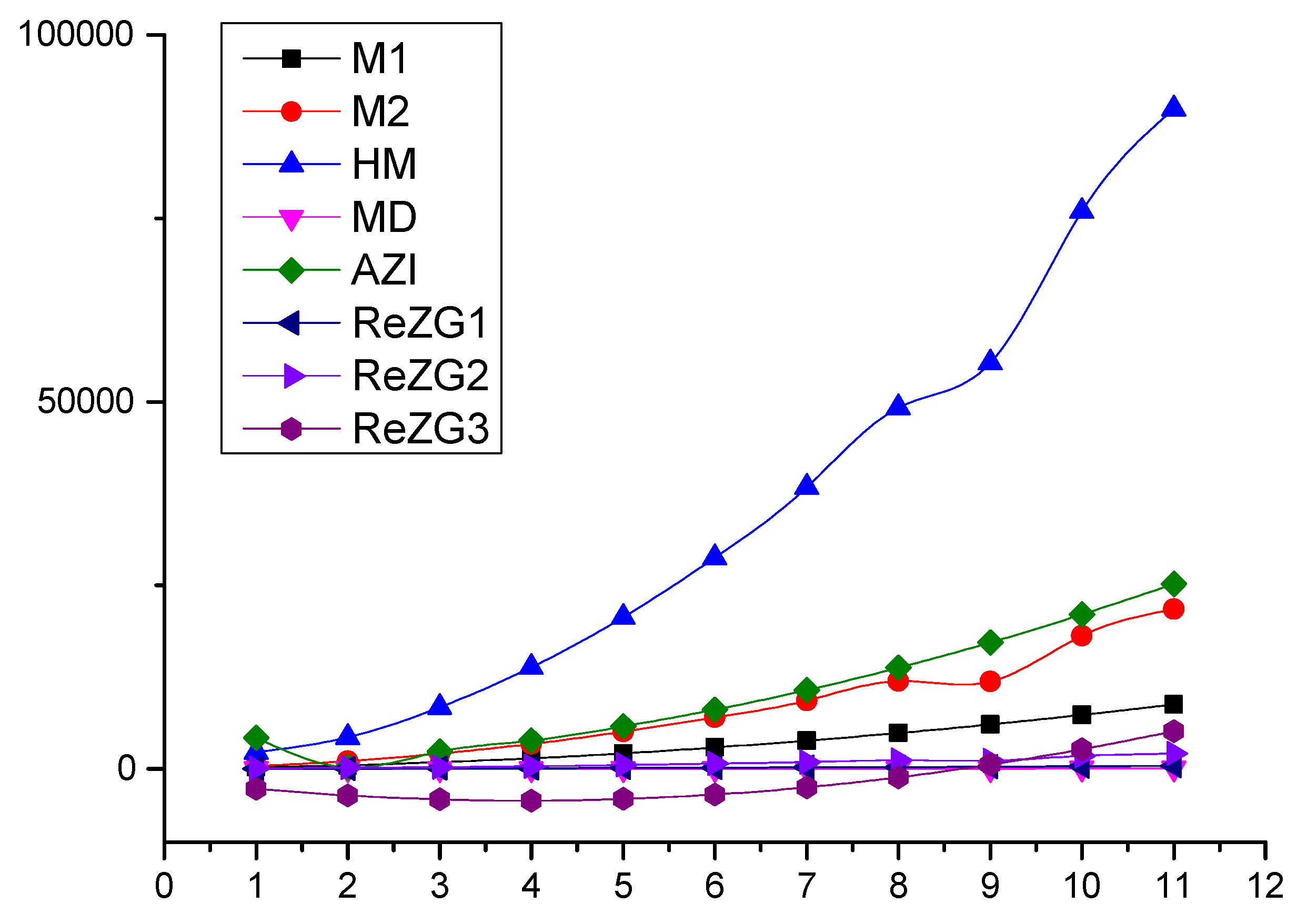
In this section, we present a numerical comparison of Zagreb indices in Table 2 and graphical comparison in Figure 2 of Zagreb polynomials for and for the chain of silicates .

Table 2.
Zagreb topological indices of , for 2, .
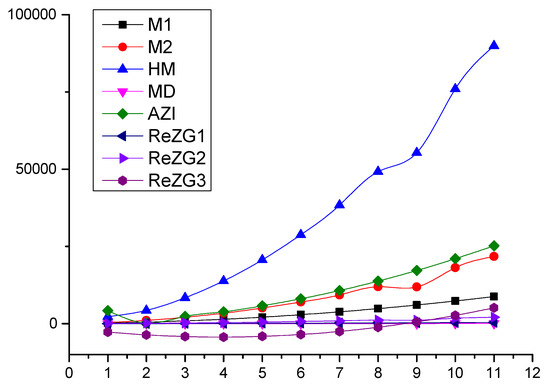
Figure 2.
Graphical comparison of Zagreb indices for 2, .
4. Zagreb Polynomials and Indices for and p Are Odd
Here, in the chain of silicates , we observed for that p is odd and the atom–bond on the basis of the valency of every atom of changed. So, on the basis of valency, Table 3 provides the partition of the set of atom–bonds.

Table 3.
Atom–bond partition of ; p is odd and .
Theorem 9.
Let p be odd and . Then the first Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the first Zagreb polynomial (1), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 9 at , we obtain the first Zagreb index of the silicate network as follows: Let p be odd and . Then the first Zagreb index of is .
Theorem 10.
Let p be odd and . Then the second Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the second Zagreb polynomial (2), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 10 at , we obtain the second Zagreb index of the chain of silicates as follows: Let p be odd and . Then the second Zagreb index of is .
Theorem 11.
Let p be odd and . Then the hyper-Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the hyper-Zagreb polynomial (3), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 11 at , we obtain the hyper-Zagreb index of the chain of silicates as follows: Let p be odd and . Then the hyper-Zagreb index of is .
Theorem 12.
Let p be odd and . Then the modified Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the modified Zagreb polynomial (4), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 12 at , we obtain the modified Zagreb index of the chain of silicates as follows: Let p be odd and . Then the modified Zagreb index of is .
Theorem 13.
Let p be odd and . Then the augmented Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the augmented Zagreb polynomial (5), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 13 at , we obtain the augmented Zagreb index of the chain of silicates as follows: Let p be odd and . Then the augmented Zagreb index of is .
Theorem 14.
Let p be odd and . Then the first redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the first redefined Zagreb polynomial (6), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 14 at , we obtain the first redefined Zagreb index of the chain of silicates as follows: Let p be odd and . Then the first redefined Zagreb index of is .
Theorem 15.
Let p be odd and . Then the second redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the second redefined Zagreb polynomial (7), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 15 at , we obtain the second redefined Zagreb index of the chain of silicates as follows: Let p be odd and . Then the second redefined Zagreb index of is .
Theorem 16.
Let p be odd and . Then the third redefined Zagreb polynomial of is .
Proof.
Using the atom–bond partition from Table 3, in the formula of the third redefined Zagreb polynomial (8), we obtain
This gives
□
By taking the first derivative of the polynomial in Theorem 16 at , we obtain the third redefined Zagreb index of the chain of silicates as follows: Let p be odd and . Then the third redefined Zagreb index of is .
Comparison
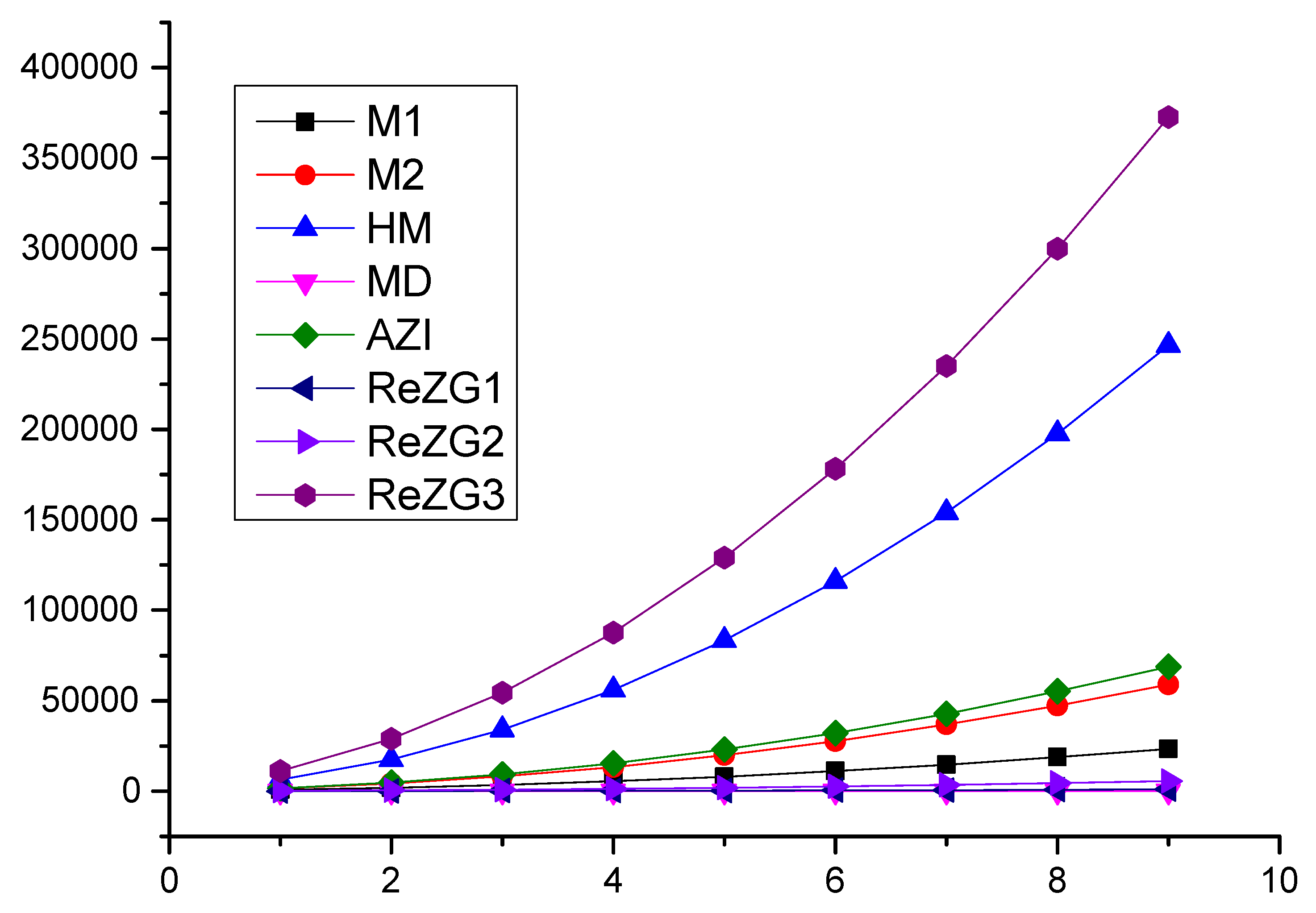
In this section, we present a numerical comparison of the Zagreb indices and a graphical comparison of the Zagreb polynomials for and p is odd; we use and for the chain of silicates (Table 4, Figure 3).

Table 4.
Zagreb indices of for and p is odd.
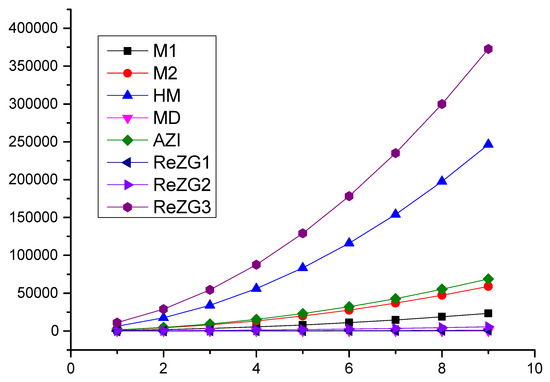
Figure 3.
Graphical comparisons of Zagreb indices for and p are odd.
5. Conclusions
In the analysis of quantitative structure-property relationships (QSPRs) and (QSARs), chemical indices are major implements used to approximate the characteristic features of biological activities, and physical, biomedicine, and molecular compounds. It is ordinary for questions to emerge about the characterization of silicate networks on the bases of the nature of Zagreb polynomials. We computed Zagreb polynomials for the chain of silicates under various situations in this research article. We obtained the first Zagreb, second Zagreb, hyper-Zagreb, augmented Zagreb, redefined first Zagreb, redefined second Zagreb, and redefined third Zagreb indices for the chain of silicates from these Zagreb polynomials. For instance, topological indices or Zagreb indices are used to create quantitative structure–activity relationships (QSARs) that connect the chemical structure of molecules to the biological activities or other characteristics of such compounds.
Open problems: For the characterization of the chain of silicates, followers are invited to discuss or research the following open problem:
- Are Zagreb polynomials and Zagreb indices affected when both p and q are even or odd?
- The results will be interesting when .
Author Contributions
Conceptualization, M.U.G., S.D. and F.S.; methodology, M.U.G.; software, A.A.; validation, M.U.G. and E.S.M.T.E.D.; formal analysis, E.S.M.T.E.D.; investigation, M.U.G.; resources, F.S. and A.A.; data curation, F.S.; writing—original draft preparation, F.S. and F.M.A.; writing—review and editing, J.-B.L.; visualization, E.S.M.T.E.D.; supervision, F.M.A.; project administration, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were used to support this study.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Ghani, M.U.; Sultan, F.; Tag El Din, E.S.M.; Khan, A.R.; Liu, J.B.; Cancan, M. A Paradigmatic Approach to Find the Valency-Based K-Banhatti and Redefined Zagreb Entropy for Niobium Oxide and a Metal—Organic Framework. Molecules 2022, 27, 6975. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ul Haq Bokhary, S.A.; Rehman, M.U.; Ali, U.; Mubeen, H.; Iqbal, Q.; Liu, J.B. Degree-Based Indices of Some Complex Networks. J. Math. 2021, 2021, 5531357. [Google Scholar] [CrossRef]
- Irfan, M.; Rehman, H.U.; Almusawa, H.; Rasheed, S.; Baloch, I.A. M-Polynomials and Topological Indices for Line Graphs of Chain Silicate Network and H-Naphtalenic Nanotubes. J. Math. 2021, 2021, 5551825. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, T.; Hayat, S. The Calculations of Topological Indices on Certain Networks. J. Math. 2021, 2021, 6694394. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.J. CuBr-catalyzed efficient alkynylation of sp3 C- H bonds adjacent to a nitrogen atom. J. Am. Chem. Soc. 2004, 126, 11810–11811. [Google Scholar] [CrossRef]
- Shi, L.; Xia, W. Photoredox functionalization of C–H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 2012, 41, 7687–7697. [Google Scholar] [CrossRef]
- Ashraful Alam, M.; Ghani, M.U.; Kamran, M.; Shazib Hameed, M.; Hussain Khan, R.; Baig, A. Degree-Based Entropy for a Non-Kekulean Benzenoid Graph. J. Math. 2022, 2022, 2288207. [Google Scholar] [CrossRef]
- Chu, Y.M.; Khan, A.R.; Ghani, M.U.; Ghaffar, A.; Inc, M. Computation of zagreb polynomials and zagreb indices for benzenoid triangular & hourglass system. Polycycl. Aromat. Compd. 2022, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ghani, M.U.; Sultan, F.; Inc, M.; Cancan, M. Connecting SiO 4 in Silicate and Silicate Chain Networks to Compute Kulli Temperature Indices. Molecules 2022, 27, 7533. [Google Scholar] [CrossRef]
- Ghani, M.U.; Sultan, F.; El Sayed, M.; Cancan, M.; Ali, S. SiO4 characterization in a chain and C6 H6 embedded in a Non-kekulean structure for Kulli Temperature indices. Eur. PMC 2022. [Google Scholar] [CrossRef]
- Costa, P.; Evangelista, J.S.; Leal, I.; Miranda, P.C. Chemical Graph Theory for Property Modeling in QSAR and QSPR—Charming QSAR & QSPR. Mathematics 2021, 9, 60. [Google Scholar]
- Mondal, S.; Dey, A.; De, N.; Pal, A. QSPR analysis of some novel neighbourhood degree-based topological descriptors. Complex Intell. Syst. 2021, 7, 977–996. [Google Scholar] [CrossRef]
- Al-Ahmadi, B.; Saleh, A.; Al-Shammakh, W. Downhill Zagreb Polynomials of Graphs. Res. Rev. Discret. Math. Struct. 2021, 7, 15–26. [Google Scholar]
- Zakharov, A.B.; Tsarenko, D.K.; Ivanov, V.V. Topological characteristics of iterated line graphs in the QSAR problem: A multigraph in the description of properties of unsaturated hydrocarbons. Struct. Chem. 2021, 32, 1629–1639. [Google Scholar] [CrossRef]
- Natarajan, V.; Kumar, P.N.; Ahmad, M.; Sharma, J.P.; Chaudhary, A.K.; Sharma, P.K. Effect of electron-phonon interaction and valence band edge shift for carrier-type reversal in layered ZnS/rGO nanocomposites. J. Colloid Interface Sci. 2021, 586, 39–46. [Google Scholar] [CrossRef]
- Gutman, I.; Trinajstić, N. Graph theory and molecular orbitals. Total φ-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Das, K.C.; Gutman, I. Some properties of the second Zagreb index. MATCH Commun. Math. Comput. Chem 2004, 52, 3-1. [Google Scholar]
- Shirdel, G.; Rezapour, H.; Sayadi, A. The hyper-Zagreb index of graph operations. Iran. J. Math. Chem. 2013, 4, 213–220. [Google Scholar]
- Vukičević, D.; Graovac, A. Valence connectivity versus Randić, Zagreb and modified Zagreb index: A linear algorithm to check discriminative properties of indices in acyclic molecular graphs. Croat. Chem. Acta 2004, 77, 501–508. [Google Scholar]
- Furtula, B.; Graovac, A.; Vukičević, D. Augmented zagreb index. J. Math. Chem. 2010, 48, 370–380. [Google Scholar] [CrossRef]
- Ranjini, P.; Lokesha, V.; Usha, A. Relation between phenylene and hexagonal squeeze using harmonic index. Int. J. Graph Theory 2013, 1, 116–121. [Google Scholar]
- Ghani, M.U.; Kashif Maqbool, M.; George, R.; Ofem, A.E.; Cancan, M. Entropies Via Various Molecular Descriptors of Layer Structure of H3BO3. Mathematics 2022, 10, 4831. [Google Scholar] [CrossRef]
- Koubisy, M.; Shaaban, K.S.; Wahab, E.A.; Sayyed, M.; Mahmoud, K. Synthesis, structure, mechanical and radiation shielding features of 50SiO 2–(48+ X) Na 2 B 4 O 7–(2- X) MnO 2 glasses. Eur. Phys. J. Plus 2021, 136, 1–18. [Google Scholar] [CrossRef]
- Mandlimath, T.R.; Balaji, D.; Kumar, S.P. Synthesis, structural and thermal expansion investigation of La, Ce and Eu substituted Bi4 (SiO4) 3. Mater. Chem. Phys. 2021, 270, 124841. [Google Scholar] [CrossRef]
- Liebau, F. Structural Chemistry of Silicates: Structure, Bonding, and Classification; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).