Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions
Abstract
1. Introduction
2. Results
2.1. ATR-FTIR Fingerprint and Chemical Composition of CEO
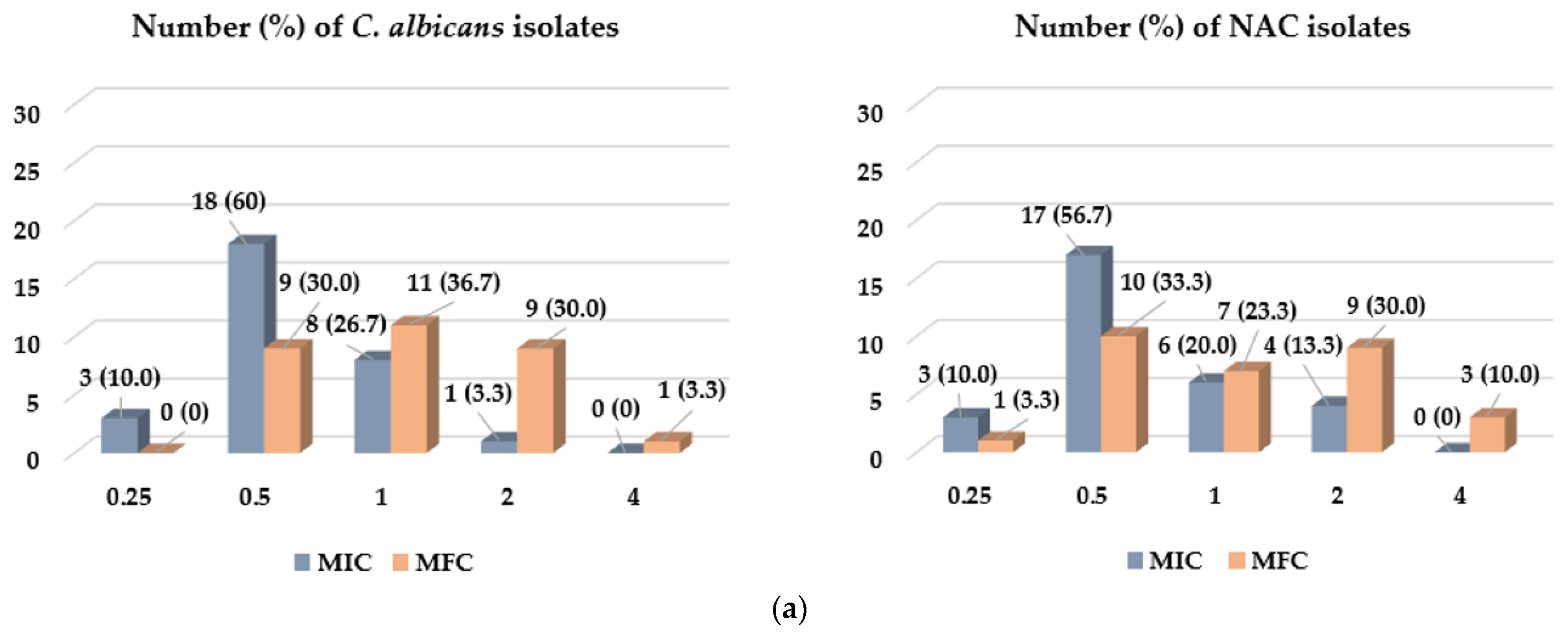
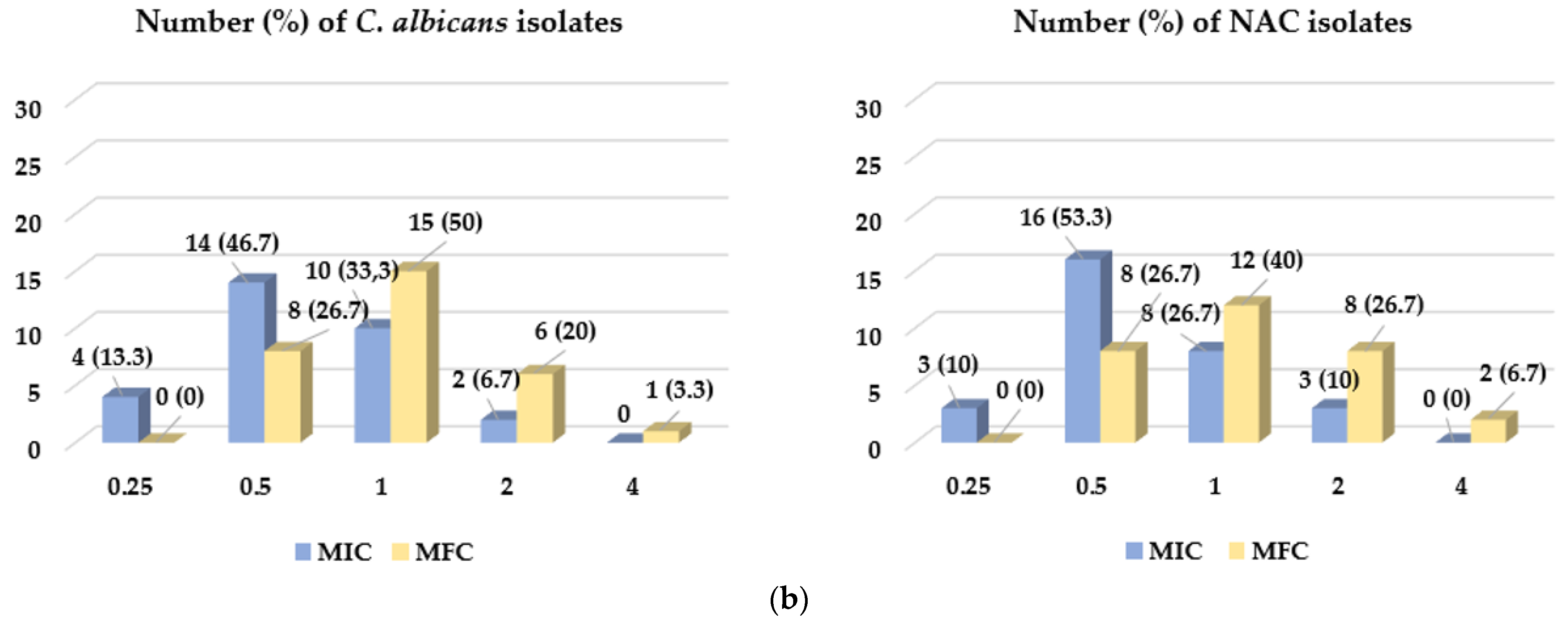
2.2. The Antifungal Activity Assessment of CEO and EUG
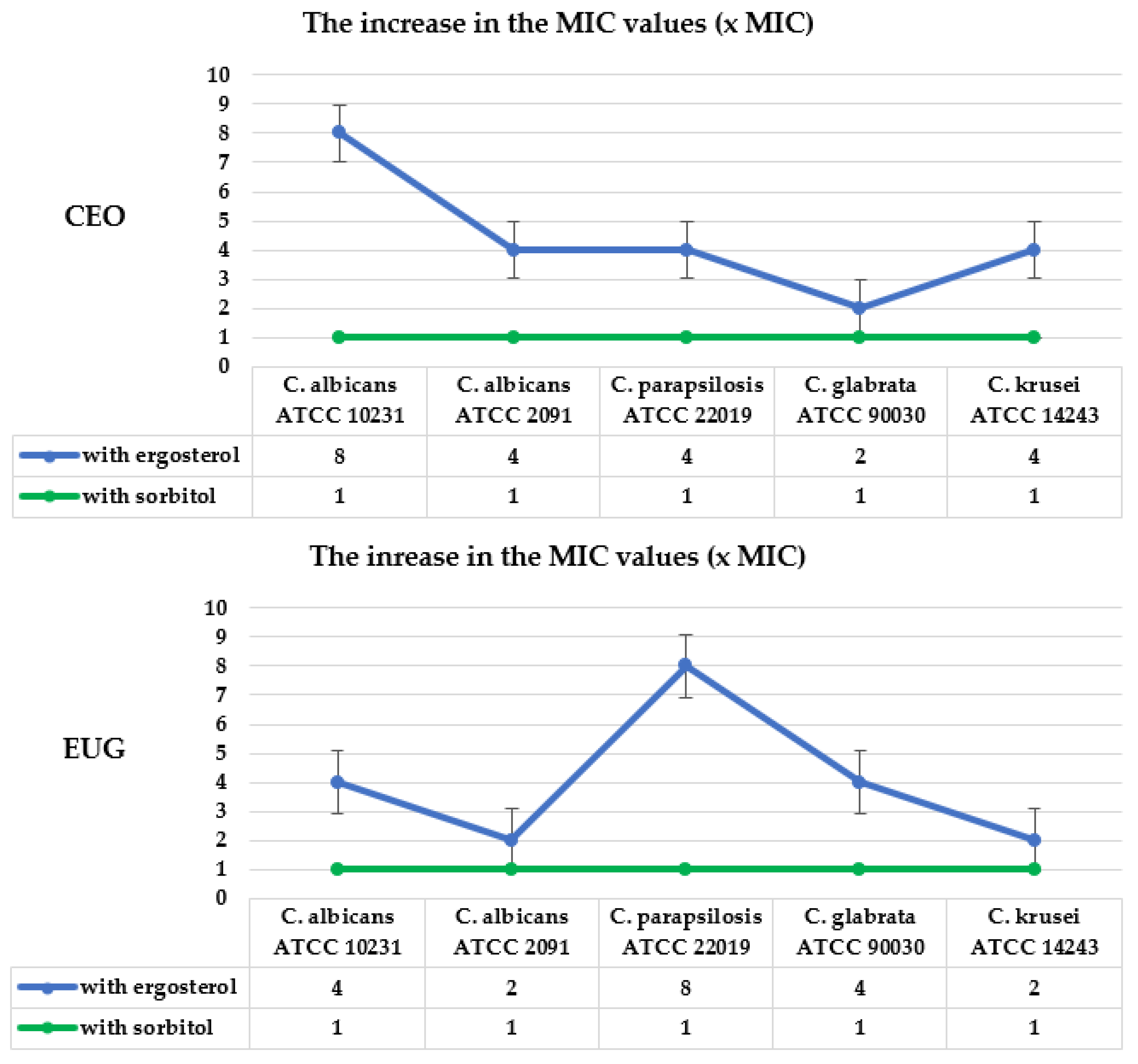
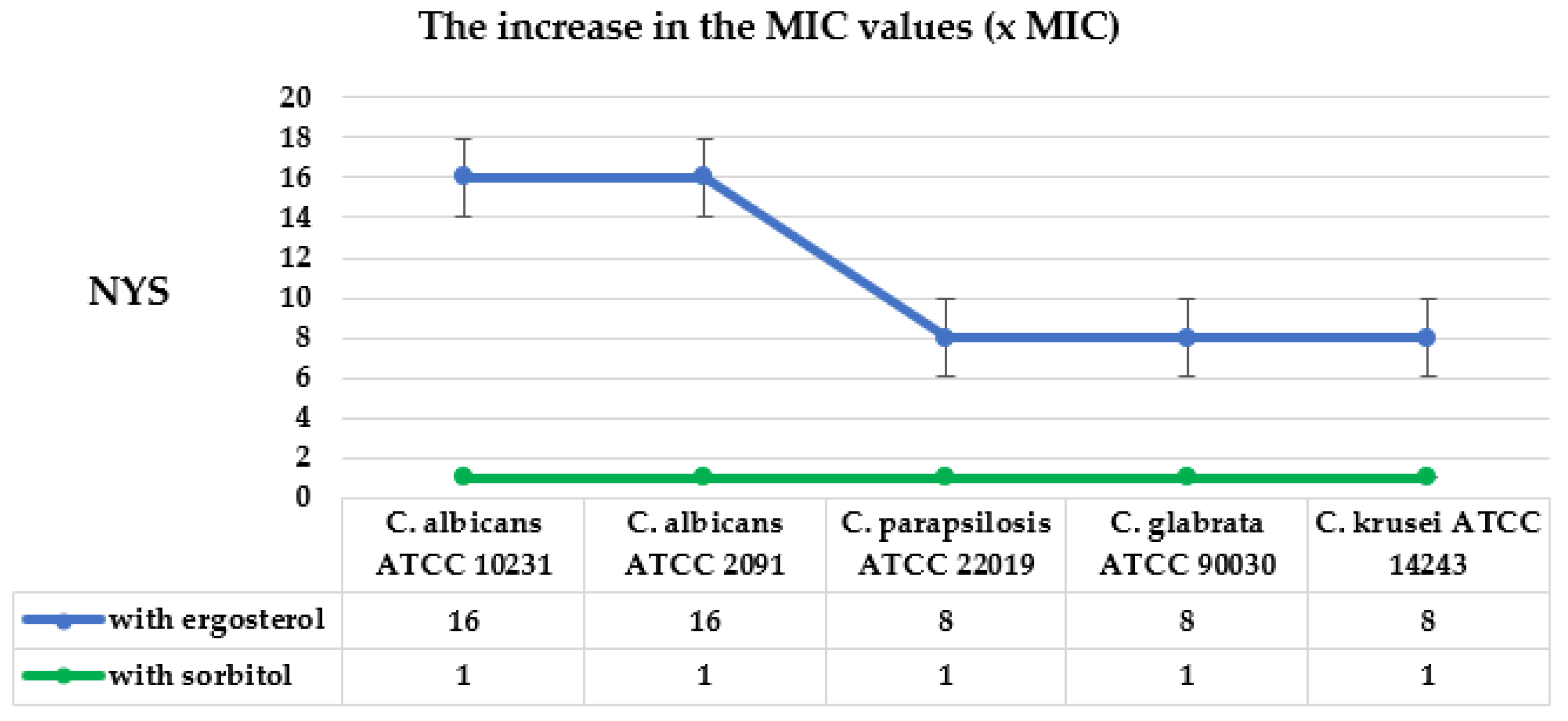
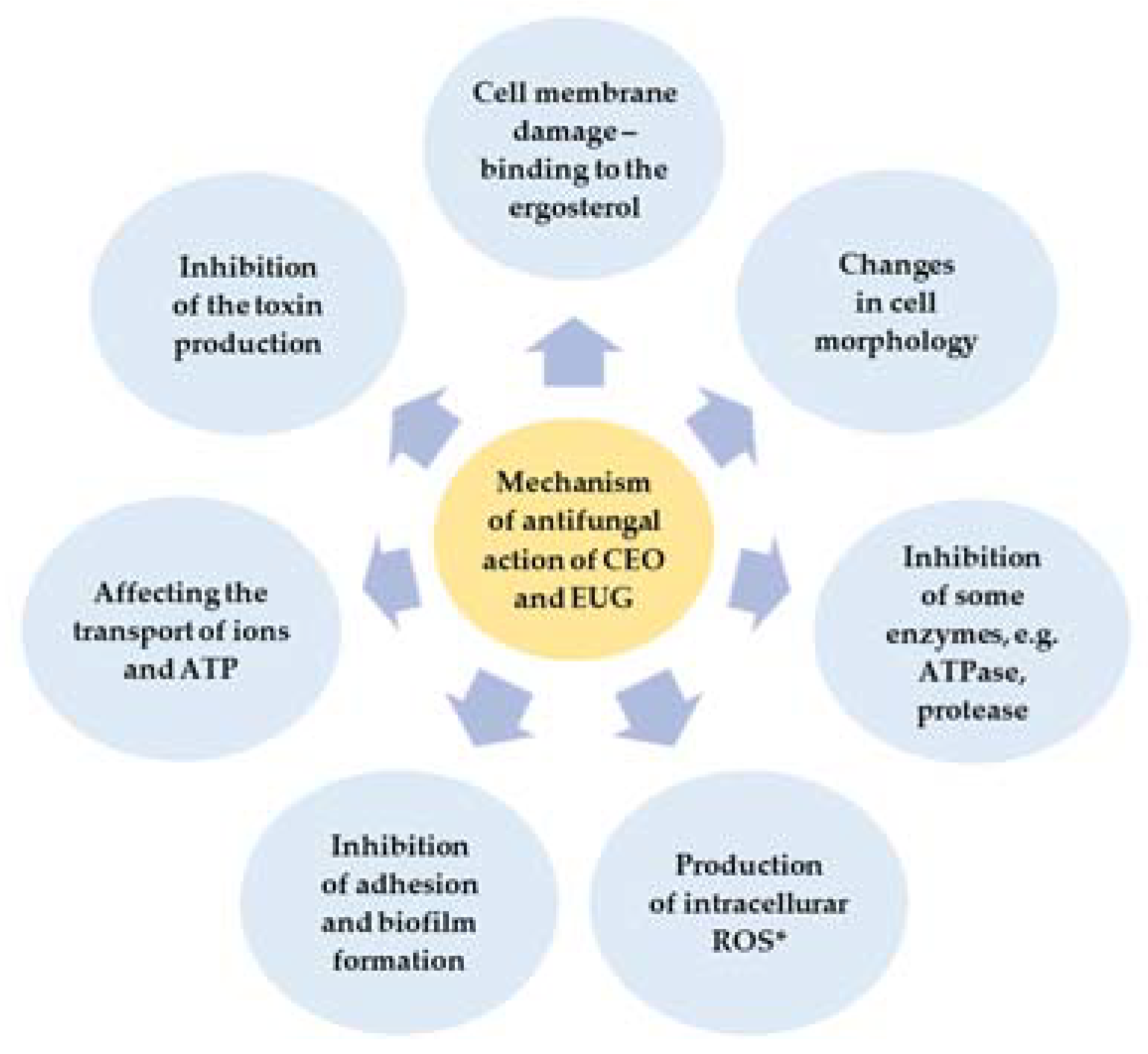
2.3. Mode of Antifungal Action of CEO and EUG
2.4. Investigation of Interaction of CEO and EUG with Selected Antimycotics
3. Discussion
3.1. The Antifungal Activity Assessment of CEO and EUG
3.2. Mode of Antifungal Action of CEO and EUG
3.3. Investigation of Interaction of CEO and EUG with Selected Antimycotics
4. Materials and Methods
4.1. Materials
4.1.1. The Studied Compounds
4.1.2. Microorganisms
4.2. Methods
4.2.1. Attenuated Total Reflection–Fourier Transform Infrared (ATR–FTIR)
4.2.2. Gas Chromatography
4.2.3. In Vitro Antifungal Activity Assay of CEO and EUG
4.2.4. Mode of Antifungal Action of CEO and EUG
Sorbitol Assay
Ergosterol Assay
4.2.5. Investigation of Interaction of the Natural Compounds and Selected Antimycotics
4.2.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biernasiuk, A.; Berecka-Rycerz, A.; Gumieniczek, A.; Malm, M.; Łączkowski, K.Z.; Szymańska, J.; Malm, A. The newly synthesized thiazole derivatives as potential antifungal compounds against Candida albicans. Appl. Microbiol. Biotechnol. 2021, 105, 6355–6367. [Google Scholar] [CrossRef] [PubMed]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal activity and mechanism of action of the Co(III) coordination complexes with diamine chelate ligands against reference and clinical strains of Candida spp. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.R. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2). VirusDisease 2016, 27, 170–178. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef]
- de Oliveira, A.D.N.; Leite Lima, E.T.; de Oliveira, D.T.; Angélica, R.S.; de Aguiar Andrade, E.H.; da Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; et al. GC-MS characterization of antibacterial, antioxidant, and antitrypanosomal activity of Syzygium aromaticum essential oil and eugenol. Evid.-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Koba, K.; Nenonene, A.Y.; Raynaud, C.; Chaumont, J.P.; Sanda, K. Antibacterial activities of the buds essential oil of Syzygium aromaticum (L.) Merr. & Perry from Togo. J. Biol. Act. Prod. Nat. 2011, 1, 42–51. [Google Scholar]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef]
- Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, H.; Moneim, H.; Farid, M.; Gohar, A.A. Clove oil cream: A new effective treatment for chronic anal fissure. Colorectal Dis. 2007, 9, 549–552. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Gwak, K.S.; Yang, I.; Choi, W.S.; Jo, H.J.; Chang, J.W.; Jeung, E.B.; Choi, I.G. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J. Microbiol. 2007, 45, 460–465. [Google Scholar] [PubMed]
- Sharifzadeh, A.; Shokri, H. In vitro synergy of eugenol on the antifungal effects of voriconazole against Candida tropicalis and Candida krusei strains isolated from the genital tract of mares. Equine Vet. J. 2021, 53, 94–101. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Tarhan, İ.; Bakır, M.R.; Kalkan, O.; Yöntem, M.; Kara, H. Rapid determination of adulteration of clove essential oil with benzyl alcohol and ethyl acetate: Towards quality control analysis by FTIR with chemometrics. Vib. Spectrosc. 2022, 118, 103339. [Google Scholar] [CrossRef]
- Blanco, A.R.; Nostro, A.; D’Angelo, V.; D’Arrigo, M.; Mazzone, M.G.; Marino, A. Efficacy of a fixed combination of tetracycline, chloramphenicol, and colistimethate sodium for treatment of Candida albicans keratitis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal activity and potential mechanism of N-butylphthalide alone and in combination with fluconazole against Candida albicans. Front. Microbiol. 2019, 10, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Terças, A.L.; Marques, S.G.; Moffa, E.B.; Alves, M.B.; de Azevedo, C.M.; Siqueira, W.L.; Monteiro, C.A. Antifungal drug susceptibility of Candida species isolated from HIV-positive patients recruited at a public hospital in São Luís, Maranhão, Brazil. Front. Microbiol. 2017, 2, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Satthanakul, P.; Taweechaisupapong, S.; Luengpailin, S.; Khunkitti, W. The antifungal efficacy of essential oils in combination with chlorhexidine against Candida spp. Songklanakarin J. Sci. Technol. 2019, 41, 144–150. [Google Scholar]
- Sharma, Y.; Khan, L.A.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Med. 2016, 26, 244–254. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowak, A.; Kunicka-Styczyńska, A.; Siadur, A. Biological effects of various chemically characterized essential oils: Investigation of the mode of action against Candida albicans and HeLa cells. RSC Adv. 2016, 6, 97199–97207. [Google Scholar] [CrossRef]
- Khan, M.S.; Malik, A.; Ahmad, I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med. Mycol. 2012, 50, 33–42. [Google Scholar] [CrossRef]
- da Silva, I.C.G.; Santos, H.B.P.; Cavalcanti, Y.W.; Nonaka, C.F.W.; de Sousa, S.A.; de Castro, R.D. Antifungal activity of eugenol and its association with nystatin on Candida albicans. Pesqui. Bras. Odontopediatria Clin. Integr. 2017, 17, e3235. [Google Scholar] [CrossRef]
- Leite, M.C.; Bezerra, A.P.; de Sousa, J.P.; Guerra, F.Q.; Lima, E.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 378280. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Filho, A.A.; de Oliveira, H.M.B.F.; de Sousa, J.P.; Meireles, D.; de Azevedo Maia, G.L.; Filho, J.M.B.; Lima, E.O. In vitro anti-Candida activity and mechanism of action of the flavonoid isolated from Praxelis clematidea against Candida albicans species. J. App. Pharm. Sci. 2016, 6, 66–69. [Google Scholar] [CrossRef][Green Version]
- Lima, I.O.; de Medeiros Nóbrega, F.; de Oliveira, W.A.; de Oliveira Lima, E.; Albuquerque Menezes, E.; Afrânio Cunha, F.; de Fátima Formiga Melo Diniz, M. Anti-Candida albicans effectiveness of citral and investigation of mode of action. Pharm. Biol. 2012, 50, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.D.; Lima, E.O. Anti-Candida activity and chemical composition of Cinnamomum zeylanicum blume essential oil. Braz. Arch. Biol. Technol. 2013, 56, 749–755. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhao, I.S.; Duffin, S.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. Revitalising silver nitrate for caries management. Int. J. Environ. Res. Public Health 2018, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.B.; Karuppayil, S.M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans. SpringerPlus 2013, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Serra, E.; Hidalgo-Bastida, L.A.; Verran, J. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens 2018, 7, 15. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid.-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Thilakan, A.; Sabu, N.; Ramankutty, R.; Vidya, K.C.; Thomas, N.A.; Jobe, J. Antimicrobial activity of 0.05 N and 0.1 N silver nitrate mouthwash against Streptococcus mutans and Candida albicans: An in vitro study. J. Int. Oral Health 2022, 14, 101–105. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Lee, M.H. Triclosan: An update on biochemical and molecular mechanisms. Oxidative Med. Cell. Longev. 2019, 2, 1607304. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic interaction of eugenol and antimicrobial drugs in eradication of single and mixed biofilms of Candida albicans and Streptococcus mutans. AMB Express 2020, 10, 185–194. [Google Scholar] [CrossRef] [PubMed]
- de Paula, S.B.; Bartelli, T.F.; Raimo, V.D.; Santos, J.P.; Morey, A.T.; Bosini, M.A.; Nakamura, C.V.; Yamauchi, L.M.; Yamada-Ogatta, S.F. Effect of eugenol on cell surface hydrophobicity, adhesion, and biofilm of Candida tropicalis and Candida dubliniensis isolated from oral cavity of HIV-infected patients. Evid.-Based Complement. Altern. Med. 2014, 2014, 505204. [Google Scholar] [CrossRef]
- Araújo, M.I.F.; Freitas, F.O.R.; Morais, A.M.B.; Brustein, V.P.; Nogueira, T.B.; Sá, S.; Nogueira, R.B.; Sá, S.; Sousa, M.N.A.; Uchoa, D.P.L.; et al. Antifungal activity and in silico toxicology of the o-eugenol. Phytochem. Res. 2018, 10, 284–290. [Google Scholar]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, anti-MRSA activity and toxicity of essential oils from Cymbopogon species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations(MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- O’Donnell, F.; Smyth, T.J.; Ramachandran, V.T.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolones. Int. J. Antimicrob. Agents 2011, 35, 30–38. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
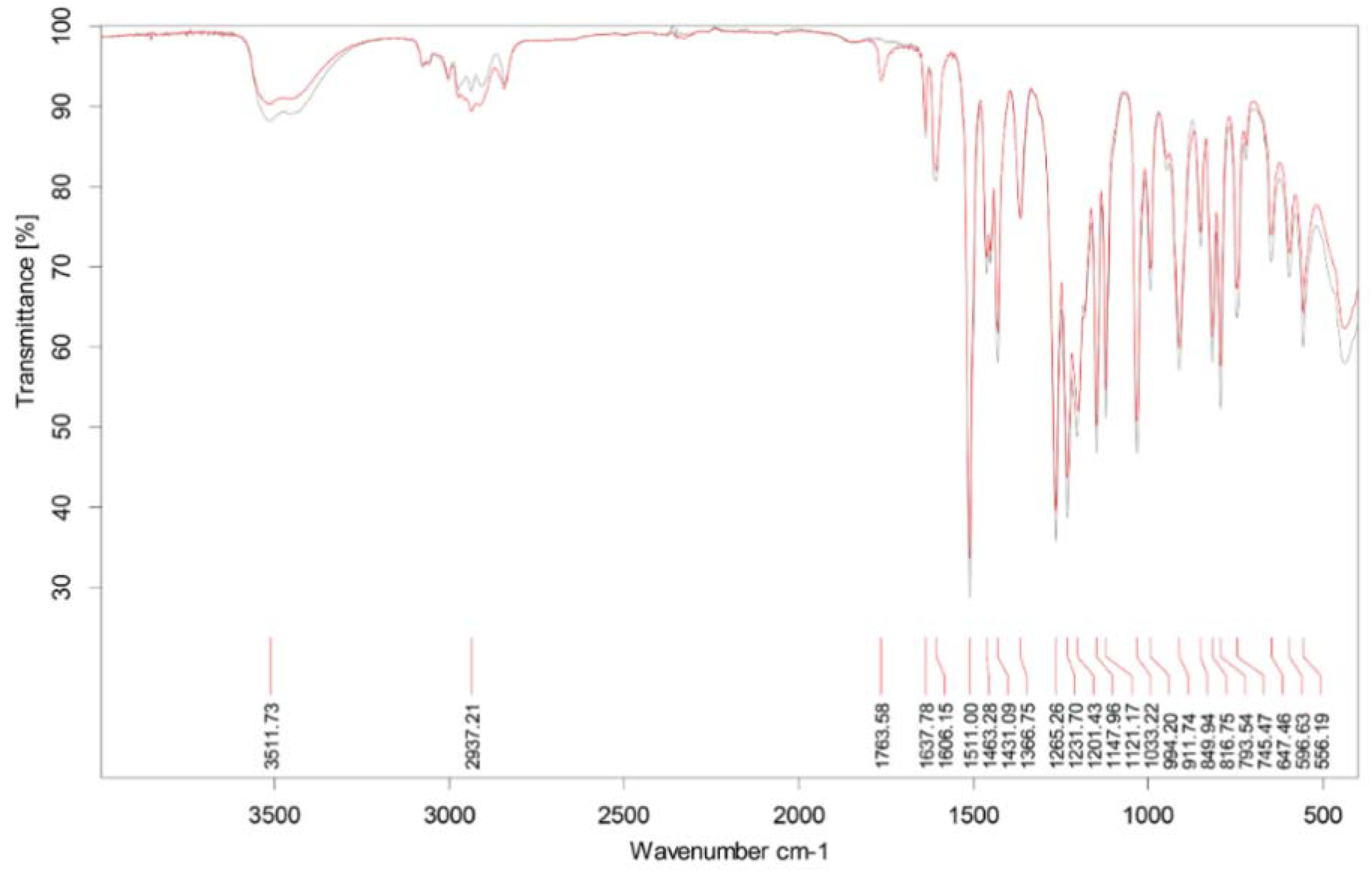
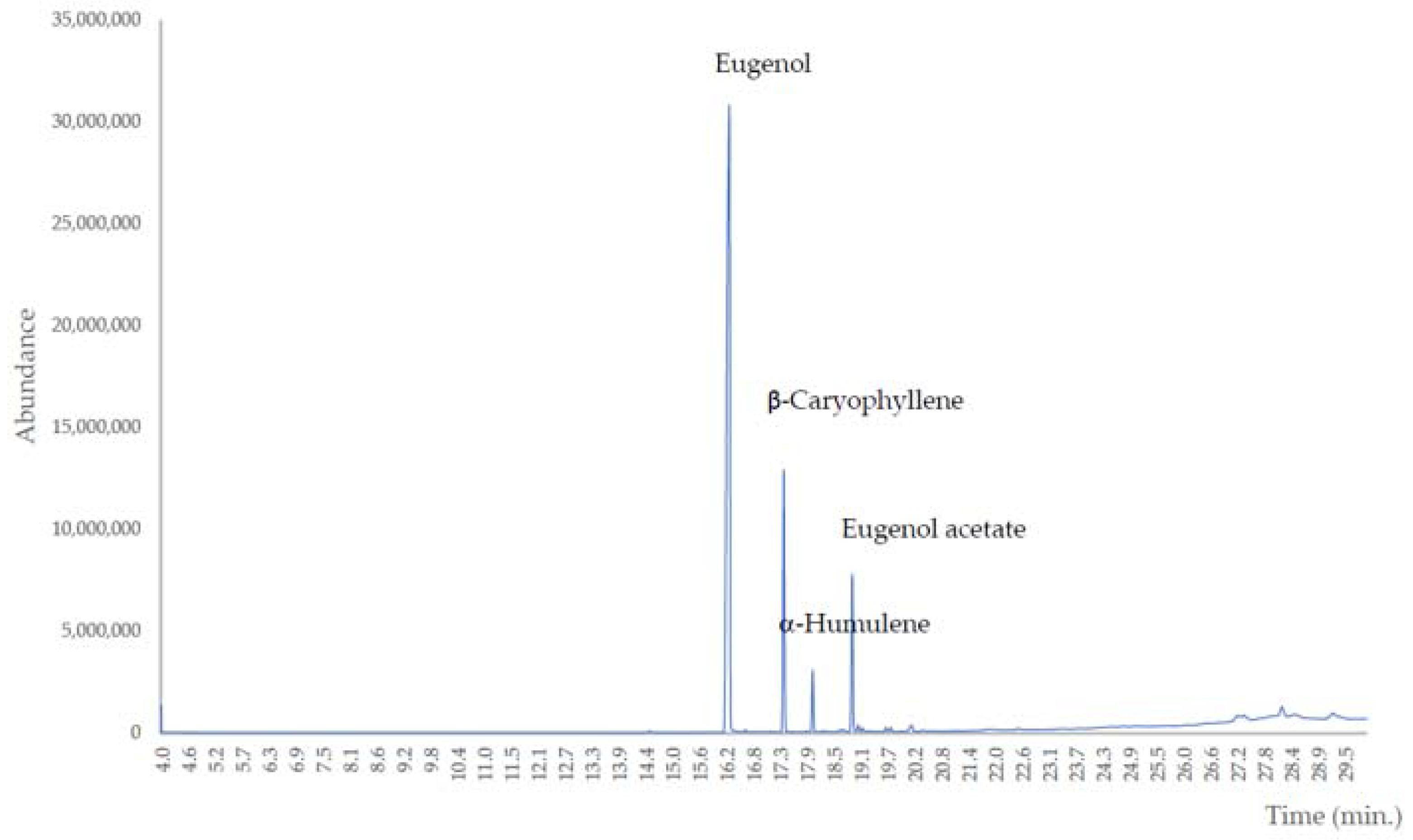
| No | Retention Time (min.) | Retention Index * | Composition (%) | Compound |
|---|---|---|---|---|
| 1 | 16.250 | 1366 | 66.81 | Eugenol |
| 2 | 17.423 | 1433 | 14.11 | β-Caryophyllene |
| 3 | 18.047 | 1469 | 3.19 | α-Humulene |
| 4 | 18.903 | 1521 | 8.33 | Eugenol acetate |
| Reference Strains | CEO | EUG | ||||
|---|---|---|---|---|---|---|
| Range of MIC | Range of MFC | MFC/MIC Ratio | Range of MIC | Range of MFC | MFC/MIC Ratio | |
| C. albicans ATCC 10231 | 1–2 | 2–4 | 1–2 | 1–2 | 2–4 | 2 |
| C. albicans ATCC 2091 | 0.5–1 | 1–2 | 2 | 0.5–1 | 1–2 | 2 |
| C. parapsilosis ATCC 22019 | 1–2 | 2–4 | 2 | 0.5–1 | 1–2 | 2 |
| C. glabrata ATCC 90030 | 1–2 | 2–4 | 2 | 1–2 | 2–4 | 2 |
| C. krusei ATCC 14243 | 0.5–2 | 1–4 | 2 | 0.25–1 | 1–2 | 2 |
| Clinical Isolates | CEO | EUG | ||||||
|---|---|---|---|---|---|---|---|---|
| Range of MIC | Range of MFC | MIC50 /MIC90 | MFC50 /MFC90 | Range of MIC | Range of MFC | MIC50 /MIC90 | MFC50 /MFC90 | |
| C. albicans | 0.25–2 | 0.5–4 | 0.5/1 | 1/2 | 0.25–2 | 0.5–4 | 0.5/1 | 1/2 |
| non-albicans Candida spp. | 0.25–2 | 0.25–4 | 0.5/2 | 1/2 | 0.25–2 | 0.5–4 | 0.5/1 | 1/2 |
| MFC/MIC Ratio | Number (Percentage) of Clinical Isolates | |||
|---|---|---|---|---|
| C. albicans | non-albicans Candida spp. | |||
| CEO | EUG | CEO | EUG | |
| 1 | 8 (26.7) | 6 (20) | 11 (36.7) | 9 (30) |
| 2 | 19 (63.3) | 20 (66.7) | 15 (50.0) | 18 (60) |
| 4 | 3 (10.0) | 4 (13.3) | 4 (13.3) | 3 (10) |
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. albicans | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| CEO | 1000 | 500 | 0.5 | 1.5 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| cetylpyridinium | 3.91 | 0.98 | 0.25 | ||
| CEO | 1000 | 250 | 0.25 | 0.375 | synergism |
| chlorhexidine | 7.81 | 0.98 | 0.125 | ||
| CEO | 1000 | 500 | 0.5 | 1 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| CEO | 1000 | 500 | 0.5 | 1 | addition |
| triclosan | 7.81 | 3.91 | 0.5 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. glabrata | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| CEO | 1000 | 250 | 0.25 | 1.25 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| cetylpyridinium | 0.98 | 0.24 | 0.25 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| chlorhexidine | 7.81 | 1.95 | 0.25 | ||
| CEO | 1000 | 250 | 0.25 | 0.75 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| triclosan | 15.62 | 3.91 | 0.25 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. krusei | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| CEO | 1000 | 500 | 0.5 | 1.5 | indifference |
| nystatin | 0.98 | 0.98 | 1 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| cetylpyridinium | 0.98 | 0.24 | 0.25 | ||
| CEO | 1000 | 125 | 0.125 | 0.375 | synergism |
| chlorhexidine | 1.95 | 0.48 | 0.25 | ||
| CEO | 1000 | 250 | 0.25 | 0.75 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| CEO | 1000 | 250 | 0.25 | 0.75 | addition |
| triclosan | 15.62 | 7.81 | 0.5 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. parapsilosis | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| CEO | 1000 | 250 | 0.25 | 1.25 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| CEO | 1000 | 125 | 0.125 | 0.375 | synergism |
| cetylpyridinium | 1.95 | 0.48 | 0.25 | ||
| CEO | 1000 | 62.5 | 0.062 | 0.562 | addition |
| chlorhexidine | 0.98 | 0.48 | 0.5 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| silver nitrate | 7.81 | 1.95 | 0.25 | ||
| CEO | 1000 | 250 | 0.25 | 0.5 | synergism |
| triclosan | 15.62 | 3.91 | 0.25 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. albicans | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| EUG | 1000 | 250 | 0.25 | 1.25 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| EUG | 1000 | 250 | 0.25 | 0.5 | synergism |
| cetylpyridinium | 3.91 | 0.98 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.5 | synergism |
| chlorhexidine | 7.81 | 1.95 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.75 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| EUG | 1000 | 250 | 0.25 | 0.75 | addition |
| triclosan | 7.81 | 3.91 | 0.5 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. glabrata | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| EUG | 1000 | 250 | 0.25 | 1.25 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| EUG | 1000 | 250 | 0.25 | 0.5 | synergism |
| cetylpyridinium | 0.98 | 0.24 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.5 | synergism |
| chlorhexidine | 7.81 | 1.95 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.75 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| EUG | 1000 | 250 | 0.25 | 0.5 | synergism |
| triclosan | 15.62 | 3.91 | 0.25 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. krusei | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| EUG | 1000 | 500 | 0.5 | 1.5 | indifference |
| nystatin | 0.98 | 0.98 | 1 | ||
| EUG | 1000 | 250 | 0.25 | 0.375 | synergism |
| cetylpyridinium | 0.98 | 0.12 | 0.125 | ||
| EUG | 1000 | 125 | 0.125 | 0.375 | synergism |
| chlorhexidine | 1.95 | 0.48 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.75 | addition |
| silver nitrate | 7.81 | 3.91 | 0.5 | ||
| EUG | 1000 | 125 | 0.125 | 0.625 | addition |
| triclosan | 15.62 | 7.81 | 0.5 | ||
| Antifungal Agent | MIC of Antifungal Agent (µg/mL) against C. parapsilosis | FIC | Ʃ FIC (FICI) | Interpretation | |
|---|---|---|---|---|---|
| Alone | Combination | ||||
| EUG | 1000 | 250 | 0.5 | 1.25 | indifference |
| nystatin | 0.48 | 0.48 | 1 | ||
| EUG | 1000 | 250 | 0.25 | 0.375 | synergism |
| cetylpyridinium | 1.95 | 0.24 | 0.125 | ||
| EUG | 1000 | 62.5 | 0.125 | 0.562 | addition |
| chlorhexidine | 0.98 | 0.48 | 0.25 | ||
| EUG | 1000 | 250 | 0.25 | 0.375 | synergism |
| silver nitrate | 7.81 | 0.98 | 0.5 | ||
| EUG | 1000 | 250 | 0.125 | 0.5 | synergism |
| triclosan | 15.62 | 3.91 | 0.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernasiuk, A.; Baj, T.; Malm, A. Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules 2023, 28, 215. https://doi.org/10.3390/molecules28010215
Biernasiuk A, Baj T, Malm A. Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules. 2023; 28(1):215. https://doi.org/10.3390/molecules28010215
Chicago/Turabian StyleBiernasiuk, Anna, Tomasz Baj, and Anna Malm. 2023. "Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions" Molecules 28, no. 1: 215. https://doi.org/10.3390/molecules28010215
APA StyleBiernasiuk, A., Baj, T., & Malm, A. (2023). Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules, 28(1), 215. https://doi.org/10.3390/molecules28010215








