Modern Developments in Bifunctional Chelator Design for Gallium Radiopharmaceuticals
Abstract
1. Introduction
2. Aqueous Gallium Chemistry and Gallium Radioisotopes
3. Bifunctional Chelator Design for Gallium Radiopharmaceuticals
4. DOTA and Other Tetraazamacrocyclic-Based Bifunctional Chelator Development
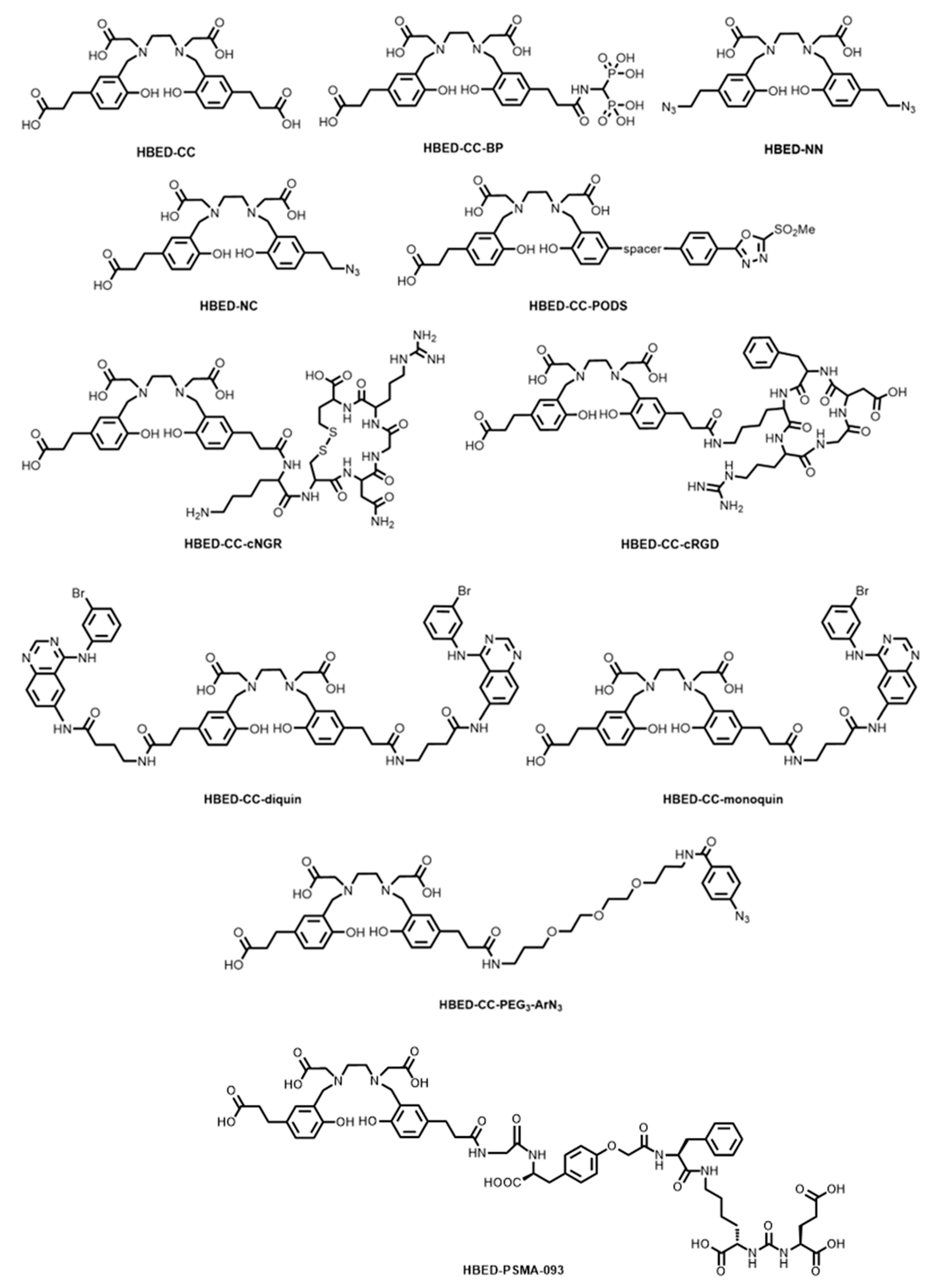
5. HBED-Based Bifunctional Chelator Development
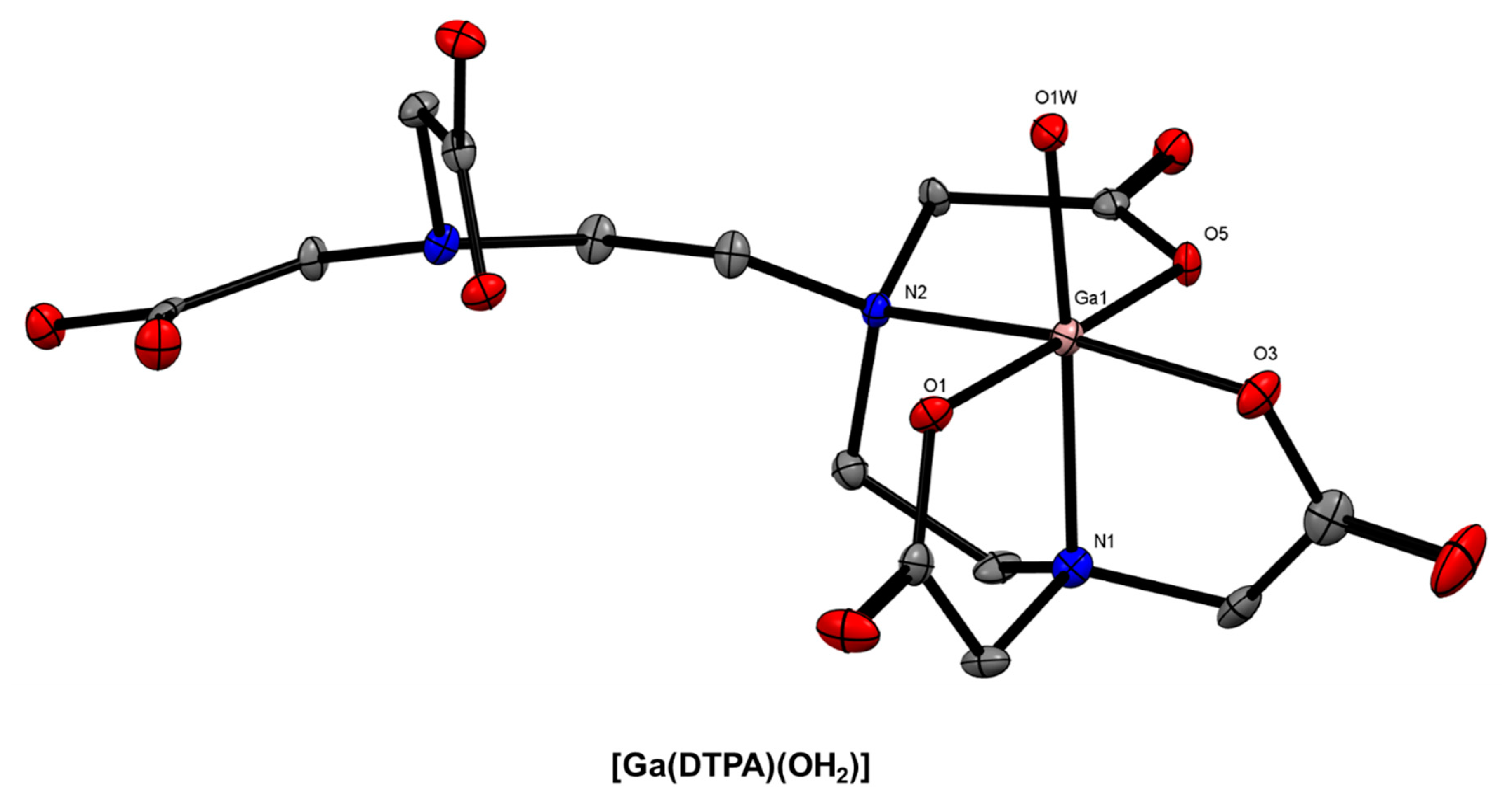
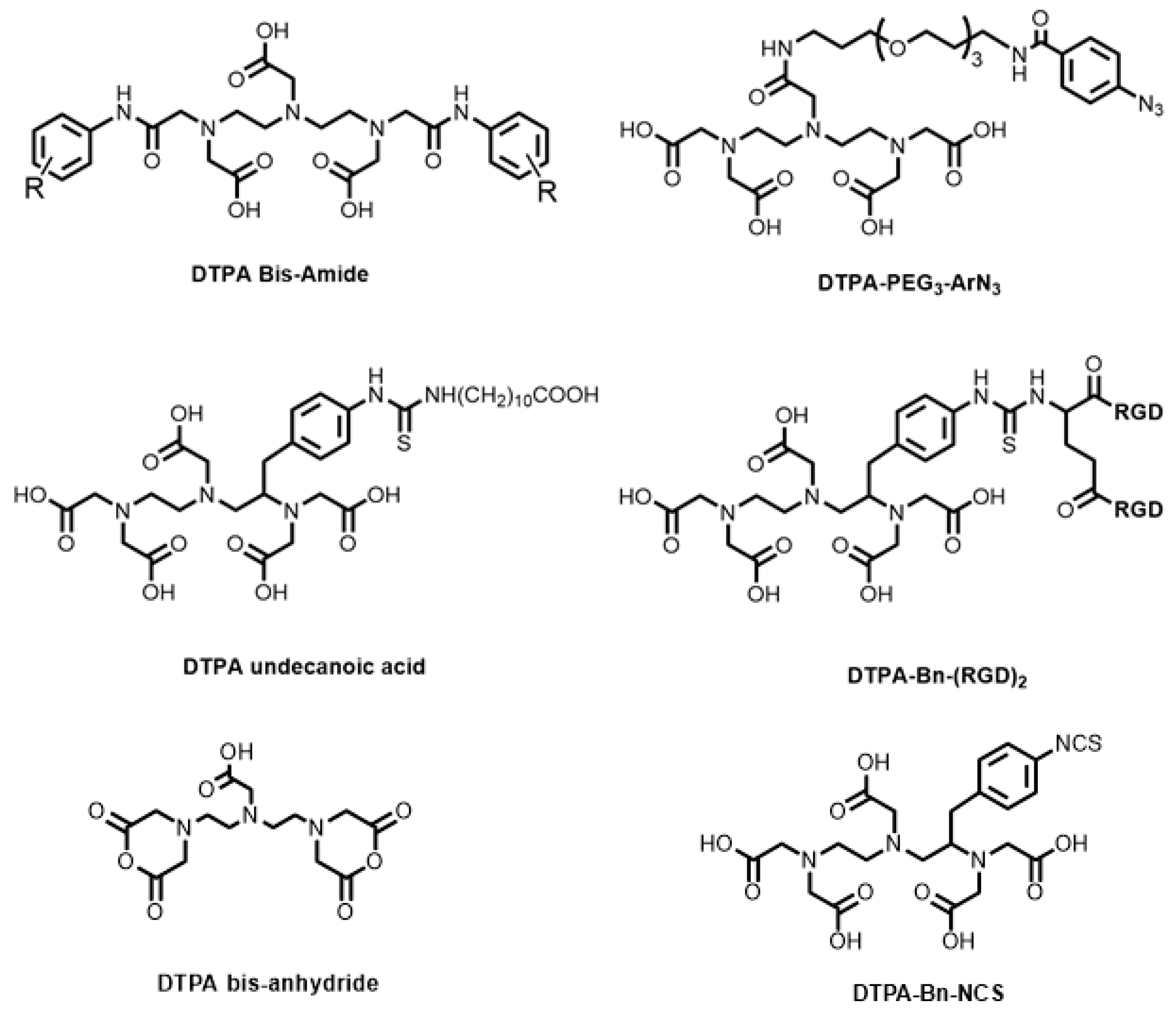
6. DTPA-Based Bifunctional Chelator Development
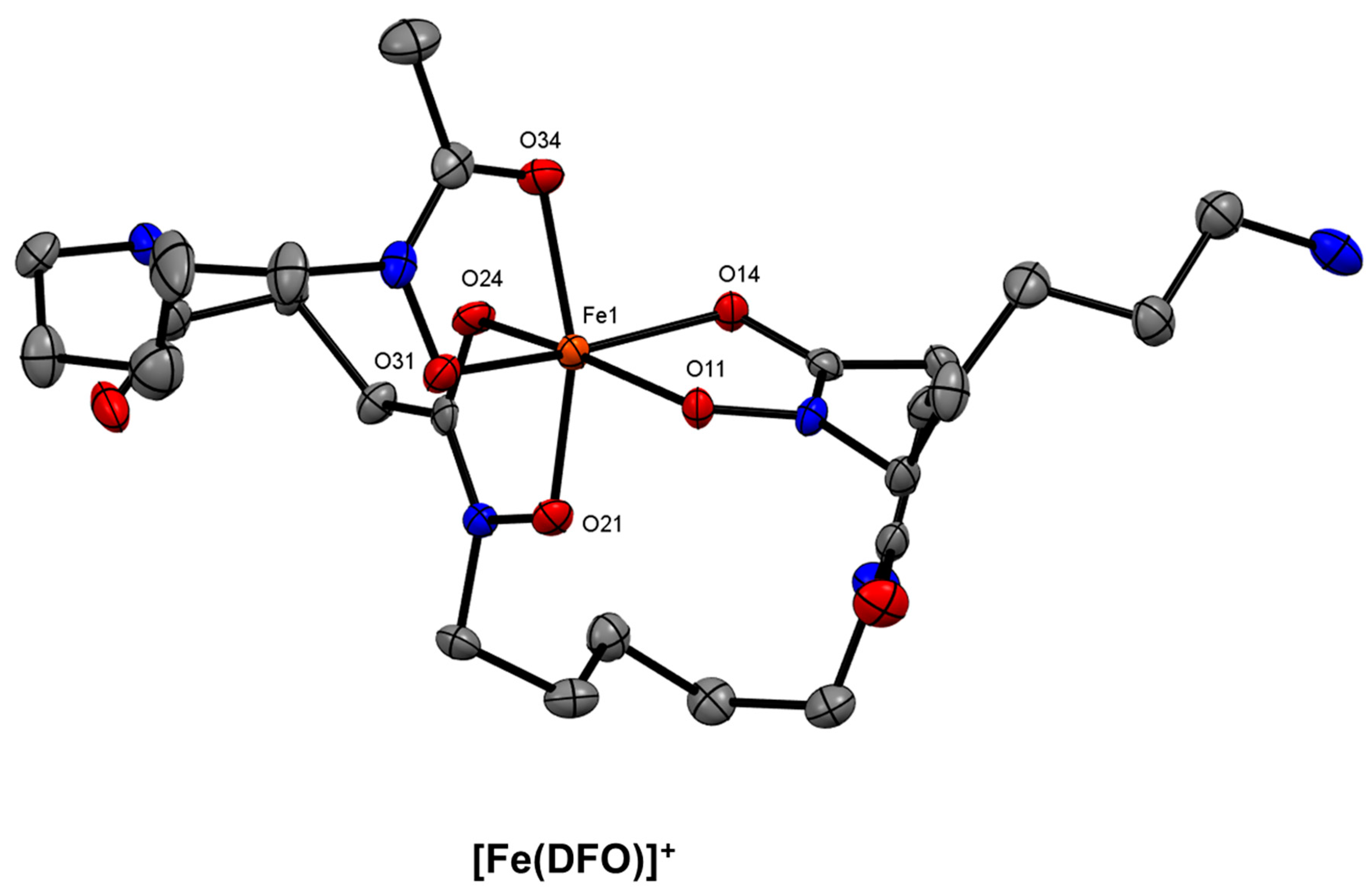
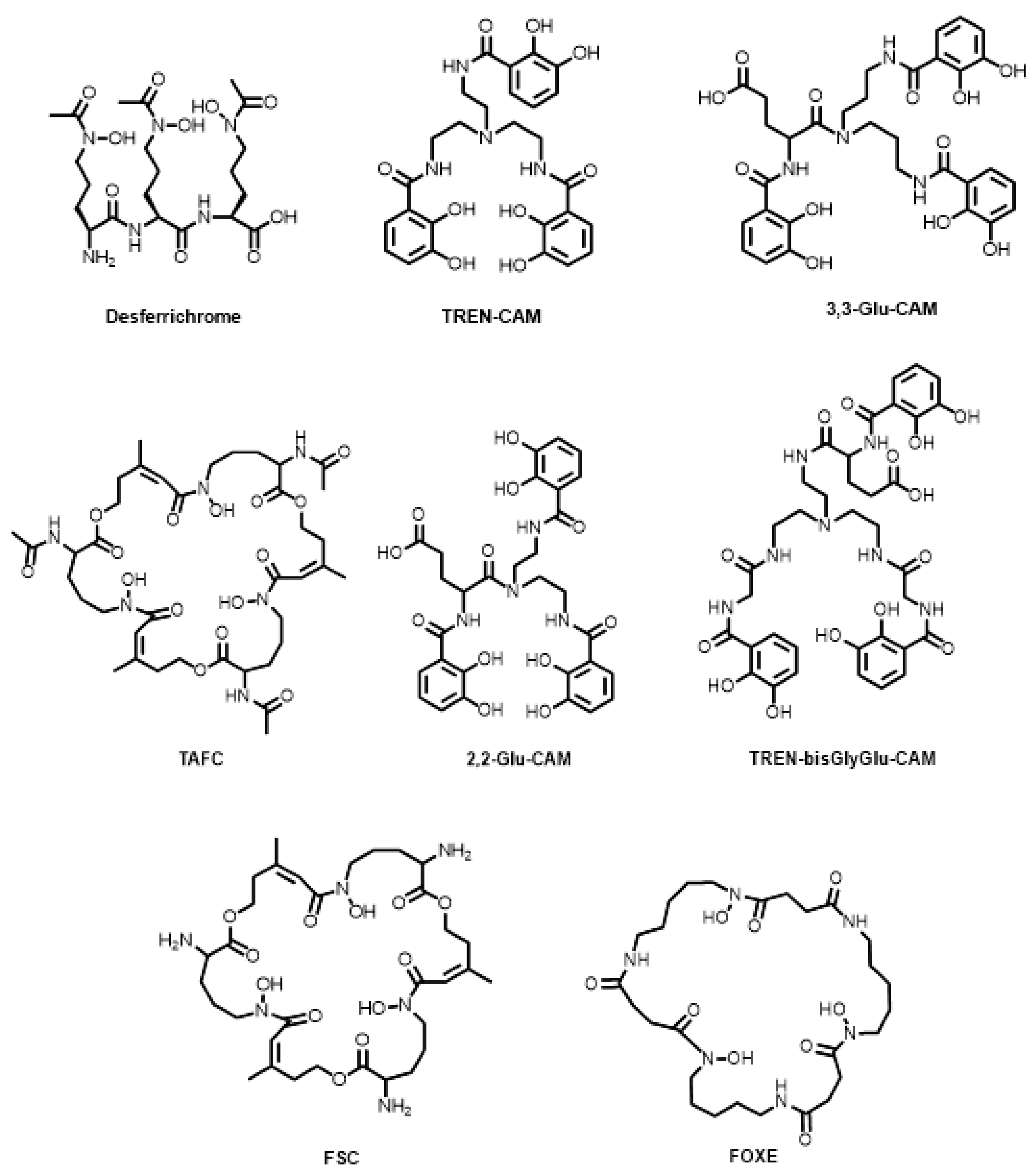
7. Siderophore-Based Bifunctional Chelator Development
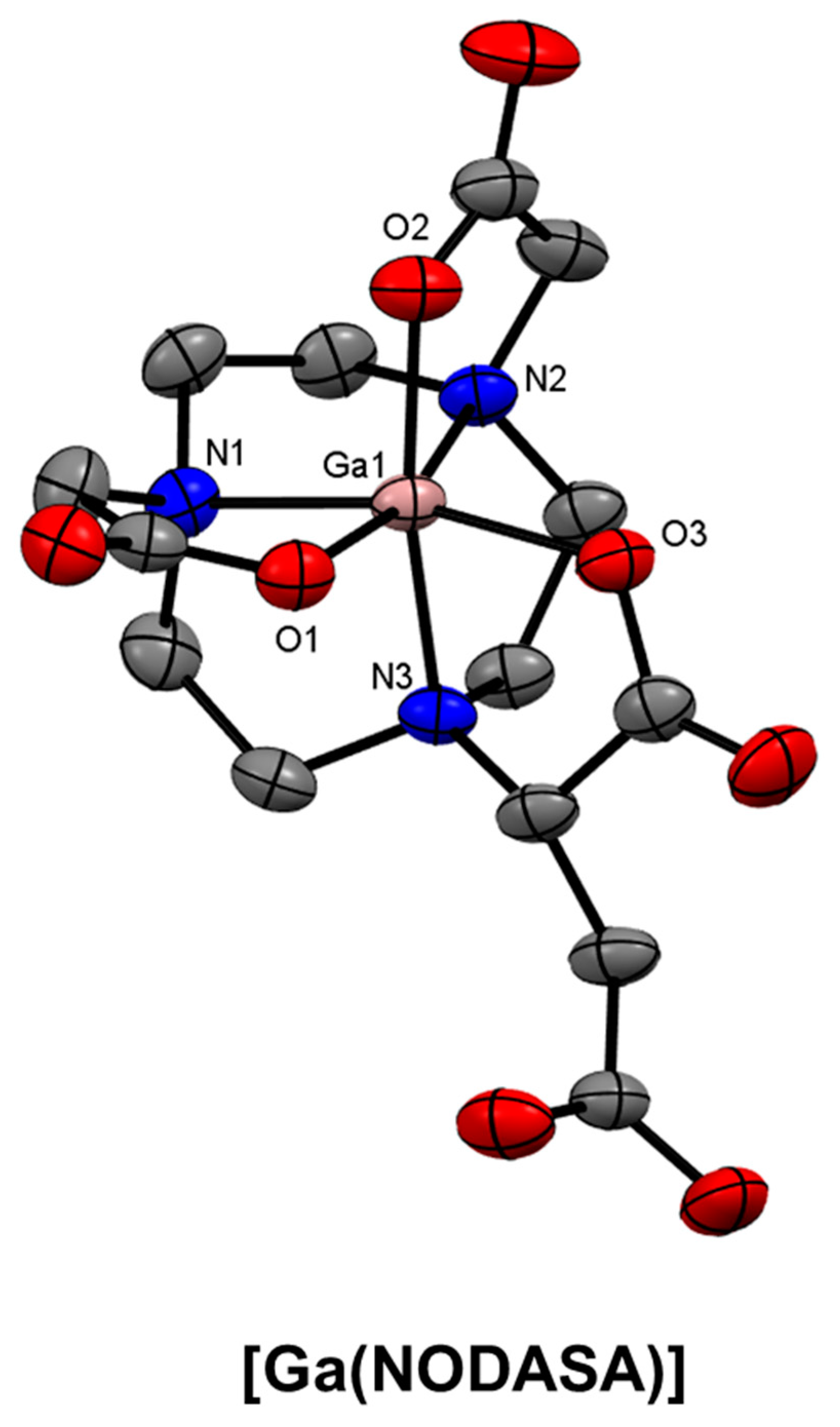
8. Pyridinecarboxylate-Based Bifunctional Chelator Development
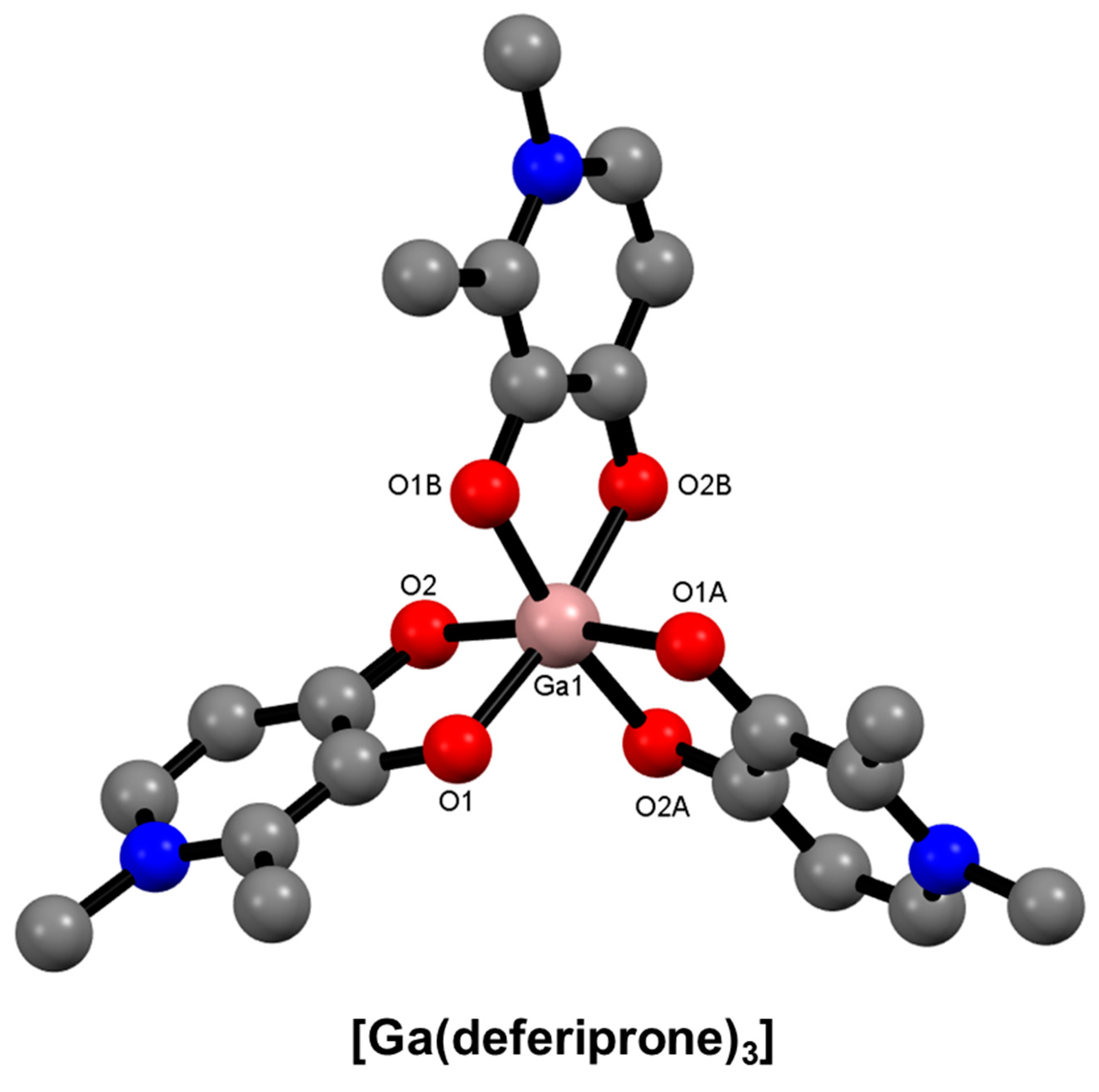
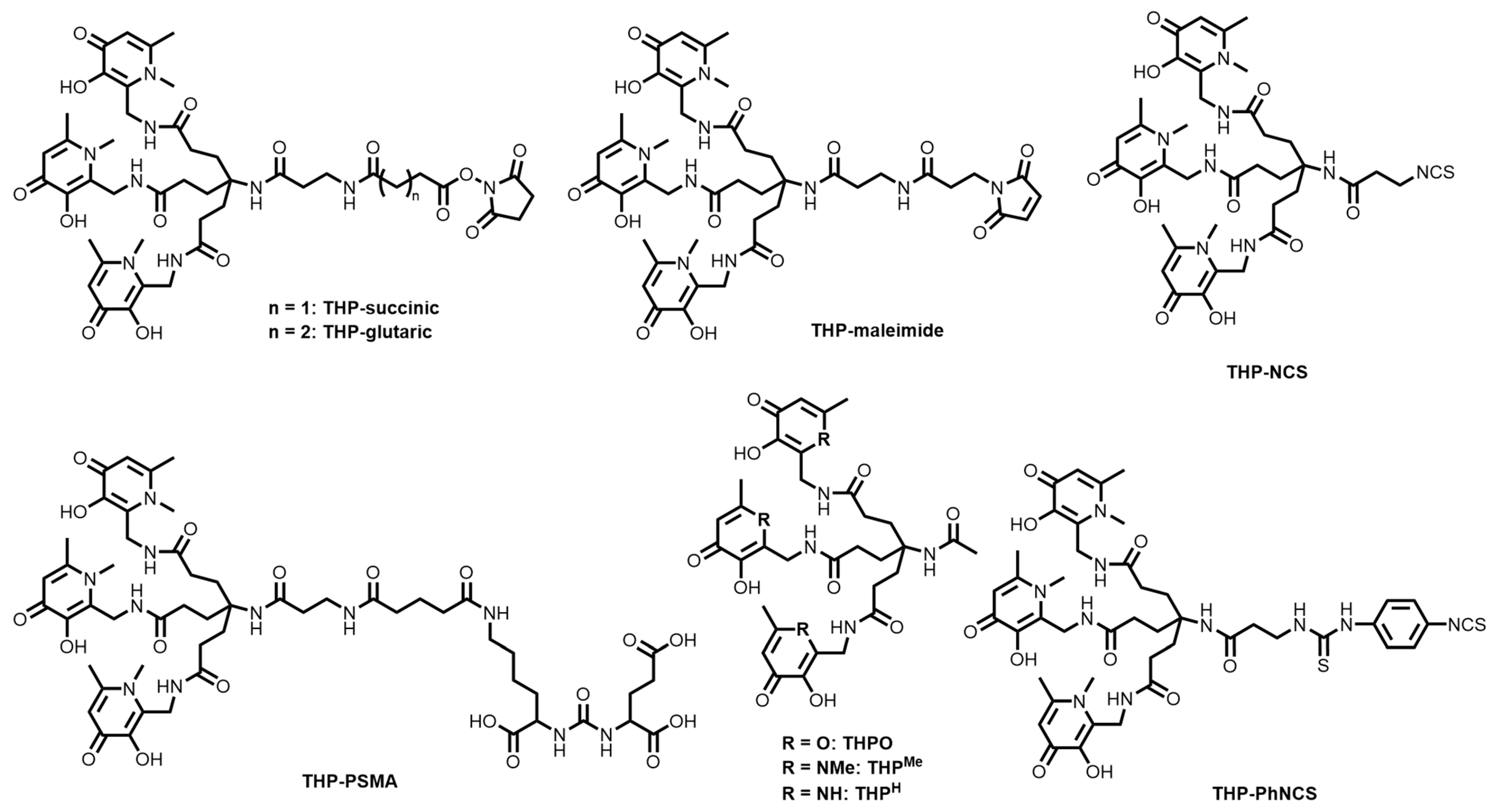
9. Hydroxypyridinone-Based Bifunctional Chelator Development
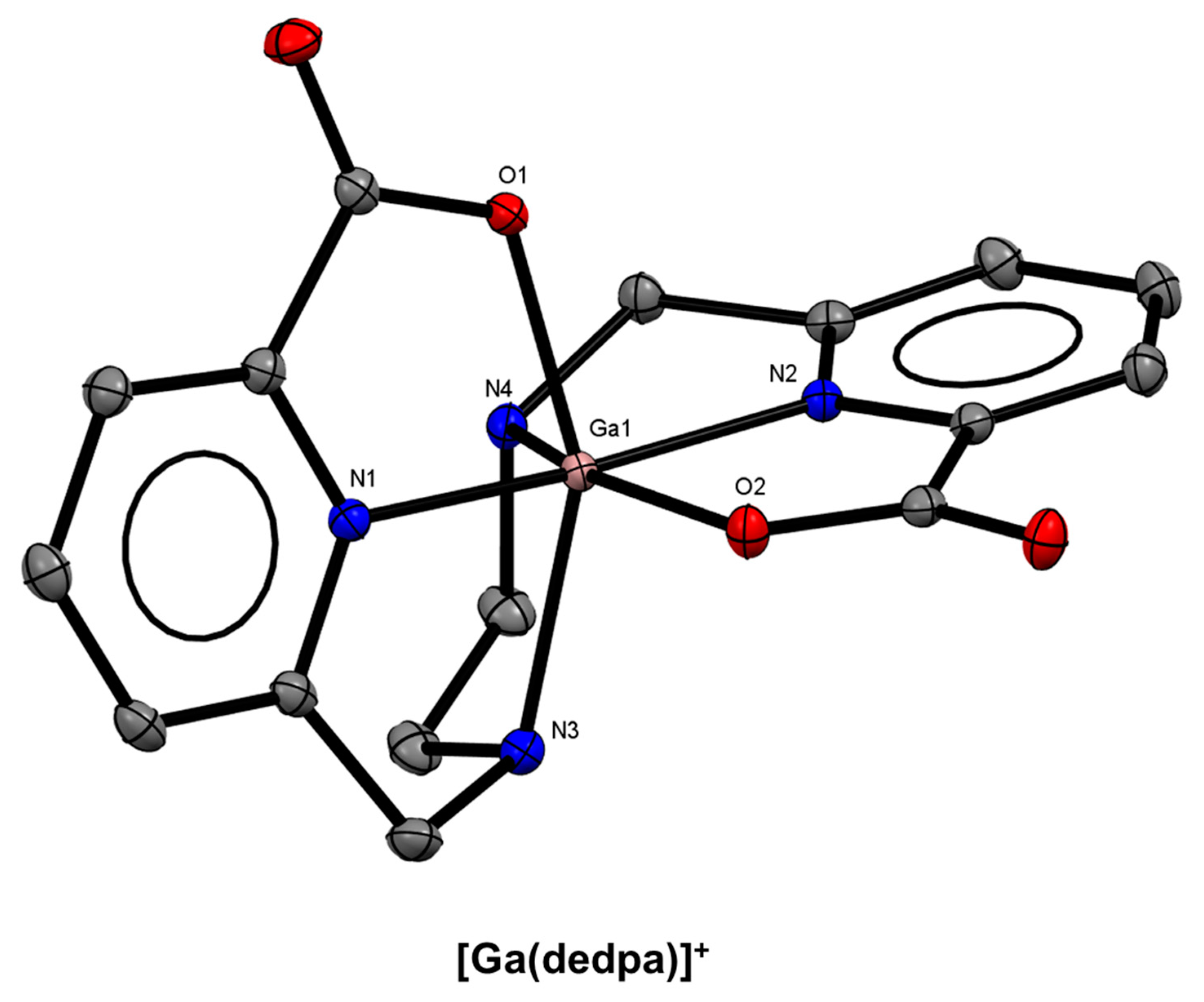
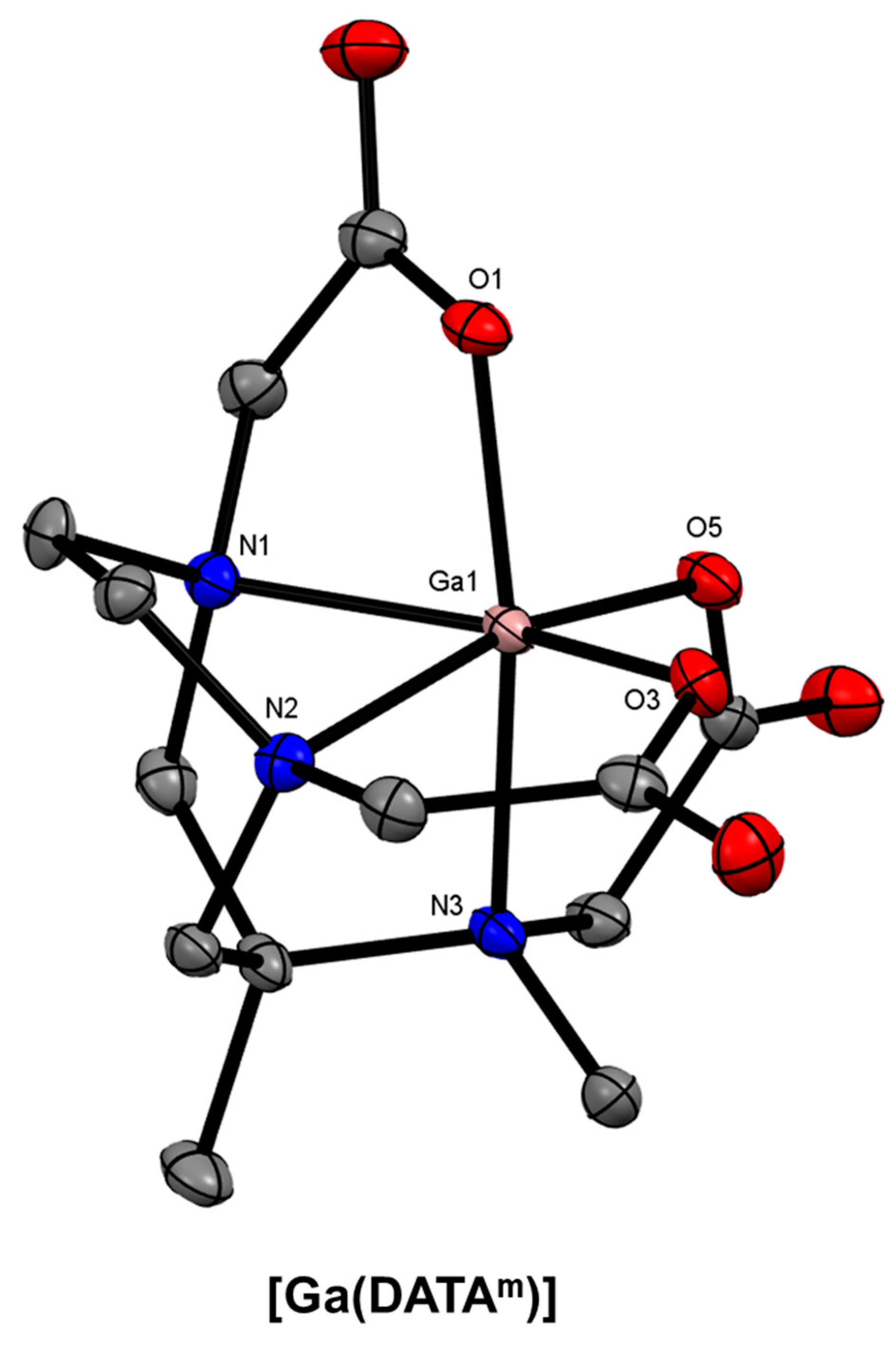
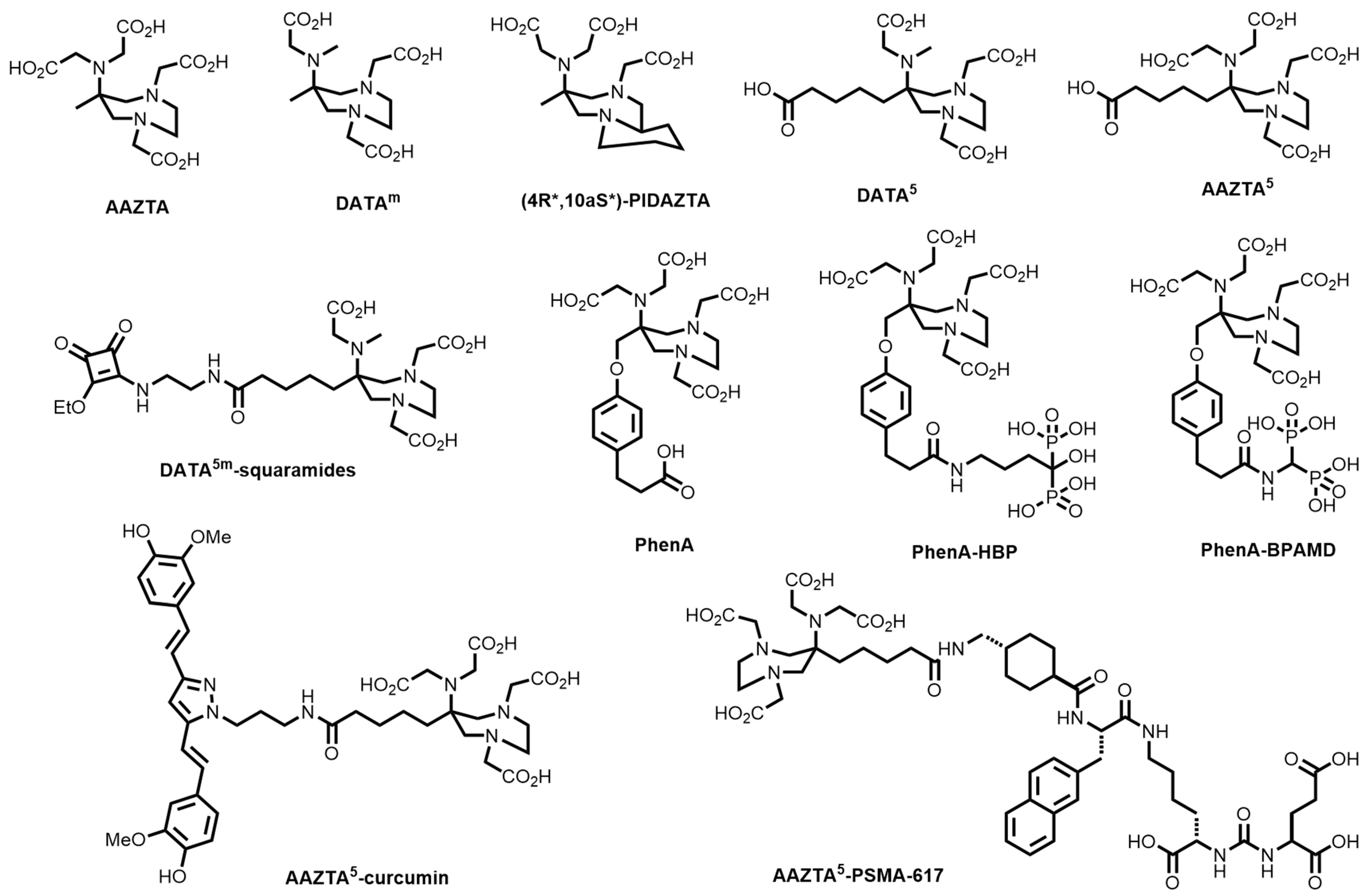
10. Diazepine-Based Bifunctional Chelator Development
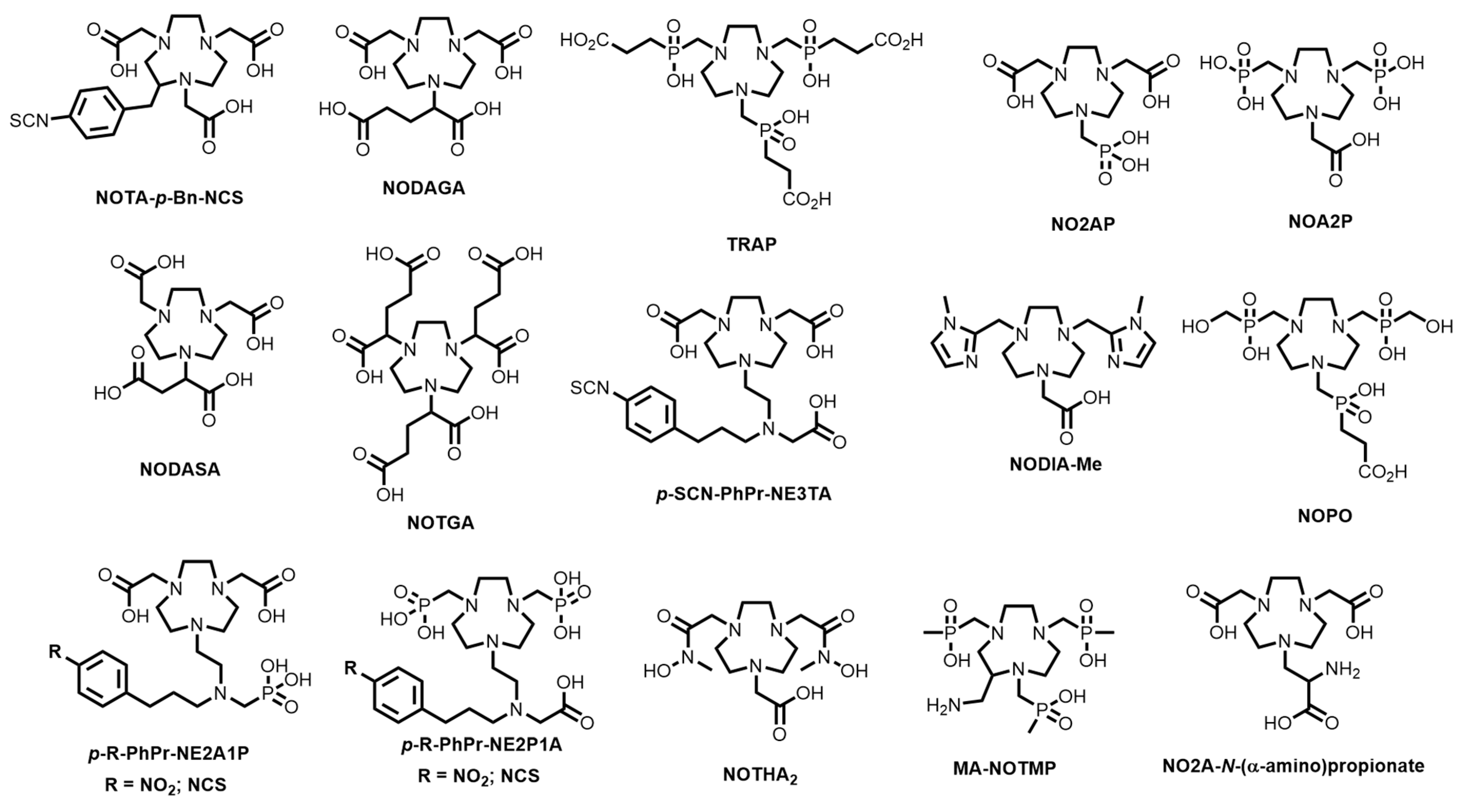
11. TACN-Based Bifunctional Chelator Development
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Bartholomä, M.D.; Louie, A.S.; Valliant, J.F.; Zubieta, J. Technetium and gallium derived radiopharmaceuticals: Comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem. Rev. 2010, 110, 2903–2920. [Google Scholar] [CrossRef]
- Pathuri, G.; Hedrick, A.F.; January, S.E.; Galbraith, W.K.; Awasthi, V.; Arnold, C.D.; Cowley, B.D.; Gali, H. Synthesis and in vivo evaluation of gallium-68-labeled glycine and hippurate conjugates for positron emission tomography renography. J. Label. Compd. Radiopharm. 2015, 58, 14–19. [Google Scholar] [CrossRef]
- Smith, D.L.; Breeman, W.A.P.; Sims-Mourtada, J. The untapped potential of Gallium 68-PET: The next wave of ⁶⁸Ga-agents. Appl. Radiat. Isot. 2013, 76, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- Turlakow, A.; Yeung, H.W.; Pui, J.; Macapinlac, H.; Liebovitz, E.; Rusch, V.; Goy, A.; Larson, S.M. Fludeoxyglucose Positron Emission Tomography in the Diagnosis of Giant Cell Arteritis. Arch. Intern. Med. 2001, 161, 1003–1007. [Google Scholar] [CrossRef]
- McInnes, L.E.; Rudd, S.E.; Donnelly, P.S. Copper, gallium and zirconium positron emission tomography imaging agents: The importance of metal ion speciation. Coord. Chem. Rev. 2017, 352, 499–516. [Google Scholar] [CrossRef]
- FDA-Approved Radiopharmaceuticals. Cardinal Health, Denver, CO. 2022. Available online: https://www.cardinalhealth.com/content/dam/corp/web/documents/fact-sheet/cardinal-health-fda-approved-radiopharmaceuticals.pdf (accessed on 28 July 2022).
- Rösch, F. Theranostics, Gallium-68, and Other Radionuclides; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–16. [Google Scholar]
- Schnökel, H. Formation, structure and bonding of metalloid Al and Ga clusters. A challenge for chemical efforts in nanosciences. Dalton Trans. 2008, 4344–4362. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Stasch, A. The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Fischer, R.C.; Power, P.P. π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef]
- Moerlein, S.M.; Welch, M.J. The Chemistry of Gallium and Indium as Related to Radiopharmaceutical Production. Int. J. Nucl. Med. Biol. 1981, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics (Internet Version 2016); CRC Press LLC/Taylor and Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pearson, R.G. Absolute Electronegativity and Hardness: Application to Inorganic Chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Peters, J.A.; Görls, H.; Platas-Iglesias, C. Structural Study of Ga(III), In(III), and Fe(III) Complexes of Triaza-Macrocycle Based Ligands with N3S3 Donor Set. Inorg. Chem. 2009, 48, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, C.; Levason, W.; Ratnani, R.; Reid, G.; Webster, M. Synthesis, characterisation and structures of thio-, seleno- and telluro-ether complexes of gallium(III). Dalton Trans. 2008, 6274–6282. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M.L.; Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. The Synthesis of a Bis(thiosemicarbazone) Macrocyclic Ligand and the Mn(II), Co(II), Zn(II) and 68Ga(III) Complexes. Molecules 2021, 26, 3646. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Dolmella, A.; Tisato, F.; Porchia, M.; Refosco, F. Mononuclear six-coordinated Ga(III) complexes: A comprehensive survey. Coord. Chem. Rev. 2009, 253, 56–77. [Google Scholar] [CrossRef]
- Cutler, C.S.; Giron, M.C.; Reichert, D.E.; Snyder, A.Z.; Herrero, P.; Anderson, C.J.; Quarless, D.A.; Koch, S.A.; Welch, M.J. Evaluation of Gallium-68 Tris(2-Mercaptobenzyl)Amine: A Complex with Brain and Myocardial Uptake. Nucl. Med. Biol. 1999, 26, 305–316. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Martell, A.E.; Koch, S.A.; Hwang; Quarless, D.A.; Welch, M.J. The Gallium(III) and Indium(III) Complexes of Tris(2-mercaptobenzyl)amine and Tris(2-hydroxybenzyl)amine. Inorg. Chem. 1998, 37, 5902–5911. [Google Scholar] [CrossRef]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Weiner, R.E.; Schreiber, G.J.; Hoffer, P.B.; Bushberg, J.T. Compounds Which Mediate Gallium-67 Transfer from Lactoferrin to Ferritin. J. Nucl. Med. 1985, 26, 908–916. [Google Scholar]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2013, 43, 260–290. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. 68Ga-based Radiopharmaceuticals: Production and Application Relationship. Molecules 2015, 20, 12913–12943. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Sollert, C.; Stark, D.; Clark, M.; Katsifis, A.; Hockley, B.G.; Parr, D.C.; Frigell, J.; Henderson, B.D.; Abghari-Gerst, M.; et al. Cyclotron-based production of 68Ga, [68Ga]GaCl3, and [68Ga]Ga-PSMA-11 from a liquid target. EJNMMI Radiopharm. Chem. 2020, 5, 25. [Google Scholar] [CrossRef]
- Bois, F.; Noirot, C.; Dietemann, S.; Mainta, I.C.; Zilli, T.; Garibotto, V.; Walter, M.A. [68Ga]Ga-PSMA-11 in prostate cancer: A comprehensive review. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 349–374. [Google Scholar] [PubMed]
- Young, J.D.; Abbate, V.; Imberti, C.; Meszaros, L.K.; Ma, M.T.; Terry, S.Y.; Hider, R.C.; Mullen, G.E.; Blower, P.J. 68Ga-THP-PSMA: A PET Imaging Agent for Prostate Cancer Offering Rapid, Room-Temperature, 1-Step Kit-Based Radiolabeling. J. Nucl. Med. 2017, 58, 1270–1277. [Google Scholar] [CrossRef]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-Based Post-processing of Generator-Derived ⁶⁸Ga Toward Kit-Type Preparation of ⁶⁸Ga-Radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Alban, R.; François, H.; Sandrine, J.-K.; Andrea, S.; Catherine, R.; Marc, F. Feasibility and Evaluation of Automated Methods for Radiolabeling of Radiopharmaceutical Kits with Gallium-68. Curr. Radiopharm. 2019, 12, 229–237. [Google Scholar]
- Hong, H.; Wang, G.; Ploessl, K.; Zha, Z.; Zang, J.; Zhu, Z.; Zhu, L.; Kung, H.F. Kit-based preparation of [68Ga]Ga-P16-093 (PSMA-093) using different commercial 68Ge/68Ga generators. Nucl. Med. Biol. 2022, 106–107, 1–9. [Google Scholar] [CrossRef]
- Satpati, D. Recent Breakthrough in 68Ga-Radiopharmaceuticals Cold Kits for Convenient PET Radiopharmacy. Bioconjug. Chem. 2021, 32, 430–447. [Google Scholar] [CrossRef]
- Mishoe, A.; DeNoble, P. Setting Up a Successful Radiopharmaceutical Production Facility. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 627–633. [Google Scholar]
- Sequeira, S.; Lyashchenko, S.K. The Clinical Translation Process in the United States. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 619–625. [Google Scholar]
- Blower, P.J.; Cusnir, R.; Darwesh, A.; Long, N.J.; Ma, M.T.; Osborne, B.E.; Price, T.W.; Pellico, J.; Reid, G.; Southworth, R.; et al. Advances in Inorganic Chemistry; Hubbard, C.D., van Eldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 78, pp. 1–35. [Google Scholar]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Kubeil, M.; Nsubuga, A.; Singh, G.; Gasser, G.; Stephan, H. Harnessing the Coordination Chemistry of 1,4,7-Triazacyclononane for Biomimicry and Radiopharmaceutical Applications. ChemPlusChem 2018, 83, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Kubíček, V.; Böhmová, Z.; Ševčíková, R.; Vaněk, J.; Lubal, P.; Poláková, Z.; Michalicová, R.; Kotek, J.; Hermann, P. NOTA Complexes with Copper(II) and Divalent Metal Ions: Kinetic and Thermodynamic Studies. Inorg. Chem. 2018, 57, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.L.; Yapp, D.T.T.; Mandel, D.; Gill, R.K.; Boros, E.; Wong, M.Q.; Jurek, P.; Kiefer, G.E. 68 Ga Small Peptide Imaging: Comparison of NOTA and PCTA. Bioconjug. Chem. 2012, 23, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Cawthray, J.F.; Boros, E.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. H2CHXdedpa and H4CHXoctapa—Chiral Acyclic Chelating Ligands for 67/68Ga and 111In Radiopharmaceuticals. Inorg. Chem. 2015, 54, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Seemann, J.; Waldron, B.P.; Roesch, F.; Parker, D. Approaching ‘Kit-Type’ Labelling with 68Ga: The DATA Chelators. ChemMedChem 2015, 10, 1019–1026. [Google Scholar] [CrossRef]
- Cusnir, R.; Imberti, C.; Hider, R.C.; Blower, P.J.; Ma, M.T. Hydroxypyridinone Chelators: From Iron Scavenging to Radiopharmaceuticals for PET Imaging with Gallium-68. Int. J. Mol. Sci. 2017, 18, 116. [Google Scholar] [CrossRef]
- Kubíček, V.; Havlíčková, J.; Kotek, J.; Tircsó, G.; Hermann, P.; Tóth, E.; Lukes, I. Gallium(III) Complexes of DOTA and DOTA-Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. [Google Scholar] [CrossRef]
- Viola, N.A.; Rarig, R.S.; Ouellette, W.; Doyle, R.P. Synthesis, structure and thermal analysis of the gallium complex of 1,4,7,10-tetraazacyclo-dodecane-N,N′,N′′,N′′′-tetraacetic acid (DOTA). Polyhedron 2006, 25, 3457–3462. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Edem, P.E.; Jørgensen, J.T.; Nørregaard, K.; Rossin, R.; Yazdani, A.; Valliant, J.F.; Robillard, M.; Herth, M.M.; Kjaer, A. Evaluation of a 68Ga-Labeled DOTA-Tetrazine as a PET Alternative to 111In-SPECT Pretargeted Imaging. Molecules 2020, 25, 463. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Chang, C.-H.; Rossi, E.A.; McBride, W.J.; Sharkey, R.M. Pretargeted Molecular Imaging and Radioimmunotherapy. Theranostics 2012, 2, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, G. A Revisit to the Pretargeting Concept—A Target Conversion. Front. Pharmacol. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef]
- Eidherr, H.; Girschele, F.; Mitterhauser, M.; Wadsak, W. Radiochemical Syntheses; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 321–334. [Google Scholar]
- Eichenberger, L.S.; Patra, M.; Holland, J.P. Photoactive chelates for radiolabelling proteins. Chem. Commun. 2019, 55, 2257–2260. [Google Scholar] [CrossRef]
- Notni, J.; Pohle, K.; Wester, H.-J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: Practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012, 2, 28–32. [Google Scholar] [CrossRef]
- Vatsa, R.; Shukla, J.; Kumar, S.; Chakraboarty, S.; Dash, A.; Singh, G.; Mittal, B.R. Effect of Macro-Cyclic Bifunctional Chelators DOTA and NODAGA on Radiolabeling and In Vivo Biodistribution of Ga-68 Cyclic RGD Dimer. Cancer Biother. Radiopharm. 2019, 34, 427–435. [Google Scholar] [PubMed]
- Roosenburg, S.; Laverman, P.; Joosten, L.; Cooper, M.S.; Kolenc-Peitl, P.K.; Foster, J.M.; Hudson, C.; Leyton, J.; Burnet, J.; Oyen, W.J.G.; et al. PET and SPECT Imaging of a Radiolabeled Minigastrin Analogue Conjugated with DOTA, NOTA, and NODAGA and Labeled with 64Cu, 68Ga, and 111In. Mol. Pharm. 2014, 11, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Ghai, A.; Singh, B.; Hazari, P.P.; Schultz, M.K.; Parmar, A.; Kumar, P.; Sharma, S.; Dhawan, D.; Mishra, A.K. Radiolabeling optimization and characterization of 68Ga labeled DOTA–polyamido-amine dendrimer conjugate—Animal biodistribution and PET imaging results. Appl. Radiat. Isot. 2015, 105, 40–46. [Google Scholar] [CrossRef]
- Bhadwal, M.; Das, T.; Sarma, H.D.; Banerjee, S. Radiosynthesis and Bioevaluation of [68Ga]-Labeled 5,10,15,20-Tetra(4-methylpyridyl)-porphyrin for Possible Application as a PET Radiotracer for Tumor Imaging. Mol. Imaging Biol. 2015, 17, 111–118. [Google Scholar] [CrossRef]
- Guleria, M.; Das, T.; Amirdhanayagam, J.; Sarma, H.D.; Dash, A. Comparative Evaluation of Using NOTA and DOTA Derivatives as Bifunctional Chelating Agents in the Preparation of 68Ga-Labeled Porphyrin: Impact on Pharmacokinetics and Tumor Uptake in a Mouse Model. Cancer Biother. Radiopharm. 2018, 33, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sicco, E.; Báez, J.; Margenat, J.; García, F.; Ibarra, M.; Cabral, P.; Moreno, M.; Cerecetto, H.; Calzada, V. Derivatizations of Sgc8-c aptamer to prepare metallic radiopharmaceuticals as imaging diagnostic agents: Syntheses, isolations, and physicochemical characterizations. Chem. Biol. Drug Des. 2018, 91, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; de Lombaerde, S.; Vangestel, C.; de Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, R.; Mallia, M.B.; Sarma, H.D. 68Ga-labeled PET tracers for targeting tumor hypoxia: Role of bifunctional chelators on pharmacokinetics. Nucl. Med. Biol. 2021, 96–97, 61–67. [Google Scholar] [CrossRef]
- Peukert, C.; Langer, L.N.B.; Wegener, S.M.; Tutov, A.; Bankstahl, J.P.; Karge, B.; Bengel, F.M.; Ross, T.L.; Brönstrup, M. Optimization of Artificial Siderophores as 68Ga-Complexed PET Tracers for In Vivo Imaging of Bacterial Infections. J. Med. Chem. 2021, 64, 12359–12378. [Google Scholar] [CrossRef]
- Devreux, M.; Henoumont, C.; Dioury, F.; Stanicki, D.; Boutry, S.; Larbanoix, L.; Ferroud, C.; Muller, R.N.; Laurent, S. Bimodal Probe for Magnetic Resonance Imaging and Photoacoustic Imaging Based on a PCTA-Derived Gadolinium(III) Complex and ZW800–1. Eur. J. Inorg. Chem. 2019, 2019, 3354–3365. [Google Scholar] [CrossRef]
- Enel, M.; Leygue, N.; Saffon, N.; Galaup, C.; Picard, C. Facile Access to the 12-Membered Macrocyclic Ligand PCTA and Its Derivatives with Carboxylate, Amide, and Phosphinate Ligating Functionalities. Eur. J. Org. Chem. 2018, 2018, 1765–1773. [Google Scholar] [CrossRef]
- Leygue, N.; Enel, M.; Diallo, A.; Mestre-Voegtlé, B.; Galaup, C.; Picard, C. Efficient Synthesis of a Family of Bifunctional Chelators Based on the PCTA[12] Macrocycle Suitable for Bioconjugation. Eur. J. Org. Chem. 2019, 2019, 2899–2913. [Google Scholar] [CrossRef]
- Pandey, U.; Gamre, N.; Kumar, Y.; Shetty, P.; Sarma, H.D.; Dash, A. A systematic evaluation of the potential of PCTA-NCS ligand as a bifunctional chelating agent for design of 177Lu radiopharmaceuticals. J. Radioanal. Nucl. Chem. 2016, 307, 187–194. [Google Scholar] [CrossRef]
- Ferreira, C.; Lamsa, E.; Woods, M.; Duan, Y.; Fernando, P.; Bensimon, C.; Kordos, M.; Guenther, K.; Jurek, P.; Kiefer, G. Evaluation of Bifunctional Chelates for the Development of Gallium-Based Radiopharmaceuticals. Bioconjug. Chem. 2010, 21, 531–536. [Google Scholar] [CrossRef]
- Yong-Sang, J.; Dioury, F.; Meneyrol, V.; Ait-Arsa, I.; Idoumbin, J.-P.; Guibbal, F.; Patché, J.; Gimié, F.; Khantalin, I.; Couprie, J.; et al. Development, synthesis, and 68Ga-Labeling of a Lipophilic complexing agent for atherosclerosis PET imaging. Eur. J. Med. Chem. 2019, 176, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Azad, B.B.; Cho, C.-F.; Lewis, J.D.; Luyt, L.G. Synthesis, radiometal labeling and in vitro evaluation of a targeted PPIX derivative. Appl. Radiat. Isot. 2012, 70, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bryden, F.; Savoie, H.; Rosca, E.V.; Boyle, R.W. PET/PDT theranostics: Synthesis and biological evaluation of a peptide-targeted gallium porphyrin. Dalton Trans. 2015, 44, 4925–4932. [Google Scholar] [CrossRef] [PubMed]
- Enakieva, Y.Y.; Volostnykh, M.V.; Nefedov, S.E.; Kirakosyan, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y.; Bessmertnykh-Lemeune, A.G.; Stern, C.; Guilard, R. Gallium(III) and Indium(III) Complexes with meso-Monophosphorylated Porphyrins: Synthesis and Structure. A First Example of Dimers Formed by the Self-Assembly of meso-Porphyrinylphosphonic Acid Monoester. Inorg. Chem. 2017, 56, 3055–3070. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, Y.; Jalilian, A.R.; Amini, M.M.; Ardaneh, K.; Rahiminejad, A.; Bolourinovin, F.; Moradkhani, S.; Majdabadi, A. Development of a 68Ga-Fluorinated Porphyrin Complex as a Possible PET Imaging Agent. Nucl. Med. Mol. Imaging 2012, 46, 20–26. [Google Scholar] [CrossRef]
- Zoller, F.; Riss, P.J.; Montforts, F.-P.; Kelleher, D.K.; Eppard, E.; Rösch, F. Radiolabelling and preliminary evaluation of 68Ga-tetrapyrrole derivatives as potential tracers for PET. Nucl. Med. Biol. 2013, 40, 280–288. [Google Scholar] [CrossRef]
- L’Eplattenier, F.; Murase, I.; Martell, A. New multidentate ligands. VI. Chelating tendencies of N,N′-Di(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid. J. Am. Chem. Soc. 1967, 89, 837–843. [Google Scholar] [CrossRef]
- Bartholomä, M.D. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in Positron Emission Tomography. Inorg. Chim. Acta 2012, 389, 36–51. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Zha, Z.; Ploessl, K.; Choi, S.R.; Wu, Z.; Zhu, L.; Kung, H.F. Synthesis and evaluation of a novel urea-based 68Ga-complex for imaging PSMA binding in tumor. Nucl. Med. Biol. 2018, 59, 36–47. [Google Scholar] [CrossRef]
- Wang, G.; Hong, H.; Zang, J.; Liu, Q.; Jiang, Y.; Fan, X.; Zhu, Z.; Zhu, L.; Kung, H.F. Head-to-head comparison of [68Ga]Ga-P16-093 and [68Ga]Ga-PSMA-617 in dynamic PET/CT evaluation of the same group of recurrent prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Wu, Z.; Choi, S.R.; Ploessl, K.; Smith, M.; Alexoff, D.; Zhu, L.; Kung, H.F. A New [68Ga]Ga-HBED-CC-Bisphosphonate as a Bone Imaging Agent. Mol. Pharm. 2020, 17, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Ploessl, K.; Zha, Z.; Wang, H.; Guo, R.; Xie, Q.; Zhu, H.; Yang, Z.; Zhu, L.; Kung, H.F. Development and validation of a kit formulation of [68Ga]Ga-P15-041 as a bone imaging agent. Appl. Radiat. Isot. 2021, 169, 109485. [Google Scholar] [CrossRef] [PubMed]
- Satpati, D.; Sharma, R.; Kumar, C.; Sarma, H.D.; Dash, A. 68Ga-Chelation and comparative evaluation of N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. MedChemComm 2017, 8, 673–679. [Google Scholar] [CrossRef]
- Fay, R.; Gut, M.; Holland, J.P. Photoradiosynthesis of 68Ga-Labeled HBED-CC-Azepin-MetMAb for Immuno-PET of c-MET Receptors. Bioconjug. Chem. 2019, 30, 1814–1820. [Google Scholar] [CrossRef]
- Klika, K.D.; da Pieve, C.; Kopka, K.; Smith, G.; Makarem, A. Synthesis and application of a thiol-reactive HBED-type chelator for development of easy-to-produce Ga-radiopharmaceutical kits and imaging probes. Org. Biomol. Chem. 2021, 19, 1722–1726. [Google Scholar] [CrossRef]
- Adumeau, P.; Davydova, M.; Zeglis, B.M. Thiol-Reactive Bifunctional Chelators for the Creation of Site-Selectively Modified Radioimmunoconjugates with Improved Stability. Bioconjug. Chem. 2018, 29, 1364–1372. [Google Scholar] [CrossRef]
- Liolios, C.; Shegani, A.; Roupa, I.; Kiritsis, C.; Makarem, A.; Paravatou-Petsotas, M.; Pelecanou, M.; Bouziotis, P.; Papadopoulos, M.; Kopka, K.; et al. Synthesis, characterization and evaluation of 68Ga labelled monomeric and dimeric quinazoline derivatives of the HBED-CC chelator targeting the epidermal growth factor receptor. Bioorg. Chem. 2020, 100, 103855. [Google Scholar] [CrossRef]
- Makarem, A.; Klika, K.D.; Litau, G.; Remde, Y.; Kopka, K. HBED-NN: A Bifunctional Chelator for Constructing Radiopharmaceuticals. J. Org. Chem. 2019, 84, 7501–7508. [Google Scholar] [CrossRef]
- Makarem, A.; Sarvestani, M.K.; Klika, K.D.; Kopka, K. A Multifunctional HBED-Type Chelator with Dual Conjugation Capabilities for Radiopharmaceutical Development. Synlett 2019, 30, 1795–1798. [Google Scholar] [CrossRef]
- McDonagh, A.W.; McNeil, B.L.; Rousseau, J.; Roberts, R.J.; Merkens, H.; Yang, H.; Bénard, F.; Ramogida, C.F. Development of a multi faceted platform containing a tetrazine, fluorophore and chelator: Synthesis, characterization, radiolabeling, and immuno-SPECT imaging. EJNMMI Radiopharm. Chem. 2022, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Jerzyk, K.; Kludkiewicz, D.; Pijarowska-Kruszyna, J.; Jaron, A.; Maurin, M.; Sikora, A.; Kordowski, L.; Garnuszek, P. Synthesis of HBED–CC–tris(tert-butyl ester) using a solid phase and a microwave reactor. Tetrahedron 2021, 84, 132018. [Google Scholar] [CrossRef]
- Hider, R.C.; Hall, A.D. Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 28, pp. 41–173. [Google Scholar]
- Wallin, M.; Turner, P.; Katsifis, A.; Yang, M.; Chan, H.-K. Crystal structure of aqua(2-{[2-({2-[bis(carboxylato-κO-methyl)amino-κN]ethyl}(carboxylato-κO-methyl)amino-κN)ethyl](carboxymethyl)azaniumyl}acetato)gallium(III) trihydrate. Acta Cryst. E 2018, 74, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Waters, S.E.; Robb, B.H.; Marshak, M.P. Effect of Chelation on Iron–Chromium Redox Flow Batteries. ACS Energy Lett. 2020, 5, 1758–1762. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Greiser, J.; Hagemann, T.; Niksch, T.; Traber, P.; Kupfer, S.; Gräfe, S.; Görls, H.; Weigand, W.; Freesmeyer, M. Synthesis and Characterization of GaIII, InIII and LuIII Complexes of a Set of dtpa Bis-Amide Ligands. Eur. J. Inorg. Chem. 2015, 2015, 4125–4137. [Google Scholar] [CrossRef]
- Vergara, I.; Castillo, E.Y.; Romero-Piña, M.E.; Torres-Viquez, I.; Paniagua, D.; Boyer, L.V.; Alagón, A.; Medina, L.A. Biodistribution and Lymphatic Tracking of the Main Neurotoxin of Micrurus fulvius Venom by Molecular Imaging. Toxins 2016, 8, 85. [Google Scholar] [CrossRef]
- Gut, M.; Holland, J.P. Synthesis and Photochemical Studies on Gallium and Indium Complexes of DTPA-PEG3-ArN3 for Radiolabeling Antibodies. Inorg. Chem. 2019, 58, 12302–12310. [Google Scholar] [CrossRef]
- Patra, M.; Eichenberger, L.S.; Fischer, G.; Holland, J.P. Photochemical Conjugation and One-Pot Radiolabelling of Antibodies for Immuno-PET. Angew. Chem. Int. Ed. 2019, 58, 1928–1933. [Google Scholar] [CrossRef]
- Patra, M.; Klingler, S.; Eichenberger, L.S.; Holland, J.P. Simultaneous Photoradiochemical Labeling of Antibodies for Immuno-Positron Emission Tomography. iScience 2019, 13, 416–431. [Google Scholar] [CrossRef]
- Jain, A.; Chakraborty, S.; Sarma, H.D.; Dash, A. A Systematic Comparative Evaluation of 68Ga-Labeled RGD Peptides Conjugated with Different Chelators. Nucl. Med. Mol. Imaging 2018, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mathur, A.; Pandey, U.; Sarma, H.D.; Dash, A. 68Ga labeled fatty acids for cardiac metabolic imaging: Influence of different bifunctional chelators. Bioorg. Med. Chem. Lett. 2016, 26, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Hoh, C.K.; Olson, E.S.; Jahromi, A.H.; Hall, D.J.; Barback, C.V.; You, Y.-H.; Yanagita, M.; Sharma, K.; Vera, D.R. Molecular Imaging of the Glomerulus via Mesangial Cell Uptake of Radiolabeled Tilmanocept. J. Nucl. Med. 2019, 60, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Sattarzadeh, E.; Amini, M.M.; Kakaei, S.; Khanchi, A. 68Ga-radiolabeled magnetic nanoparticles for PET–MRI imaging. J. Radioanal. Nucl. Chem. 2018, 317, 1333–1339. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254. [Google Scholar] [CrossRef]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef]
- Ettlinger, L.; Corbaz, R.; Hütter, R. Zur Systematik der Actinomyceten. Archiv. Mikrobiol. 1958, 31, 326–358. [Google Scholar] [CrossRef]
- Yokoyama, A.; Ohmomo, Y.; Horiuchi, K.; Saji, H.; Tanaka, H.; Yamamoto, K.; Ishil, Y.; Torizuka, K. Deferoxamine, A Promising Bifunctional Chelating Agent for Labeling Proteins with Gallium: Ga-67 DF-HSA: Concise Communication. J. Nucl. Med. 1982, 23, 909–914. [Google Scholar]
- Joaqui-Joaqui, M.A.; Pandey, M.K.; Bansal, A.; Raju, M.V.R.; Armstrong-Pavlik, F.; Dundar, A.; Wong, H.L.; DeGrado, T.R.; Pierre, V.C. Catechol-Based Functionalizable Ligands for Gallium-68 Positron Emission Tomography Imaging. Inorg. Chem. 2020, 59, 12025–12038. [Google Scholar] [CrossRef] [PubMed]
- Govindan, S.V.; Michel, R.B.; Griffiths, G.L.; Goldenberg, D.M.; Mattes, M.J. Deferoxamine as a chelator for 67Ga in the preparation of antibody conjugates. Nucl. Med. Biol. 2005, 32, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Caraco, C.; Aloj, L.; Eckelman, W.C. The gallium–deferoxamine complex: Stability with different deferoxamine concentrations and incubation conditions. Appl. Radiat. Isot. 1998, 49, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Ryser, J.E.; Rose, K.; Jones, R.; Pelegrin, A.; Donath, A.; Egeli, R.; Smith, A.; Offord, R.E. Elimination of Free Radionuclide by a Chelating Agent Improves Tumor-to-Nontumor Ratios Following Radioimmunotargeting with Antibody Labeled with 67Ga. Nucl. Med. Biol. 1998, 25, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.; Fischer, E.; Rosillo-Lopez, M.; Salzmann, C.G.; Holland, J.P. Multi-functionalised graphene nanoflakes as tumour-targeting theranostic drug-delivery vehicles. Chem. Sci. 2019, 10, 8880–8888. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, S.; White, P.S.; Crumbliss, A.L. Crystal structure of ferrioxamine B: A comparative analysis and implications for molecular recognition. J. Biol. Inorg. Chem. 2001, 6, 810–818. [Google Scholar] [CrossRef]
- Gourni, E.; del Pozzo, L.; Bartholomä, M.; Kiefer, Y.; Meyer, P.T.; Maecke, H.R.; Holland, J.P. Radiochemistry and Preclinical PET Imaging of 68Ga-Desferrioxamine Radiotracers Targeting Prostate-Specific Membrane Antigen. Mol. Imaging 2017, 16, 1536012117737010. [Google Scholar] [CrossRef]
- Ioppolo, J.A.; Caldwell, D.; Beiraghi, O.; Llano, L.; Blacker, M.; Valliant, J.F.; Berti, P.J. 67Ga-labeled deferoxamine derivatives for imaging bacterial infection: Preparation and screening of functionalized siderophore complexes. Nucl. Med. Biol. 2017, 52, 32–41. [Google Scholar] [CrossRef]
- Brandt, M.; Cowell, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. Radiolabelling of the octadentate chelators DFO* and oxoDFO* with zirconium-89 and gallium-68. J. Biol. Inorg. Chem. 2020, 25, 789–796. [Google Scholar] [CrossRef]
- Brown, C.J.M.; Gotsbacher, M.P.; Codd, R. Improved Access to Linear Tetrameric Hydroxamic Acids with Potential as Radiochemical Ligands for Zirconium(IV)-89 PET Imaging. Aust. J. Chem. 2020, 73, 969–978. [Google Scholar] [CrossRef]
- Richardson-Sanchez, T.; Tieu, W.; Gotsbacher, M.P.; Telfer, T.J.; Codd, R. Exploiting the biosynthetic machinery of Streptomyces pilosus to engineer a water-soluble zirconium(iv) chelator. Org. Biomol. Chem. 2017, 15, 5719–5730. [Google Scholar] [CrossRef]
- Noor, A.; van Zuylekom, J.K.; Rudd, S.E.; Roselt, P.D.; Haskali, M.B.; Yan, E.; Wheatcroft, M.; Hicks, R.J.; Cullinane, C.; Donnelly, P.S. Imaging Somatostatin Positive Tumors with Tyr3-Octreotate/Octreotide Conjugated to Desferrioxamine B Squaramide Radiolabeled with either Zirconium-89 or Gallium-68. Bioconjug. Chem. 2021, 32, 1192–1203. [Google Scholar] [CrossRef]
- Noor, A.; van Zuylekom, J.K.; Rudd, S.E.; Waldeck, K.; Roselt, P.D.; Haskali, M.B.; Wheatcroft, M.P.; Yan, E.; Hicks, R.J.; Cullinane, C.; et al. Bivalent Inhibitors of Prostate-Specific Membrane Antigen Conjugated to Desferrioxamine B Squaramide Labeled with Zirconium-89 or Gallium-68 for Diagnostic Imaging of Prostate Cancer. J. Med. Chem. 2020, 63, 9258–9270. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova, S.; Daniskova, A.; Bendova, K.; Novy, Z.; Soural, M.; Petrik, M. [68Ga]Ga-DFO-c(RGDyK): Synthesis and Evaluation of Its Potential for Tumor Imaging in Mice. Int. J. Mol. Sci. 2021, 22, 7391. [Google Scholar] [CrossRef]
- Oroujeni, M.; Xu, T.; Gagnon, K.; Rinne, S.S.; Weis, J.; Garousi, J.; Andersson, K.G.; Löfblom, J.; Orlova, A.; Tolmachev, V. The Use of a Non-Conventional Long-Lived Gallium Radioisotope 66Ga Improves Imaging Contrast of EGFR Expression in Malignant Tumours Using DFO-ZEGFR:2377 Affibody Molecule. Pharmaceutics 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Bauman, A.; Valverde, I.E.; Fischer, C.A.; Vomstein, S.; Mindt, T.L. Development of 68Ga- and 89Zr-Labeled Exendin-4 as Potential Radiotracers for the Imaging of Insulinomas by PET. J. Nucl. Med. 2015, 56, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Kaeppeli, S.A.M.; Schibli, R.; Mindt, T.L.; Behe, M. Comparison of desferrioxamine and NODAGA for the gallium-68 labeling of exendin-4. EJNMMI Radiopharm. Chem. 2019, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Hisada, H.; Temma, T.; Shimizu, Y.; Kimura, H.; Ono, M.; Nakamoto, Y.; Togashi, K.; Saji, H. Gallium-68-Labeled Anti-HER2 Single-Chain Fv Fragment: Development and In Vivo Monitoring of HER2 Expression. Mol. Imaging Biol. 2015, 17, 102–110. [Google Scholar] [CrossRef]
- Harris, W.R.; Carrano, C.J.; Cooper, S.R.; Sofen, S.R.; Avdeef, A.E.; McArdle, J.V.; Raymond, K.N. Coordination Chemistry of Microbial Iron Transport Compounds. 19. Stability Constants and Electrochemical Behavior of Ferric Enterobactin and Model Complexes. J. Am. Chem. Soc. 1979, 101, 6097–6104. [Google Scholar] [CrossRef]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Schrettl, M.; Helbok, A.; Blatzer, M.; Decristoforo, C. In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl. Med. Biol. 2012, 39, 361–369. [Google Scholar] [CrossRef]
- Petrik, M.; Umlaufova, E.; Raclavsky, V.; Palyzova, A.; Havlicek, V.; Pfister, J.; Mair, C.; Novy, Z.; Popper, M.; Hajduch, M.; et al. 68Ga-labelled desferrioxamine-B for bacterial infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 372–382. [Google Scholar] [CrossRef]
- Evers, A.; Hancock, R.D.; Martell, A.E.; Motekaitis, R.J. Metal ion recognition in ligands with negatively charged oxygen donor groups. Complexation of iron(III), gallium(III), indium(III), aluminum(III), and other highly charged metal ions. Inorg. Chem. 1989, 28, 2189–2195. [Google Scholar] [CrossRef]
- Guillou, A.; Earley, D.F.; Patra, M.; Holland, J.P. Light-induced synthesis of protein conjugates and its application in photoradiosynthesis of 89Zr-radiolabeled monoclonal antibodies. Nat. Protoc. 2020, 15, 3579–3594. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Nostra, B.V.; Viola, N. The Radiopharmaceutical Chemistry of Zirconium-89. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 371–390. [Google Scholar]
- Boros, E.; Ferreira, C.L.; Cawthray, J.F.; Price, E.W.; Patrick, B.O.; Wester, D.W.; Adam, M.J.; Orvig, C. Acyclic Chelate with Ideal Properties for 68Ga PET Imaging Agent Elaboration. J. Am. Chem. Soc. 2010, 132, 15726–15733. [Google Scholar] [CrossRef] [PubMed]
- Platas-Iglesias, C.; Mato-Iglesias, M.; Djanashvili, K.; Muller, R.N.; Elst, L.V.; Peters, J.A.; de Blas, A.; Rodríguez-Blas, T. Lanthanide Chelates Containing Pyridine Units with Potential Application as Contrast Agents in Magnetic Resonance Imaging. Chem. Eur. J. 2004, 10, 3579–3590. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Cawthray, J.F.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. Evaluation of the H2dedpa Scaffold and its cRGDyK Conjugates for Labeling with 64Cu. Inorg. Chem. 2012, 51, 6279–6284. [Google Scholar] [CrossRef]
- Boros, E.; Ferreira, C.L.; Patrick, B.O.; Adam, M.J.; Orvig, C. New Ga derivatives of the H2dedpa scaffold with improved clearance and persistent heart uptake. Nucl. Med. Biol. 2011, 38, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Ferreira, C.L.; Yapp, D.T.T.; Gill, R.K.; Price, E.W.; Adam, M.J.; Orvig, C. RGD conjugates of the H2dedpa scaffold: Synthesis, labeling and imaging with 68Ga. Nucl. Med. Biol. 2012, 39, 785–794. [Google Scholar] [CrossRef]
- Bailey, G.A.; Price, E.W.; Zeglis, B.M.; Ferreira, C.L.; Boros, E.; Lacasse, M.J.; Patrick, B.O.; Lewis, J.S.; Adam, M.J.; Orvig, C. H2azapa: A Versatile Acyclic Multifunctional Chelator for 67Ga, 64Cu, 111In, and 177Lu. Inorg. Chem. 2012, 51, 12575–12589. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Pan, J.; Ferreira, C.L.; Patrick, B.O.; Rebullar, K.; Yapp, D.T.T.; Lin, K.-S.; Adam, M.J.; Orvig, C. Nitroimidazole-Containing H2dedpa and H2CHXdedpa Derivatives as Potential PET Imaging Agents of Hypoxia with 68Ga. Inorg. Chem. 2015, 54, 4953–4965. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Murphy, L.; Cawthray, J.F.; Ross, J.D.; Adam, M.J.; Orvig, C. Novel “bi-modal” H2dedpa derivatives for radio- and fluorescence imaging. J. Inorg. Biochem. 2016, 162, 253–262. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Boros, E.; Patrick, B.O.; Zeisler, S.K.; Kumlin, J.; Adam, M.J.; Schaffer, P.; Orvig, C. Evaluation of H2CHXdedpa, H2dedpa- and H2CHXdedpa-N,N′-propyl-2-NI ligands for 64Cu(II) radiopharmaceuticals. Dalton Trans. 2016, 45, 13082–13090. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Schindler, D.; Schneider, C.; Tan, Y.L.K.; Huh, S.; Ferreira, C.L.; Adam, M.J.; Orvig, C. Synthesis and characterization of lipophilic cationic Ga(III) complexes based on the H2CHXdedpa and H2dedpa ligands and their 67/68Ga radiolabeling studies. RSC Adv. 2016, 6, 103763–103773. [Google Scholar] [CrossRef]
- Saito, K.; Watanabe, H.; Iikuni, S.; Ono, M. Development of novel 67/68Ga-labeled pyridyl benzofuran derivatives as islet amyloid imaging probes. Nucl. Med. Biol. 2022, 106–107, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jaraquemada-Peláez, M.a.d.G.; Cao, Y.; Pan, J.; Lin, K.-S.; Patrick, B.O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. 2018, 58, 2275–2285. [Google Scholar] [CrossRef]
- Wang, X.; Jaraquemada-Peláez, M.d.G.; Cao, Y.; Ingham, A.; Rodríguez-Rodríguez, C.; Pan, J.; Wang, Y.; Saatchi, K.; Häfeli, U.O.; Lin, K.-S.; et al. H2CHXhox: Rigid Cyclohexane-Reinforced Nonmacrocyclic Chelating Ligand for [nat/67/68Ga]Ga3+. Inorg. Chem. 2020, 59, 4895–4908. [Google Scholar] [CrossRef]
- Berry, D.J.; Ma, Y.; Ballinger, J.R.; Tavaré, R.; Koers, A.; Sunassee, K.; Zhou, T.; Nawaz, S.; Mullen, G.E.D.; Hider, R.C.; et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: Tris(hydroxypyridinone) ligands. Chem. Commun. 2011, 47, 7068–7070. [Google Scholar] [CrossRef]
- Imberti, C.; Adumeau, P.; Blower, J.E.; al Salemee, F.; Torres, J.B.; Lewis, J.S.; Zeglis, B.M.; Terry, S.Y.A.; Blower, P.J. Manipulating the In Vivo Behaviour of 68Ga with Tris(Hydroxypyridinone) Chelators: Pretargeting and Blood Clearance. Int. J. Mol. Sci. 2020, 21, 1496. [Google Scholar] [CrossRef]
- Dobbin, P.S.; Hider, R.C.; Hall, A.D.; Taylor, P.D.; Sarpong, P.; Porter, J.B.; Xiao, G.; van der Helm, D. Synthesis, physicochemical properties, and biological evaluation of N-substituted 2-alkyl-3-hydroxy-4(1H)-pyridinones: Orally active iron chelators with clinical potential. J. Med. Chem. 1993, 36, 2448–2458. [Google Scholar] [CrossRef]
- Yue, J.L.; Martell, A.E. Potentiometric and spectrophotometric determination of stabilities of the 1-hydroxy-2-pyridinone complexes of trivalent and divalent metal ions. Inorg. Chim. Acta 1993, 214, 103–111. [Google Scholar] [CrossRef]
- Scarrow, R.C.; Riley, P.E.; Abu-Dari, K.; White, D.L.; Raymond, K.N. Ferric ion sequestering agents. 13. Synthesis, structures, and thermodynamics of complexation of cobalt(III) and iron(III) tris complexes of several chelating hydroxypyridinones. Inorg. Chem. 1985, 24, 954–967. [Google Scholar] [CrossRef]
- Nelson, W.O.; Karpishin, T.B.; Rettig, S.J.; Orvig, C. Aluminum and gallium compounds of 3-hydroxy-4-pyridinones: Synthesis, characterization, and crystallography of biologically active complexes with unusual hydrogen bonding. Inorg. Chem. 1988, 27, 1045–1051. [Google Scholar] [CrossRef]
- Imberti, C.; Terry, S.Y.A.; Cullinane, C.; Clarke, F.; Cornish, G.H.; Ramakrishnan, N.K.; Roselt, P.; Cope, A.P.; Hicks, R.J.; Blower, P.J.; et al. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of αvβ3 Integrin Expression with Gallium-68. Bioconjug. Chem. 2017, 28, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Cullinane, C.; Waldeck, K.; Roselt, P.; Hicks, R.J.; Blower, P.J. Rapid kit-based 68Ga-labelling and PET imaging with THP-Tyr3-octreotate: A preliminary comparison with DOTA-Tyr3-octreotate. EJNMMI Res. 2015, 5, 52. [Google Scholar] [CrossRef]
- Ma, M.T.; Cullinane, C.; Imberti, C.; Torres, J.B.; Terry, S.Y.A.; Roselt, P.; Hicks, R.J.; Blower, P.J. New Tris(hydroxypyridinone) Bifunctional Chelators Containing Isothiocyanate Groups Provide a Versatile Platform for Rapid One-Step Labeling and PET Imaging with 68Ga3+. Bioconjug. Chem. 2016, 27, 309–318. [Google Scholar] [CrossRef]
- Imberti, C.; Chen, Y.-L.; Foley, C.A.; Ma, M.T.; Paterson, B.M.; Wang, Y.; Young, J.D.; Hider, R.C.; Blower, P.J. Tuning the properties of tris(hydroxypyridinone) ligands: Efficient 68Ga chelators for PET imaging. Dalton Trans. 2019, 48, 4299–4313. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Mullen, G.E.D.; Sunassee, K.; Bordoloi, J.; Blower, P.J.; Ballinger, J.R. Simple, mild, one-step labelling of proteins with gallium-68 using a tris(hydroxypyridinone) bifunctional chelator: A 68Ga-THP-scFv targeting the prostate-specific membrane antigen. EJNMMI Res. 2017, 7, 86. [Google Scholar] [CrossRef]
- Blower, J.E.; Cooper, M.S.; Imberti, C.; Ma, M.T.; Marshall, C.; Young, J.D.; Blower, P.J. The Radiopharmaceutical Chemistry of the Radionuclides of Gallium and Indium. In Radiopharmaceutical Chemistry; Lewis, J.S., Windhorst, A.D., Zeglis, B.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 255–271. [Google Scholar]
- Hofman, M.S.; Eu, P.; Jackson, P.; Hong, E.; Binns, D.; Iravani, A.; Murphy, D.; Mitchell, C.; Siva, S.; Hicks, R.J.; et al. Cold Kit for Prostate-Specific Membrane Antigen (PSMA) PET Imaging: Phase 1 Study of 68Ga-Tris(Hydroxypyridinone)-PSMA PET/CT in Patients with Prostate Cancer. J. Nucl. Med. 2018, 59, 625–631. [Google Scholar] [CrossRef]
- Kulkarni, M.; Hughes, S.; Mallia, A.; Gibson, V.; Young, J.; Aggarwal, A.; Morris, S.; Challacombe, B.; Popert, R.; Brown, C.; et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 674–686. [Google Scholar] [CrossRef]
- Floresta, G.; Keeling, G.P.; Memdouh, S.; Meszaros, L.K.; de Rosales, R.T.M.; Abbate, V. NHS-Functionalized THP Derivative for Efficient Synthesis of Kit-Based Precursors for 68Ga Labeled PET Probes. Biomedicines 2021, 9, 367. [Google Scholar] [CrossRef]
- Aime, S.; Bombieri, G.; Cavallotti, C.; Giovenzana, G.B.; Imperio, D.; Marchini, N. An unusual gadolinium ten-coordinated dimeric complex in the series of MRI contrast agents: Na[Gd(H2O)AAZTA]·3H2O. Inorg. Chim. Acta 2008, 361, 1534–1541. [Google Scholar] [CrossRef]
- Aime, S.; Calabi, L.; Cavallotti, C.; Gianolio, E.; Giovenzana, G.B.; Losi, P.; Maiocchi, A.; Palmisano, G.; Sisti, M. [Gd-AAZTA]−: A New Structural Entry for an Improved Generation of MRI Contrast Agents. Inorg. Chem. 2004, 43, 7588–7590. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Uggeri, F.; Giovenzana, G.B.; Bényei, A.; Brücher, E.; Aime, S. Equilibrium and Kinetic Properties of the Lanthanoids(III) and Various Divalent Metal Complexes of the Heptadentate Ligand AAZTA. Chem. Eur. J. 2009, 15, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Elemento, E.M.; Parker, D.; Aime, S.; Gianolio, E.; Lattuada, L. Variation of water exchange dynamics with ligand structure and stereochemistry in lanthanide complexes based on 1,4-diazepine derivatives. Org. Biomol. Chem. 2009, 7, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.A.; Neves, A.; Bortoluzzi, A.J.; Casellato, A.; Anjos, A.d.; Greatti, A.; Xavier, F.R.; Szpoganicz, B. First-Transition-Metal Complexes Containing the Ligands 6-Amino-6-methylperhydro-1,4-diazepine (AAZ) and a New Functionalized Derivative: Can AAZ Act as a Mimetic Ligand for 1,4,7-Triazacyclononane? Inorg. Chem. 2005, 44, 7690–7692. [Google Scholar] [CrossRef] [PubMed]
- Waldron, B.P.; Parker, D.; Burchardt, C.; Yufit, D.S.; Zimny, M.; Roesch, F. Structure and stability of hexadentate complexes of ligands based on AAZTA for efficient PET labelling with gallium-68. Chem. Commun. 2013, 49, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Waldron, B.P. Conformational analysis and synthetic approaches to polydentate perhydro-diazepine ligands for the complexation of gallium(III). Org. Biomol. Chem. 2013, 11, 2827–2838. [Google Scholar] [CrossRef]
- Parker, D.; Waldron, B.P.; Yufit, D.S. Crystallographic and solution NMR structural analyses of four hexacoordinated gallium(III) complexes based on ligands derived from 6-amino-perhydro-1,4-diazepine. Dalton Trans. 2013, 42, 8001–8008. [Google Scholar] [CrossRef]
- Waldron, B. Synthesis and Evaluation of New Ligands for Gallium Radiolabelling. Ph.D. Thesis, University of Durham, Durham, UK, 2013. [Google Scholar]
- Farkas, E.; Vágner, A.; Negri, R.; Lattuada, L.; Tóth, I.; Colombo, V.; Esteban-Gómez, D.; Platas-Iglesias, C.; Notni, J.; Baranyai, Z.; et al. PIDAZTA: Structurally Constrained Chelators for the Efficient Formation of Stable Gallium-68 Complexes at Physiological pH. Chem. Eur. J. 2019, 25, 10698–10709. [Google Scholar] [CrossRef]
- Wu, Z.; Zha, Z.; Choi, S.R.; Plössl, K.; Zhu, L.; Kung, H.F. New 68Ga-PhenA bisphosphonates as potential bone imaging agents. Nucl. Med. Biol. 2016, 43, 360–371. [Google Scholar] [CrossRef]
- Gugliotta, G.; Botta, M.; Giovenzana, G.B.; Tei, L. Fast and easy access to efficient bifunctional chelators for MRI applications. Bioorg. Med. Chem. Lett. 2009, 19, 3442–3444. [Google Scholar] [CrossRef]
- Nock, B.A.; Kaloudi, A.; Nagel, J.; Sinnes, J.-P.; Roesch, F.; Maina, T. Novel bifunctional DATA chelator for quick access to site-directed PET 68Ga-radiotracers: Preclinical proof-of-principle with [Tyr3]octreotide. Dalton Trans. 2017, 46, 14584–14590. [Google Scholar] [CrossRef] [PubMed]
- Sinnes, J.-P.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, M.; Moon, E.S.; D’Angelo, F.; Geissbühler, L.; Alberts, I.; Afshar-Oromieh, A.; Rösch, F.; Rominger, A.; Gourni, E. Effect of the versatile bifunctional chelator AAZTA5 on the radiometal labelling properties and the in vitro performance of a gastrin releasing peptide receptor antagonist. EJNMMI Radiopharm. Chem. 2020, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Klasen, B.; Moon, E.S.; Rösch, F. AAZTA5-squaramide ester competing with DOTA-, DTPA- and CHX-A″-DTPA-analogues: Promising tool for 177Lu-labeling of monoclonal antibodies under mild conditions. Nucl. Med. Biol. 2021, 96–97, 80–93. [Google Scholar] [CrossRef]
- Greifenstein, L.; Grus, T.; Nagel, J.; Sinnes, J.P.; Rösch, F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA5 for versatile labeling with 44Sc, 64Cu, 68Ga and 177Lu. Appl. Radiat. Isot. 2020, 156, 108867. [Google Scholar] [CrossRef]
- Sinnes, J.-P.; Bauder-Wüst, U.; Schäfer, M.; Moon, E.S.; Kopka, K.; Rösch, F. 68Ga, 44Sc and 177Lu-labeled AAZTA5-PSMA-617: Synthesis, radiolabeling, stability and cell binding compared to DOTA-PSMA-617 analogues. EJNMMI Radiopharm. Chem. 2020, 5, 28. [Google Scholar] [CrossRef]
- Orteca, G.; Sinnes, J.-P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954. [Google Scholar] [CrossRef]
- Yadav, D.; Ballal, S.; Yadav, M.P.; Tripathi, M.; Roesch, F.; Bal, C. Evaluation of [68Ga]Ga-DATA-TOC for imaging of neuroendocrine tumours: Comparison with [68Ga]Ga-DOTA-NOC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 860–869. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.; Parker, D.; Roesch, F. DATATOC: A novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature, EJNMMI Radiopharm. Chem. 2016, 1, 4. [Google Scholar]
- Sinnes, J.-P.; Nagel, J.; Waldron, B.P.; Maina, T.; Nock, B.A.; Bergmann, R.K.; Ullrich, M.; Pietzsch, J.; Bachmann, M.; Baum, R.P.; et al. Instant kit preparation of 68Ga-radiopharmaceuticals via the hybrid chelator DATA: Clinical translation of [68Ga]Ga-DATA-TOC. EJNMMI Res. 2019, 9, 48. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Geraldes, C.F.G.C.; de Lima, J.J.P. Structural and in vivo studies of metal chelates of Ga(III) relevant to biomedical imaging. J. Inorg. Biochem. 2000, 79, 359–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Notni, J.; Šimeček, J.; Hermann, P.; Wester, H.J. TRAP, a Powerful and Versatile Framework for Gallium-68 Radiopharmaceuticals. Chem. Eur. J. 2011, 17, 14718–14722. [Google Scholar] [CrossRef] [PubMed]
- Drahoš, B.; Kubíček, V.; Bonnet, C.S.; Hermann, P.; Lukeš, I.; Tóth, É. Dissociation kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.; Sherry, A.; Cacheris, W. Synthesis, Protonation Sequence, and NMR Studies of Polyazamacrocyclic Methylenephosphonates. Inorg. Chem. 1989, 28, 3336–3341. [Google Scholar] [CrossRef]
- Notni, J.; Hermann, P.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Plutnar, J.; Loktionova, N.; Riss, P.J.; Rösch, F.; Lukeš, I. A Triazacyclononane-Based Bifunctional Phosphinate Ligand for the Preparation of Multimeric 68Ga Tracers for Positron Emission Tomography. Chem. Eur. J. 2010, 16, 7174–7185. [Google Scholar] [CrossRef]
- Bianchi, A.; Calabi, L.; Giorgi, C.; Losi, P.; Mariani, P.; Paoli, P.; Rossi, P.; Valtancoli, B.; Virtuani, M. Thermodynamic and structural properties of Gd3+ complexes with functionalized macrocyclic ligands based upon 1,4,7,10-tetraazacyclododecane. J. Chem. Soc. Dalton Trans. 2000, 697–705. [Google Scholar] [CrossRef]
- Notni, J.; Šimeček, J.; Wester, H.-J. Phosphinic Acid Functionalized Polyazacycloalkane Chelators for Radiodiagnostics and Radiotherapeutics: Unique Characteristics and Applications. ChemMedChem 2014, 9, 1107–1115. [Google Scholar] [CrossRef]
- Davey, P.R.W.J.; Forsyth, C.M.; Paterson, B.M. Crystallographic and Computational Characterisation of the Potential PET Tracer 1,4,7-Triazacyclononane-1,4,7-tri(methylenephosphonato)gallium(III). ChemistrySelect 2022, 7, e202103698. [Google Scholar] [CrossRef]
- Brand, C.; Longo, V.A.; Groaning, M.; Weber, W.A.; Reiner, T. Development of a New Folate-Derived Ga-68-Based PET Imaging Agent. Mol. Imaging Biol. 2017, 19, 754–761. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, H.; Li, Y.; Wu, X.; Wang, H.; Cheng, Z. Detecting Vulnerable Atherosclerotic Plaques by 68Ga-Labeled Divalent Cystine Knot Peptide. Mol. Pharm. 2019, 16, 1350–1357. [Google Scholar] [CrossRef]
- Sneddon, D.; Cornelissen, B. Emerging chelators for nuclear imaging. Curr. Opin. Chem. Biol. 2021, 63, 152–162. [Google Scholar] [CrossRef]
- Su, T.; Wang, Y.-B.; Han, D.; Wang, J.; Qi, S.; Gao, L.; Shao, Y.-H.; Qiao, H.-Y.; Chen, J.-W.; Liang, S.-H.; et al. Multimodality Imaging of Angiogenesis in a Rabbit Atherosclerotic Model by GEBP11 Peptide Targeted Nanoparticles. Theranostics 2017, 7, 4791–4804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lang, L.; Wang, Z.; Jacobson, O.; Yung, B.; Zhu, G.; Gu, D.; Ma, Y.; Zhu, X.; Niu, G.; et al. Positron Emission Tomography Imaging of Prostate Cancer with Ga-68-Labeled Gastrin-Releasing Peptide Receptor Agonist BBN7–14 and Antagonist RM26. Bioconjug. Chem. 2018, 29, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, A.; Schottelius, M.; Schwaiger, M.; Wester, H.-J. Preclinical evaluation of [68Ga]NOTA-pentixafor for PET imaging of CXCR4 expression in vivo—A comparison to [68Ga]pentixafor. EJNMMI Res. 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Eisenwiener, K.-P.; Prata, M.I.M.; Buschmann, I.; Zhang, H.-W.; Santos, A.C.; Wenger, S.; Reubi, J.C.; Mäcke, H.R. NODAGATOC, a New Chelator-Coupled Somatostatin Analogue Labeled with [67/68Ga] and [111In] for SPECT, PET, and Targeted Therapeutic Applications of Somatostatin Receptor (hsst2) Expressing Tumors. Bioconjug. Chem. 2002, 13, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Nedrow, J.R.; White, A.G.; Modi, J.; Nguyen, K.; Chang, A.J.; Anderson, C.J. Positron Emission Tomographic Imaging of Copper 64– and Gallium 68–Labeled Chelator Conjugates of the Somatostatin Agonist Tyr3-Octreotate. Mol. Imaging 2014, 13, 7290-2014. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, X.; Zhang, Z.; Sun, Z.; Loft, M.; Ding, B.; Liu, C.; Xu, L.; Yang, M.; Jiang, Y.; et al. Preclinical Study on GRPR-Targeted 68Ga-Probes for PET Imaging of Prostate Cancer. Bioconjug. Chem. 2016, 27, 1857–1864. [Google Scholar] [CrossRef]
- D’Alessandria, C.; Pohle, K.; Rechenmacher, F.; Neubauer, S.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Kessler, H.; Beer, A.J. In vivo biokinetic and metabolic characterization of the 68Ga-labelled α5β1-selective peptidomimetic FR366. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 953–963. [Google Scholar] [CrossRef]
- Hübner, R.; Cheng, X.; Wängler, B.; Wängler, C. Functional Hybrid Molecules for the Visualization of Cancer: PESIN-Homodimers Combined with Multimodal Molecular Imaging Probes for Positron Emission Tomography and Optical Imaging: Suited for Tracking of GRPR-Positive Malignant Tissue. Chem. Eur. J. 2020, 26, 16349–16356. [Google Scholar] [CrossRef]
- Van der Veen, E.L.; Suurs, F.V.; Cleeren, F.; Bormans, G.; Elsinga, P.H.; Hospers, G.A.P.; Hooge, M.N.L.; de Vries, E.G.E.; de Vries, E.F.J.; Antunes, I.F. Development and Evaluation of Interleukin-2–Derived Radiotracers for PET Imaging of T Cells in Mice. J. Nucl. Med. 2020, 61, 1355–1360. [Google Scholar] [CrossRef]
- André, J.P.; Maecke, H.R.; Zehnder, M.; Macko, L.; Akyel, K.G. 1,4,7-Triazacyclononane-1-succinic acid-4,7-diacetic acid (NODASA): A new bifunctional chelator for radio gallium-labelling of biomolecules. Chem. Commun. 1998, 12, 1301–1302. [Google Scholar] [CrossRef]
- Le Roux, J.; Rubow, S.; Ebenhan, T. A comparison of labelling characteristics of manual and automated synthesis methods for gallium-68 labelled ubiquicidin. Appl. Radiat. Isot. 2021, 168, 109452. [Google Scholar] [CrossRef] [PubMed]
- Bracke, N.; Yao, H.; Wynendaele, E.; Verbeke, F.; Xu, X.; Gevaert, B.; Maes, A.; van de Wiele, C.; Sathekge, M.; de Saeger, S.; et al. In Vitro Functional Quality Characterization of NOTA-Modified Somatropins. Anal. Chem. 2017, 89, 2764–2772. [Google Scholar] [CrossRef]
- Pulido, J.; de Cabrera, M.; Sobczak, A.J.; Amor-Coarasa, A.; McGoron, A.J.; Wnuk, S.F. 4-N-Alkanoyl and 4-N-alkyl gemcitabine analogues with NOTA chelators for 68-gallium labelling. Bioorg. Med. Chem. 2018, 26, 5624–5630. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.-P.; Kozlowski, P.; Jackson, J.; Cunanan, K.M.; Adumeau, P.; Dilling, T.R.; Zeglis, B.M.; Lewis, J.S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60, 8201–8217. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.; Gamre, N.; Lohar, S.P.; Dash, A. A systematic study on the utility of CHX-A″-DTPA-NCS and NOTA-NCS as bifunctional chelators for 177Lu radiopharmaceuticals. Appl. Radiat. Isot. 2017, 127, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Dash, A.; Pillai, M.R.A. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl. Med. Biol. 2013, 40, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; van Leeuwen, F.W.B.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast Media Mol. Imaging 2016, 11, 328–339. [Google Scholar] [CrossRef]
- Holub, J.; Meckel, M.; Kubíček, V.; Rösch, F.; Hermann, P. Gallium(III) complexes of NOTA-bis(phosphonate) conjugates as PET radiotracers for bone imaging. Contrast Media Mol. Imaging 2015, 10, 122–134. [Google Scholar] [CrossRef]
- Passah, A.; Tripathi, M.; Ballal, S.; Yadav, M.P.; Kumar, R.; Roesch, F.; Meckel, M.; Chakraborty, P.S.; Bal, C. Evaluation of bone-seeking novel radiotracer 68Ga-NO2AP-Bisphosphonate for the detection of skeletal metastases in carcinoma breast. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–49. [Google Scholar] [CrossRef]
- Läppchen, T.; Holland, J.P.; Kiefer, Y.; Bartholomä, M.D. Preparation and preclinical evaluation of a 68Ga-labelled c(RGDfK) conjugate comprising the bifunctional chelator NODIA-Me. EJNMMI Radiopharm. Chem. 2018, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Läppchen, T.; Kiefer, Y.; Holland, J.P.; Bartholomä, M.D. In vitro and in vivo evaluation of the bifunctional chelator NODIA-Me in combination with a prostate-specific membrane antigen targeting vector. Nucl. Med. Biol. 2018, 60, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, C.; Holland, J.P.; Läppchen, T.; Scherer, H.; Maus, S.; Stemler, T.; Bohnenberger, H.; Ezziddin, S.; Kurz, P.; Bartholomä, M.D. Optimized synthesis and indium complex formation with the bifunctional chelator NODIA-Me. Org. Biomol. Chem. 2018, 16, 7503–7512. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, A.; Läppchen, T.; Weinmann, C.; Bier-Schorr, L.; Keller, M.; Kiefer, Y.; Holland, J.P.; Bartholomä, M.D. Gallium Complexation, Stability, and Bioconjugation of 1,4,7-Triazacyclononane Derived Chelators with Azaheterocyclic Arms. Inorg. Chem. 2017, 56, 9097–9110. [Google Scholar] [CrossRef]
- Šimeček, J.; Schulz, M.; Notni, J.; Plutnar, J.; Kubíček, V.; Havlíčková, J.; Hermann, P. Complexation of Metal Ions with TRAP (1,4,7-Triazacyclononane Phosphinic Acid) Ligands and 1,4,7-Triazacyclononane-1,4,7-triacetic Acid: Phosphinate-Containing Ligands as Unique Chelators for Trivalent Gallium. Inorg. Chem. 2012, 51, 577–590. [Google Scholar] [CrossRef]
- Notni, J.; Steiger, K.; Hoffmann, F.; Reich, D.; Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Kessler, H.; Wester, H.-J. Complementary, Selective PET Imaging of Integrin Subtypes α5β1 and αvβ3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J. Nucl. Med. 2016, 57, 460–466. [Google Scholar] [CrossRef]
- Šimeček, J.; Notni, J.; Kapp, T.G.; Kessler, H.; Wester, H.-J. Benefits of NOPO As Chelator in Gallium-68 Peptides, Exemplified by Preclinical Characterization of 68Ga-NOPO–c(RGDfK). Mol. Pharm. 2014, 11, 1687–1695. [Google Scholar] [CrossRef]
- Šimeček, J.; Zemek, O.; Hermann, P.; Notni, J.; Wester, H.-J. Tailored Gallium(III) Chelator NOPO: Synthesis, Characterization, Bioconjugation, and Application in Preclinical Ga-68-PET Imaging. Mol. Pharm. 2014, 11, 3893–3903. [Google Scholar] [CrossRef]
- Poty, S.; Désogère, P.; Šimeček, J.; Bernhard, C.; Goncalves, V.; Goze, C.; Boschetti, F.; Notni, J.; Wester, H.J.; Denat, F. MA-NOTMP: A Triazacyclononane Trimethylphosphinate Based Bifunctional Chelator for Gallium Radiolabelling of Biomolecules. ChemMedChem 2015, 10, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Máté, G.; Šimeček, J.; Pniok, M.; Kertész, I.; Notni, J.; Wester, H.-J.; Galuska, L.; Hermann, P. The Influence of the Combination of Carboxylate and Phosphinate Pendant Arms in 1,4,7-Triazacyclononane-Based Chelators on Their 68Ga Labelling Properties. Molecules 2015, 20, 13112–13126. [Google Scholar] [CrossRef]
- Reich, D.; Wurzer, A.; Wirtz, M.; Stiegler, V.; Spatz, P.; Pollmann, J.; Wester, H.-J.; Notni, J. Dendritic poly-chelator frameworks for multimeric bioconjugation. Chem. Commun. 2017, 53, 2586–2589. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Pollmann, J.; Schmidt, A.; Reich, D.; Wester, H.-J.; Notni, J. Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results. Mol. Pharm. 2018, 15, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
- Reichart, F.; Maltsev, O.V.; Kapp, T.G.; Räder, A.F.B.; Weinmüller, M.; Marelli, U.K.; Notni, J.; Wurzer, A.; Beck, R.; Wester, H.-J.; et al. Selective Targeting of Integrin αvβ8 by a Highly Active Cyclic Peptide. J. Med. Chem. 2019, 62, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Prata, M.I.M.; André, J.P.; Kovács, Z.; Takács, A.I.; Tircsó, G.; Tóth, I.; Geraldes, C.F.G.C. Gallium(III) chelates of mixed phosphonate-carboxylate triazamacrocyclic ligands relevant to nuclear medicine: Structural, stability and in vivo studies. J. Inorg. Biochem. 2017, 177, 8–16. [Google Scholar] [CrossRef]
- Gai, Y.; Sun, L.; Lan, X.; Zeng, D.; Xiang, G.; Ma, X. Synthesis and Evaluation of New Bifunctional Chelators with Phosphonic Acid Arms for Gallium-68 Based PET Imaging in Melanoma. Bioconjug. Chem. 2018, 29, 3483–3494. [Google Scholar] [CrossRef]
- Prata, M.I.M.; Santos, A.C.; Geraldes, C.F.G.C.; Lima, J.J.P.d. Characterisation of 67Ga3+ complexes of triaza macrocyclic ligands: Biodistribution and clearance studies. Nucl. Med. Biol. 1999, 26, 707–710. [Google Scholar] [CrossRef] [PubMed][Green Version]
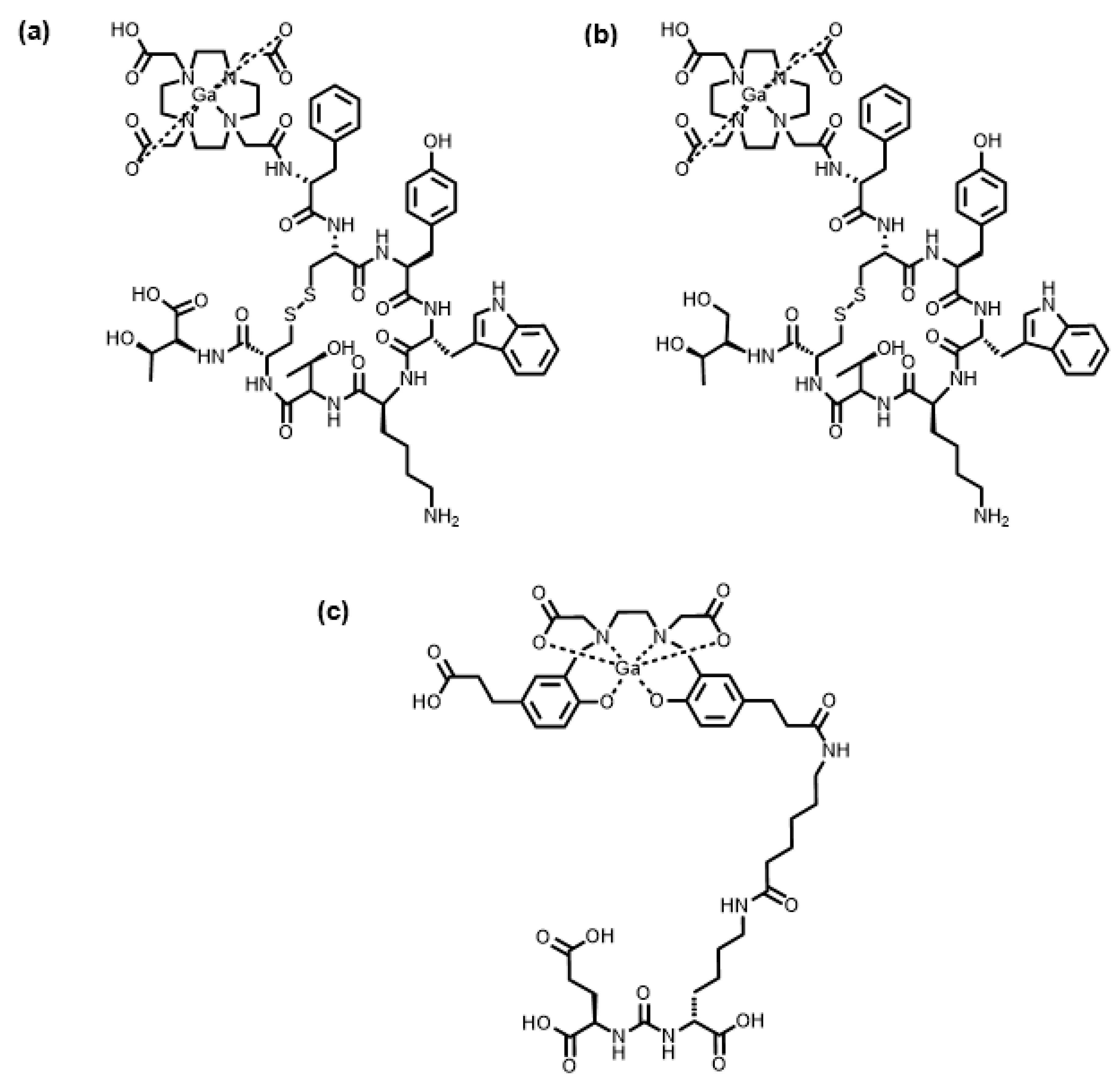
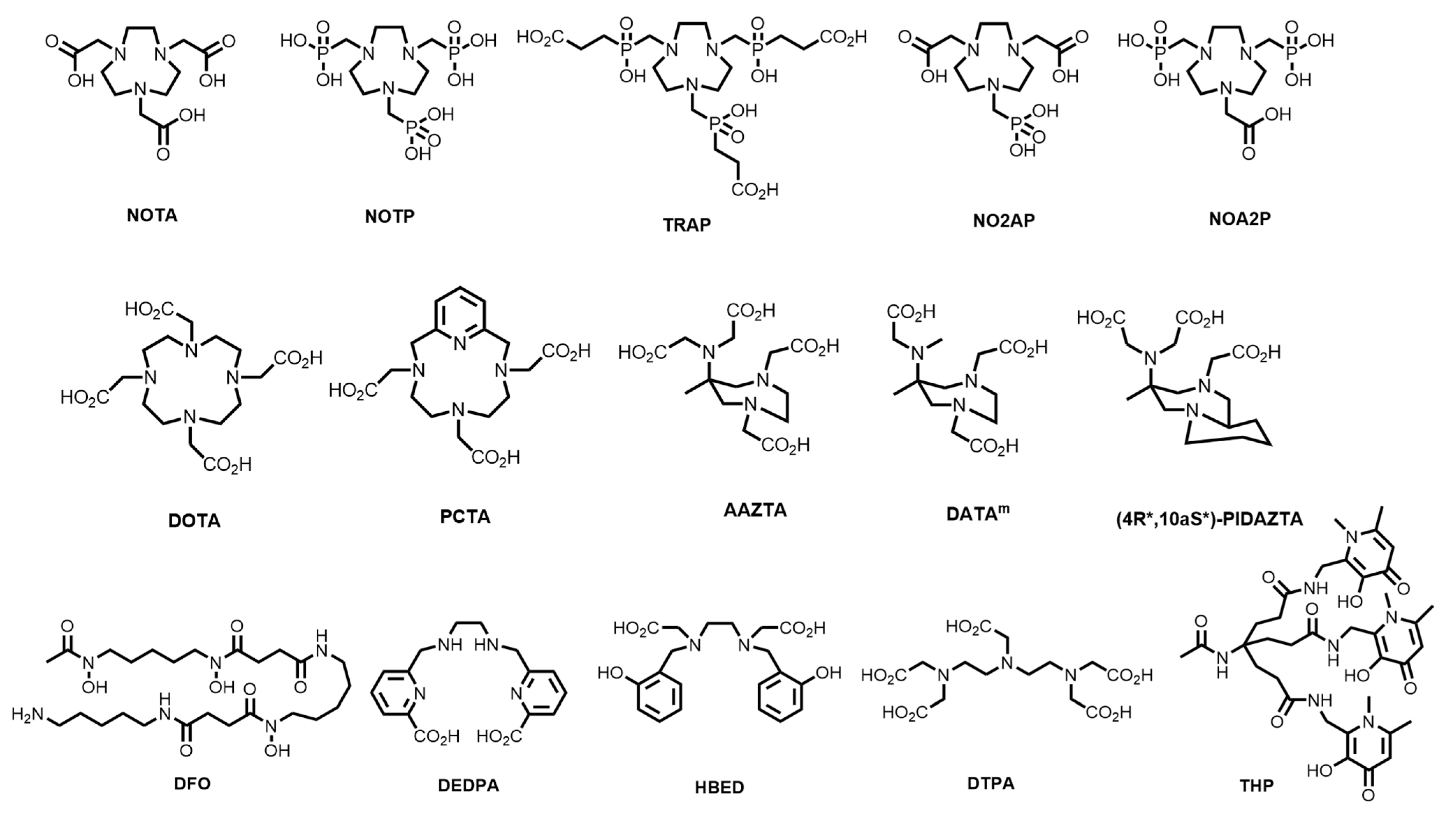
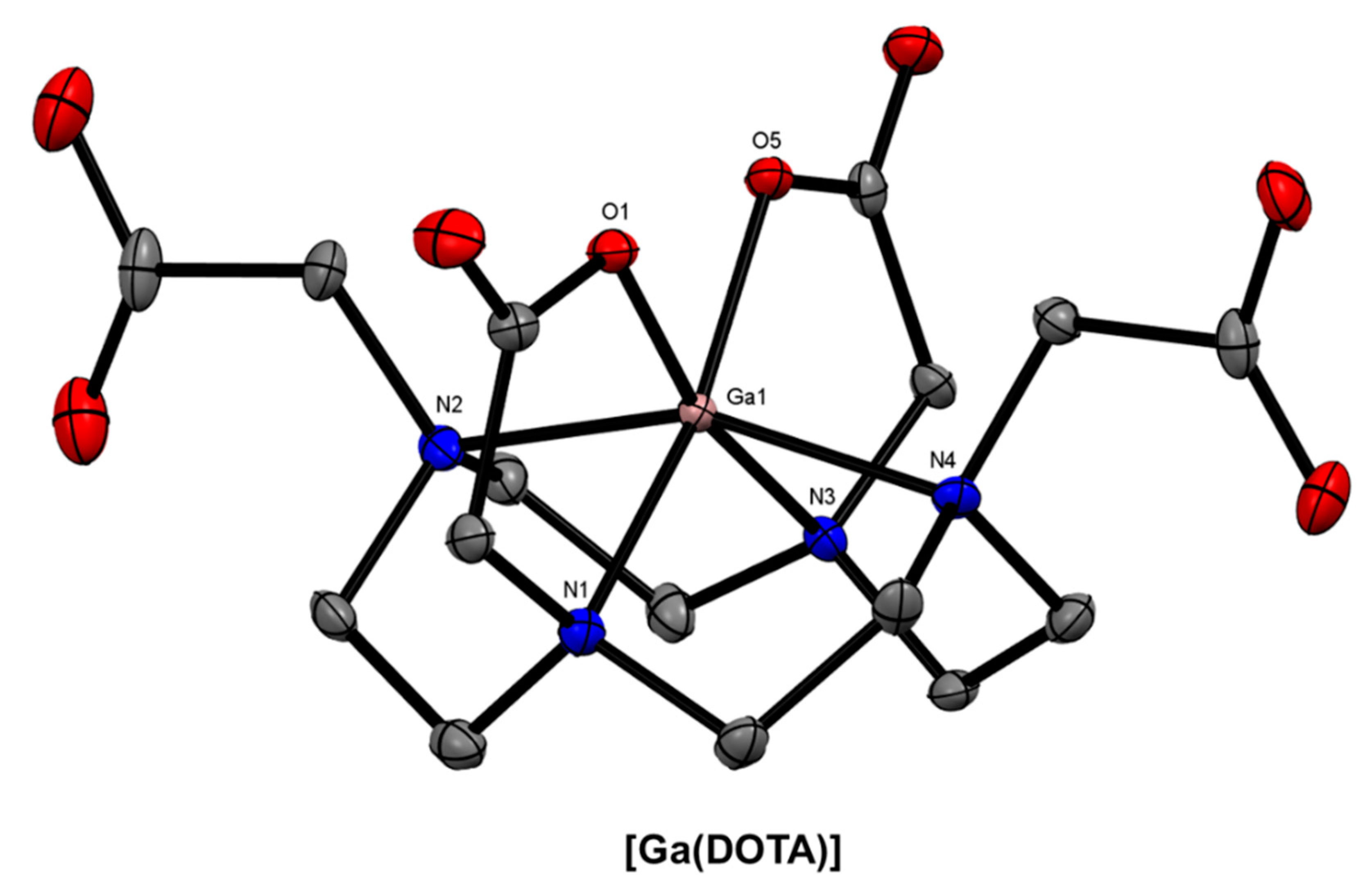

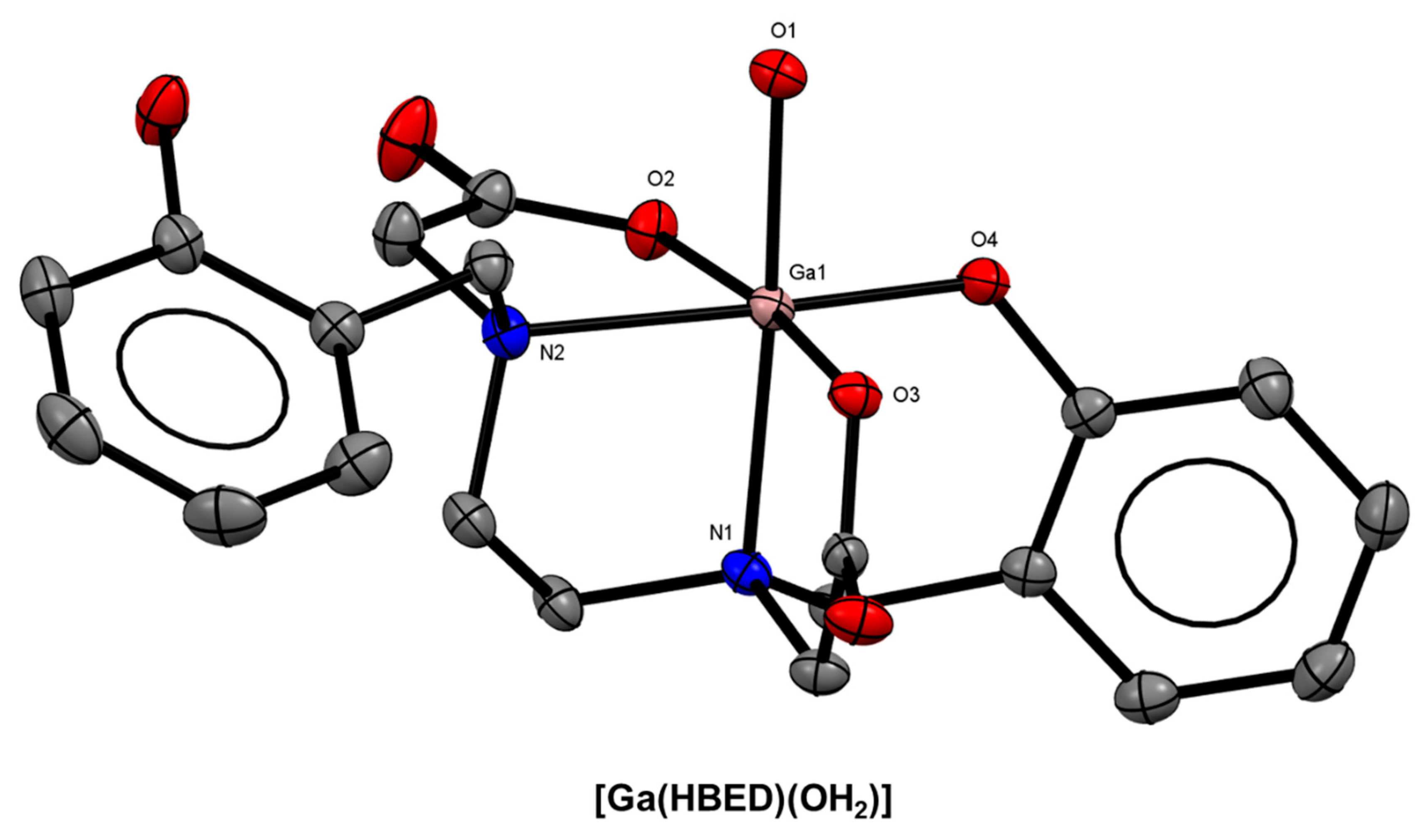




| Isotope | t1/2 | Decay Mode | E (keV) | Production Method |
|---|---|---|---|---|
| 66Ga 67Ga | 9.5 h | β+ (56%) | β+, 4150, 935 | Cyclotron, 63Cu(α,n)66Ga |
| EC (44%) | ||||
| 78.2 h | EC (100%) | γ, 93, 184, 300 | Cyclotron, 68Zn(p,2n)67Ga | |
| 68Ga | 67.7 min | β+ (90%) EC (10 %) | β+, 1880 | Cyclotron, 68Zn(p,n)68Ga; 68Ge/68Ga generator |
| Radiopharmaceutical | Manufacturer | Trade Name | Approved Indications in Adults |
|---|---|---|---|
| 67Ga-gallium citrate | Curium; Lantheus Medical Imaging | - | Detection of lymphoma, bronchogenic carcinoma, Hodgkin’s disease. |
| [68Ga]Ga-DOTATATE | Advanced Accelerator Applications | NETSPOT® | Neuroendocrine tumours (adult and paediatric patients). |
| [68Ga]Ga-DOTATOC | University of Iowa | - | Gastroenteropancreatic tumours (adult and paediatric patients). |
| [68Ga]Ga-HBED-CC-PSMA | Advanced Accelerator Applications; Telix Pharmaceuticals Inc.; University of California | [68Ga]Ga-gozetotide; LOCAMETZ®; Illucix | PSMA-positive lesions in prostate cancer and associated metastases. |
| Chelator | logKa | logK1 |
|---|---|---|
| NOTA | 13.17, 5.74, 3.22, 1.96 [185] | 29.63 [185] |
| NOTP | 11.7, 9.1, 7.5, 5.8, 3.1, 0.9 [186] | - |
| TRAP | 11.48, 5.44, 4.84, 4.23, 3.45, 1.66 [187] | 26.24 [187] |
| DFO | 10.79, 9.55, 8.96, 8.32 [130] | 28.65 [130] |
| DOTA | 11.74, 9.76, 4.68, 4.11, 2.37 [188] | 26.05 [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davey, P.R.W.J.; Paterson, B.M. Modern Developments in Bifunctional Chelator Design for Gallium Radiopharmaceuticals. Molecules 2023, 28, 203. https://doi.org/10.3390/molecules28010203
Davey PRWJ, Paterson BM. Modern Developments in Bifunctional Chelator Design for Gallium Radiopharmaceuticals. Molecules. 2023; 28(1):203. https://doi.org/10.3390/molecules28010203
Chicago/Turabian StyleDavey, Patrick R. W. J., and Brett M. Paterson. 2023. "Modern Developments in Bifunctional Chelator Design for Gallium Radiopharmaceuticals" Molecules 28, no. 1: 203. https://doi.org/10.3390/molecules28010203
APA StyleDavey, P. R. W. J., & Paterson, B. M. (2023). Modern Developments in Bifunctional Chelator Design for Gallium Radiopharmaceuticals. Molecules, 28(1), 203. https://doi.org/10.3390/molecules28010203





