Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission
Abstract
:1. Introduction
2. Search Methods
3. The Relationship between Neurotransmitter Regulation and Food Consumption
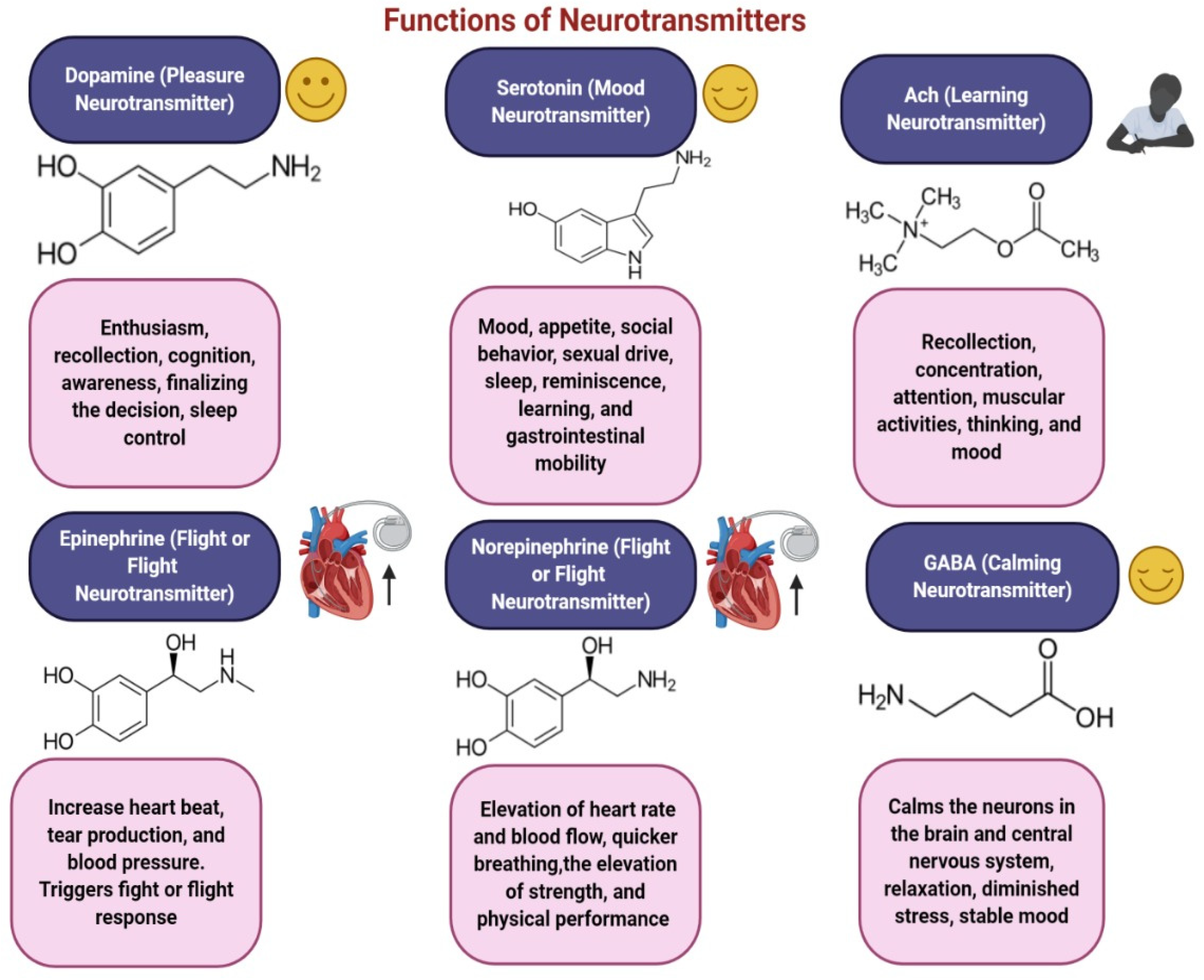
3.1. Dopamine
3.2. Acetylcholine (ACh)
3.3. Serotonin
3.4. Gamma-Aminobutyric Acid (GABA)
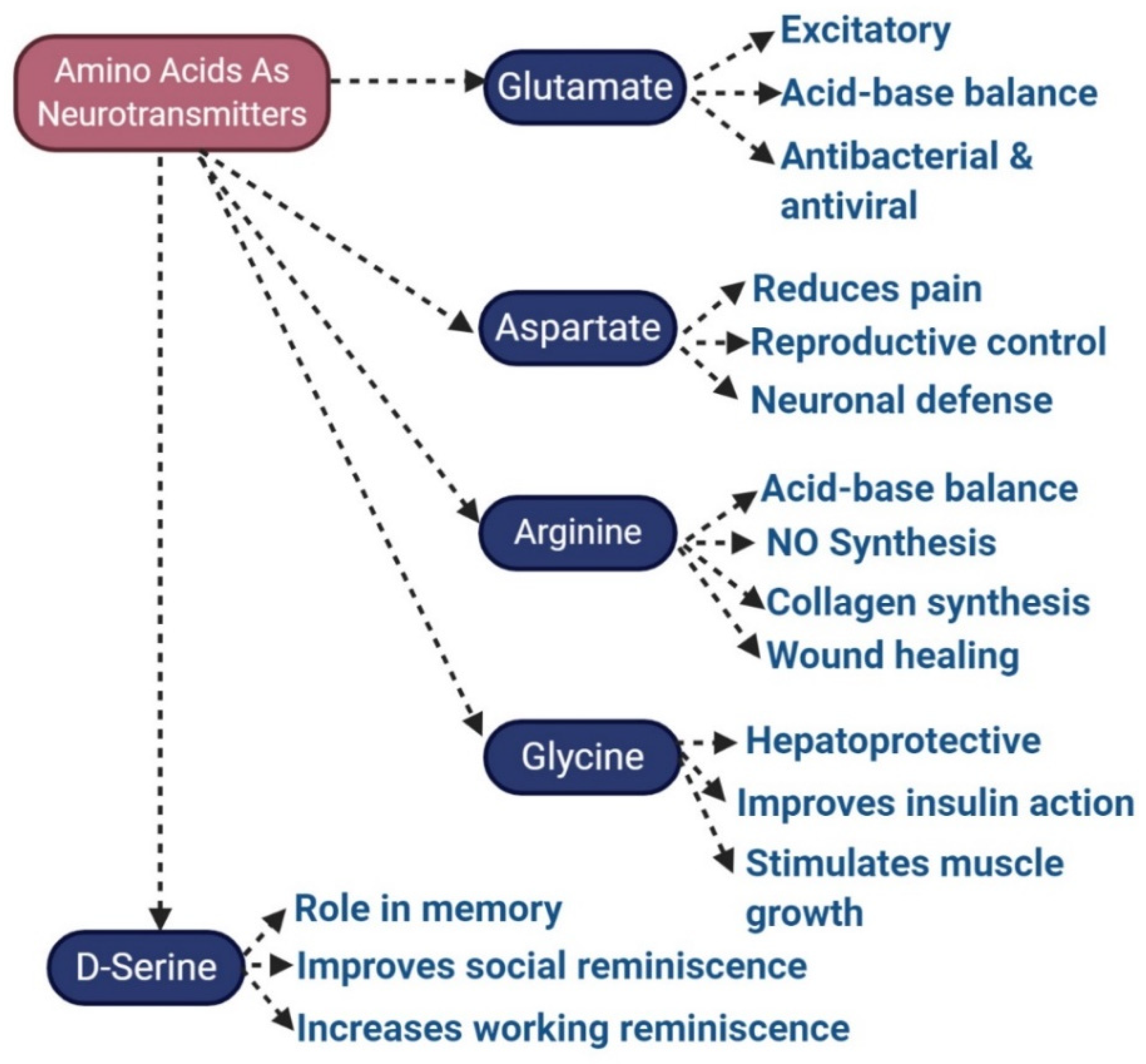
3.5. Glutamate
3.6. Norepinephrine
3.7. Epinephrine
3.8. Histamine
3.9. Aspartate
4. Discussion
5. Limitations of the Study and Future Remarks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
References
- Kavalali, E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Borodinsky, L.N.; Belgacem, Y.H.; Swapna, I.; Sequerra, E.B. Dynamic regulation of neurotransmitter specification: Relevance to nervous system homeostasis. Neuropharmacology 2014, 78, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frerking, M.; Wondolowski, J. Regulation of Neurotransmitter Release by Presynaptic Receptors. In Molecular Mechanisms of Neurotransmitter Release; Wang, Z.-W., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 297–314. [Google Scholar]
- Gao, H.F.; Wu, G.Y.; Frost, B.J.; Wang, S.R. Excitatory and inhibitory neurotransmitters in the nucleus rotundus of pigeons. Vis. Neurosci. 1995, 12, 819–825. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R.; Hills, J.M. GABA as an autonomic neurotransmitter: Studies on intrinsic GABAergic neurons in the myenteric plexus of the gut. Trends Neurosci. 1987, 10, 255–262. [Google Scholar] [CrossRef]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, J.; Zhou, P.; Li, J.; Gao, H.; Xia, Y.; Wang, Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer′s disease and Parkinson′s disease. Prog. Neurobiol. 2012, 97, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurvits, I.G.; Koenigsberg, H.W.; Siever, L.J. Neurotransmitter dysfunction in patients with borderline personality disorder. Psychiatr. Clin. N. Am. 2000, 23, 27–40. [Google Scholar] [CrossRef]
- Sarter, M.; Bruno, J.P.; Parikh, V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: Toward concepts of dynamic and function-specific dysregulation. Neuropsychopharmacology 2007, 32, 1452–1461. [Google Scholar] [CrossRef]
- Swoboda, K.J.; Hyland, K. Diagnosis and treatment of neurotransmitter-related disorders. Neurol. Clin. 2002, 20, 1143–1161. [Google Scholar] [CrossRef]
- Bahrani, E.; Nunneley, C.E.; Hsu, S.; Kass, J.S. Cutaneous Adverse Effects of Neurologic Medications. CNS Drugs 2016, 30, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Dell′Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurtman, R.J. Effects of Nutrients on Neurotransmitter Release. In Food Components to Enhance Performance: An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations; Marriott, B.M., Ed.; Institute of Medicine: Washington, DC, USA, 1994. [Google Scholar]
- Dhailappan, A.; Samiappan, S. Impact of Diet on Neurotransmitters. In Role of Nutrients in Neurological Disorders; Rajagopal, S., Ramachandran, S., Sundararaman, G., Gadde Venkata, S., Eds.; Springer: Singapore, 2022; pp. 363–383. [Google Scholar]
- Kumar, S.; Mishra, T.; Prajapati, A.; Sethi, P. Nutrition, Neurotransmitters, and Behavior. In Nutrition and Psychiatric Disorders; Mohamed, W., Kobeissy, F., Eds.; Springer Nature: Singapore, 2022; pp. 89–108. [Google Scholar]
- Roshchina, V.V. New Trends and Perspectives in the Evolution of Neurotransmitters in Microbial, Plant, and Animal Cells. In Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health; Lyte, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–77. [Google Scholar]
- Millstine, D.; Chen, C.Y.; Bauer, B. Complementary and integrative medicine in the management of headache. BMJ 2017, 357, j1805. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Dell’Osso, B.; Galentino, R.; Zanaboni Dina, C.; Banfi, G.; Porta, M. Tics and obsessive-compulsive disorder in relation to diet: Two case reports. Encephale 2018, 44, 479–481. [Google Scholar] [CrossRef]
- Guallar, E.; Stranges, S.; Mulrow, C.; Appel, L.J.; Miller, E.R. Enough Is Enough: Stop Wasting Money on Vitamin and Mineral Supplements. Ann. Intern. Med. 2013, 159, 850–851. [Google Scholar] [CrossRef]
- Choi, S.; DiSilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Effect of chronic protein ingestion on tyrosine and tryptophan levels and catecholamine and serotonin synthesis in rat brain. Nutr. Neurosci. 2011, 14, 260–267. [Google Scholar] [CrossRef]
- Fallon, S.; Shearman, E.; Sershen, H.; Lajtha, A. Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem. Res. 2007, 32, 1772–1782. [Google Scholar] [CrossRef]
- Bender, A.; Hagan, K.E.; Kingston, N. The association of folate and depression: A meta-analysis. J. Psychiatr. Res. 2017, 95, 9–18. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Roshchina, V.V. Neurotransmitters in Plants: Perspectives and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—Existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef] [Green Version]
- Shawky, E. Determination of synephrine and octopamine in bitter orange peel by HPTLC with densitometry. J. Chromatogr. Sci. 2014, 52, 899–904. [Google Scholar] [CrossRef]
- Bruinsma, K.; Taren, D.L. Chocolate: Food or drug? J. Am. Diet. Assoc. 1999, 99, 1249–1256. [Google Scholar] [CrossRef]
- Martin-Du Pan, R.C.; Wurtman, R.J. The role of nutrition in the synthesis of neurotransmitters and in cerebral functions: Clinical implications. Schweiz. Med. Wochenschr. 1981, 111, 1422–1434. [Google Scholar] [PubMed]
- Georgieff, M.K. Nutrition and the developing brain: Nutrient priorities and measurement. Am. J. Clin. Nutr. 2007, 85, 614S–620S. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D.; Roth, R.H. Dopamine synthesis, uptake, metabolism, and receptors: Relevance to gene therapy of Parkinson’s disease. Exp. Neurol. 1997, 144, 4–9. [Google Scholar] [CrossRef]
- Pifl, C.; Wolf, A.; Rebernik, P.; Reither, H.; Berger, M.L. Zinc regulates the dopamine transporter in a membrane potential and chloride dependent manner. Neuropharmacology 2009, 56, 531–540. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Ashkenazi, R.; Yehuda, S. Brain iron and dopamine receptor function. Adv. Biochem. Psychopharmacol. 1983, 37, 309–321. [Google Scholar]
- Wenzel, J.M.; Rauscher, N.A.; Cheer, J.F.; Oleson, E.B. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: A review of the neurochemical literature. ACS Chem. Neurosci. 2015, 6, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Baliki, M.N.; Mansour, A.; Baria, A.T.; Huang, L.; Berger, S.E.; Fields, H.L.; Apkarian, A.V. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J. Neurosci. 2013, 33, 16383–16393. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Rathitharan, G.; Meyer, J.H.; Furukawa, Y.; Ang, L.C.; Boileau, I.; Guttman, M.; Hornykiewicz, O.; Kish, S.J. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain 2017, 140, 2460–2474. [Google Scholar] [CrossRef] [Green Version]
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more D2 receptors. Proc. Natl. Acad. Sci USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A.; Herrera, A.J.; Venero, J.L.; Santiago, M.; De Pablos, R.M.; Villaran, R.F.; Espinosa-Oliva, A.M.; Arguelles, S.; Sarmiento, M.; Delgado-Cortes, M.J.; et al. Peripheral inflammation increases the damage in animal models of nigrostriatal dopaminergic neurodegeneration: Possible implication in Parkinson’s disease incidence. Parkinsons Dis. 2011, 2011, 393769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Self, D. Neurobiology: Dopamine as chicken and egg. Nature 2003, 422, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, J.; Zhang, J.G.; Xie, J.X. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural. Regen. Res. 2015, 10, 308–313. [Google Scholar] [CrossRef]
- Wichers, H.J.; Visser, J.F.; Huizing, H.J.; Pras, N. Occurrence of L-DOPA and dopamine in plants and cell cultures ofMucuna pruriens and effects of 2,4-d and NaCl on these compounds. Plant Cell Tissue Organ Cult. 1993, 33, 259–264. [Google Scholar] [CrossRef]
- Longo, R.; Castellani, A.; Sberze, P.; Tibolla, M. Distribution of l-dopa and related amino acids in Vicia. Phytochemistry 1974, 13, 167–171. [Google Scholar] [CrossRef]
- Ingle, P. L-Dopa bearing plants. Nat. Prod. Rad. 2003, 2, 126–133. [Google Scholar]
- Hryhorczuk, C.; Florea, M.; Rodaros, D.; Poirier, I.; Daneault, C.; Des Rosiers, C.; Arvanitogiannis, A.; Alquier, T.; Fulton, S. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology 2016, 41, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef]
- Odjakova, M.; Hadjiivanova, C. Animal neurotransmitter substances in plants. Bulg. J. Plant Physiol. 1997, 23, 94–102. [Google Scholar]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naneix, F.; Peters, K.Z.; Young, A.M.J.; McCutcheon, J.E. Age-dependent effects of protein restriction on dopamine release. Neuropsychopharmacology 2021, 46, 394–403. [Google Scholar] [CrossRef]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A. Acetylcholine: A neurotransmitter for learning and memory? Brain Res. Rev. 1995, 21, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Walker, M.; Grace, J.; Perry, R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci. 1999, 22, 273–280. [Google Scholar] [CrossRef]
- Bowman, W.C. 3 Mechanisms of Neuromuscular Blockade. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1962; Volume 2, pp. 88–131. [Google Scholar]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Menezes, M.P.; North, K.N. Inherited neuromuscular disorders: Pathway to diagnosis. J. Paediatr. Child Health 2012, 48, 458–465. [Google Scholar] [CrossRef]
- Sugiyama, K.; Tezuka, T. Acetylcholine promotes the emergence and elongation of lateral roots of Raphanus sativus. Plant Signal Behav. 2011, 6, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.A.; Nasir, M.; Pasha, T.N.; Javid, I.; Ullah, A.; Iqbal, M.A.; Ahmed, S.; Nazir, M.M.; Gondal, T.A.; Imran, M.; et al. Association of life style and dietary habits with blood choline and cardiovascular outcome. Cell. Mol. Biol. 2020, 66, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferre, M.; Hu, F.B.; Ruiz-Canela, M.; Bullo, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gomez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites From Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention With Mediterranean Diet) Study. J. Am. Heart Assoc. 2017, 6, e006524. [Google Scholar] [CrossRef]
- Kim, D.Y.; Camilleri, M. Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 2000, 95, 2698–2709. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Giridhar, P.; Ravishankar, G.A. Phytoserotonin: A review. Plant Signal Behav. 2011, 6, 800–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, D.; Kang, K.; Choi, J.Y.; Ishihara, A.; Back, K.; Lee, S.G. HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J. Med. Food 2008, 11, 385–389. [Google Scholar] [CrossRef]
- Kang, S.; Back, K. Enriched production of N-hydroxycinnamic acid amides and biogenic amines in pepper (Capsicum annuum) flowers. Sci. Hortic. 2006, 108, 337–341. [Google Scholar] [CrossRef]
- Feldman, J.M.; Lee, E.M. Serotonin content of foods: Effect on urinary excretion of 5-hydroxyindoleacetic acid. Am. J. Clin. Nutr. 1985, 42, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Udenfriend, S.; Lovenberg, W.; Sjoerdsma, A. Physiologically active amines in common fruits and vegetables. Arch. Biochem. Biophys. 1959, 85, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Regula, I. 5-hydroxytryptamine in the Leaves of the Crown of Pineapple Fruit [Ananas comosus (Stickm.) Merrill]. Acta Bot. Croat. 1977, 36, 83–86. [Google Scholar]
- Lampariello, L.R.; Cortelazzo, A.; Guerranti, R.; Sticozzi, C.; Valacchi, G. The Magic Velvet Bean of Mucuna pruriens. J. Tradit. Complement. Med. 2012, 2, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Di Viesti, V.; Zavatti, M.; Zanoli, P. Anxiolytic-like effect of Griffonia simplicifolia Baill. seed extract in rats. Phytomedicine 2011, 18, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan. Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorm, A.F.; Christensen, H.; Griffiths, K.M.; Parslow, R.A.; Rodgers, B.; Blewitt, K.A. Effectiveness of complementary and self-help treatments for anxiety disorders. Med. J. Aust. 2004, 181, S29–S46. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.R. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular Med. 2008, 10, 247–258. [Google Scholar] [CrossRef]
- Shabbir, F.; Patel, A.; Mattison, C.; Bose, S.; Krishnamohan, R.; Sweeney, E.; Sandhu, S.; Nel, W.; Rais, A.; Sandhu, R.; et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem. Int. 2013, 62, 324–329. [Google Scholar] [CrossRef]
- Davis, M.; Myers, K.M. The role of glutamate and gamma-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biol. Psychiatry 2002, 52, 998–1007. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Lewis, D.A. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008, 34, 944–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möhler, H. Physiology and Pharmacology of the GABA System: Focus on GABA Receptors. In GABA and Sleep: Molecular, Functional and Clinical Aspects; Monti, J.M., Pandi-Perumal, S.R., Möhler, H., Eds.; Springer: Basel, Switzerland, 2010; pp. 3–23. [Google Scholar]
- Sudhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, N.M.; Madsen, K.K.; Schousboe, A.; Steve White, H. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem. Int. 2012, 61, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Sepkuty, J.P.; Cohen, A.S.; Eccles, C.; Rafiq, A.; Behar, K.; Ganel, R.; Coulter, D.A.; Rothstein, J.D. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J. Neurosci. 2002, 22, 6372–6379. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Dragunow, M.; Faull, R.L. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 2000, 97, 505–519. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef]
- Oh, S.-H.; Moon, Y.-J.; Oh, C.-H. γ-Aminobutyric acid (GABA) content of selected uncooked foods. Prev. Nutr. Food Sci. 2003, 8, 75–78. [Google Scholar] [CrossRef]
- Carratu, B.; Boniglia, C.; Giammarioli, S.; Mosca, M.; Sanzini, E. Free amino acids in botanicals and botanical preparations. J. Food Sci. 2008, 73, C323–C328. [Google Scholar] [CrossRef]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S5), 106–113. [Google Scholar] [CrossRef]
- Ito, S. GABA and glycine in the developing brain. J. Physiol. Sci. 2016, 66, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Sarawagi, A.; Soni, N.D.; Patel, A.B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front. Psychiatry 2021, 12, 637863. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konradi, C.; Heckers, S. Molecular aspects of glutamate dysregulation: Implications for schizophrenia and its treatment. Pharmacol. Ther. 2003, 97, 153–179. [Google Scholar] [CrossRef]
- McEntee, W.J.; Crook, T.H. Glutamate: Its role in learning, memory, and the aging brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Cho, C.H. New mechanism for glutamate hypothesis in epilepsy. Front. Cell. Neurosci. 2013, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Tennant, D.R. Review of Glutamate Intake from Both Food Additive and Non-Additive Sources in the European Union. Ann. Nutr. Metab. 2018, 73 (Suppl. S5), 21–28. [Google Scholar] [CrossRef]
- Valluzzi, R.L.; Fierro, V.; Arasi, S.; Mennini, M.; Pecora, V.; Fiocchi, A. Allergy to food additives. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 256–262. [Google Scholar] [CrossRef]
- Ginguay, A.; Cynober, L.; Curis, E.; Nicolis, I. Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways. Biology 2017, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Bylund, D.B.; Bylund, K.C. Norepinephrine. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 614–616. [Google Scholar]
- Dahlstroem, A.; Fuxe, K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol. Scand. Suppl. 1964, 62 (Suppl. S232), 231–255. [Google Scholar]
- Herrmann, N.; Lanctot, K.L.; Khan, L.R. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, L. Pheochromocytomas, Paragangliomas and Disorders of the Sympathoadrenal System; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Leiter, O.; Bernas, S.N.; Seidemann, S.; Overall, R.W.; Horenburg, C.; Kowal, S.; Kempermann, G.; Walker, T.L. The systemic exercise-released chemokine lymphotactin/XCL1 modulates in vitro adult hippocampal precursor cell proliferation and neuronal differentiation. Sci. Rep. 2019, 9, 11831. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Zafar, T.A. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188. [Google Scholar] [CrossRef]
- Lee, J.; Lee, A.; Kim, J.H.; Shin, Y.M.; Kim, S.J.; Cho, W.D.; Lee, S.I. Effect of Omega-3 and Korean Red Ginseng on Children with Attention Deficit Hyperactivity Disorder: An Open-Label Pilot Study. Clin. Psychopharmacol. Neurosci. 2020, 18, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Huang, W.C.; Cheng, C.C.; Ke, M.W.; Tsai, J.S.; Hung, Y.M.; Huang, N.C.; Huang, M.S.; Wann, S.R. Effects of epinephrine on heart rate variability and cytokines in a rat sepsis model. Bosn. J. Basic Med. Sci. 2020, 20, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Banerji, A.; Rudders, S.A.; Corel, B.; Garth, A.M.; Clark, S.; Camargo, C.A., Jr. Repeat epinephrine treatments for food-related allergic reactions that present to the emergency department. Allergy Asthma Proc. 2010, 31, 308–316. [Google Scholar] [CrossRef]
- Hough, L. Histamine actions in the central nervous system. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, G.J., Agranoff, B.W., Albers, R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Nuutinen, S.; Panula, P. Histamine in Neurotransmission and Brain Diseases. In Histamine in Inflammation; Thurmond, R.L., Ed.; Springer: Boston, MA, USA, 2010; pp. 95–107. [Google Scholar]
- Ito, C. The role of the central histaminergic system on schizophrenia. Drug News Perspect 2004, 17, 383–387. [Google Scholar] [CrossRef]
- Torrealba, F.; Riveros, M.E.; Contreras, M.; Valdes, J.L. Histamine and motivation. Front. Syst. Neurosci. 2012, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Nuutinen, S.; Vanhanen, J.; Maki, T.; Panula, P. Histamine h3 receptor: A novel therapeutic target in alcohol dependence? Front. Syst. Neurosci. 2012, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Passani, M.B.; Ballerini, C. Histamine and neuroinflammation: Insights from murine experimental autoimmune encephalomyelitis. Front. Syst. Neurosci. 2012, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landete, J.M.; Ferrer, S.; Pardo, I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control 2007, 18, 1569–1574. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.D.; Zhu, L.J.; Shen, Y.J.; Wei, E.Q. Effects of histidine, a precursor of histamine, on pentylenetetrazole-induced seizures in rats. Acta Pharmacol. Sin. 2002, 23, 361–366. [Google Scholar] [PubMed]
- Yoshikawa, T.; Nakamura, T.; Shibakusa, T.; Sugita, M.; Naganuma, F.; Iida, T.; Miura, Y.; Mohsen, A.; Harada, R.; Yanai, K. Insufficient intake of L-histidine reduces brain histamine and causes anxiety-like behaviors in male mice. J. Nutr. 2014, 144, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Veciana-Nogues, M.T.; Vidal-Carou, M.C. Control of biogenic amines in fermented sausages: Role of starter cultures. Front. Microbiol. 2012, 3, 169. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Švecová, S.; Pelikánová, T. Levels of biogenic amines in typical vegetable products. Food Chem. 2002, 77, 349–351. [Google Scholar] [CrossRef]
- Shruti, S.; Jong-Kyu, K.; Myunghee, K. Occurrence of Biogenic Amines in Soybean Food Products. In Soybean and Health; Hany, E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 9. [Google Scholar]
- Kim, S.H.; An, H.; Wei, C.I.; Visessanguan, W.; Benjakul, S.; Morrissey, M.T.; Su, Y.C.; Pitta, T.P. Molecular Detection of a Histamine Former, Morganella morganii, in Albacore, Mackerel, Sardine, and a Processing Plant. J. Food Sci. 2003, 68, 453–457. [Google Scholar] [CrossRef]
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- D’Aniello, A.; Luongo, L.; Romano, R.; Iannotta, M.; Marabese, I.; Boccella, S.; Belardo, C.; de Novellis, V.; Arra, C.; Barbieri, A.; et al. d-Aspartic acid ameliorates painful and neuropsychiatric changes and reduces beta-amyloid Abeta(1-42) peptide in a long lasting model of neuropathic pain. Neurosci. Lett. 2017, 651, 151–158. [Google Scholar] [CrossRef]
- Boccella, S.; Vacca, V.; Errico, F.; Marinelli, S.; Squillace, M.; Guida, F.; Di Maio, A.; Vitucci, D.; Palazzo, E.; De Novellis, V.; et al. D-aspartate modulates nociceptive-specific neuron activity and pain threshold in inflammatory and neuropathic pain condition in mice. Biomed. Res. Int. 2015, 2015, 905906. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Jander, G.; Joshi, V. Recent progress in deciphering the biosynthesis of aspartate-derived amino acids in plants. Mol. Plant 2010, 3, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Sekine, M.; Furuchi, T.; Katane, M.; Nimura, N.; Shimamoto, K.; Nakajima, T.; Homma, H. A novel L-glutamate transporter inhibitor reveals endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Life Sci. 2005, 76, 2933–2944. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, S.; Somorjai, I.; Garcia-Fernandez, J.; Topo, E.; D’Aniello, A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB J. 2011, 25, 1014–1027. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, S.; Katane, M.; Maeda, K.; Kaneko, Y.; Saitoh, Y.; Miyamoto, T.; Sekine, M.; Homma, H. Biosynthesis of D-aspartate in mammals: The rat and human homologs of mouse aspartate racemase are not responsible for the biosynthesis of D-aspartate. Amino Acids 2015, 47, 975–985. [Google Scholar] [CrossRef]
- Cristino, L.; Luongo, L.; Squillace, M.; Paolone, G.; Mango, D.; Piccinin, S.; Zianni, E.; Imperatore, R.; Iannotta, M.; Longo, F.; et al. d-Aspartate oxidase influences glutamatergic system homeostasis in mammalian brain. Neurobiol. Aging 2015, 36, 1890–1902. [Google Scholar] [CrossRef]
- Kapalka, G.M. Chapter 4—Substances Involved in Neurotransmission. In Nutritional and Herbal Therapies for Children and Adolescents; Kapalka, G.M., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 71–99. [Google Scholar]
- Pardridge, W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999, 5, 556–569. [Google Scholar] [CrossRef] [Green Version]
- Logan, A.C.; Jacka, F.N. Nutritional psychiatry research: An emerging discipline and its intersection with global urbanization, environmental challenges and the evolutionary mismatch. J. Physiol. Anthropol. 2014, 33, 22. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasmi, A.; Nasreen, A.; Menzel, A.; Gasmi Benahmed, A.; Pivina, L.; Noor, S.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules 2023, 28, 210. https://doi.org/10.3390/molecules28010210
Gasmi A, Nasreen A, Menzel A, Gasmi Benahmed A, Pivina L, Noor S, Peana M, Chirumbolo S, Bjørklund G. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules. 2023; 28(1):210. https://doi.org/10.3390/molecules28010210
Chicago/Turabian StyleGasmi, Amin, Aniqa Nasreen, Alain Menzel, Asma Gasmi Benahmed, Lyudmila Pivina, Sàdaf Noor, Massimiliano Peana, Salvatore Chirumbolo, and Geir Bjørklund. 2023. "Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission" Molecules 28, no. 1: 210. https://doi.org/10.3390/molecules28010210
APA StyleGasmi, A., Nasreen, A., Menzel, A., Gasmi Benahmed, A., Pivina, L., Noor, S., Peana, M., Chirumbolo, S., & Bjørklund, G. (2023). Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules, 28(1), 210. https://doi.org/10.3390/molecules28010210







