Structural and Electrochemical Properties of Li2O-V2O5-B2O3-Bi2O3 Glass and Glass-Ceramic Cathodes for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization of LVBB Glass
2.2. Electrochemical Properties of LVBB Glass Cathode
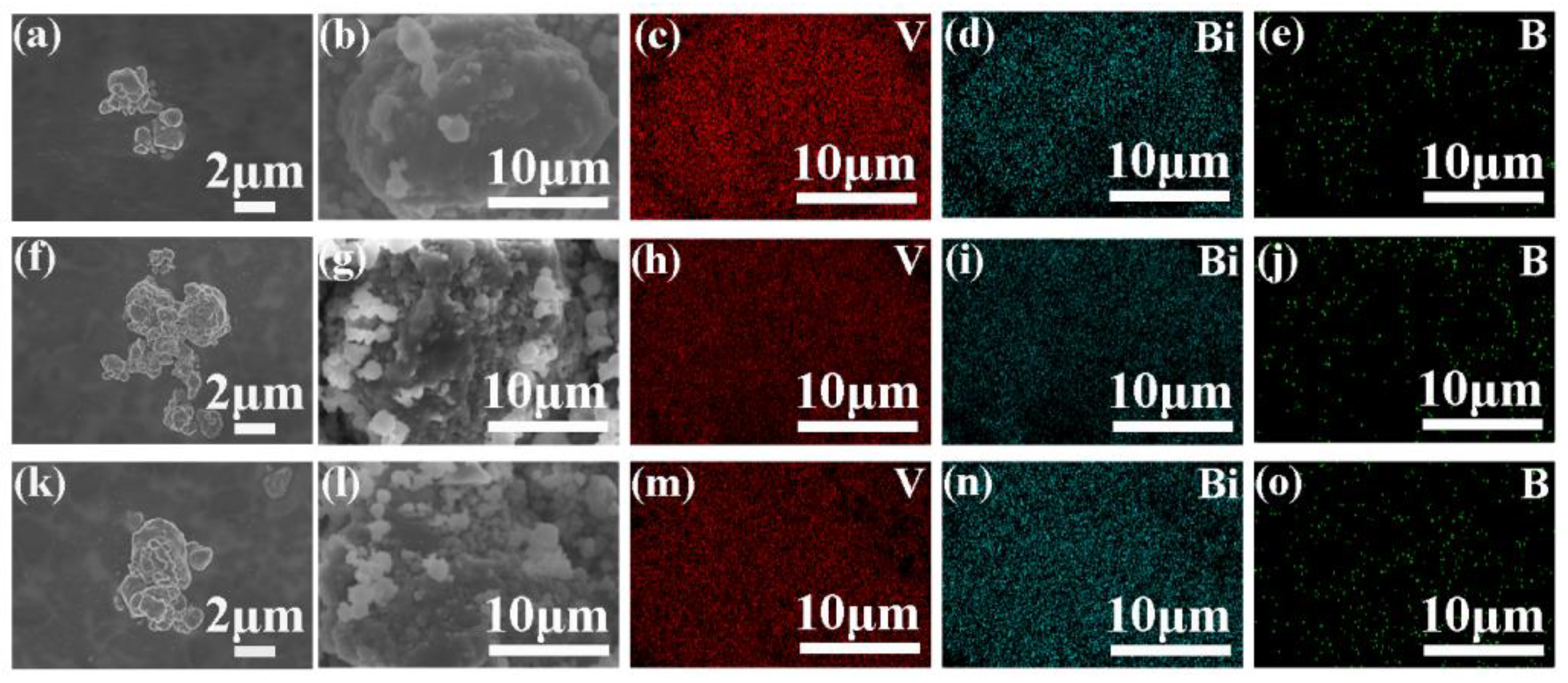
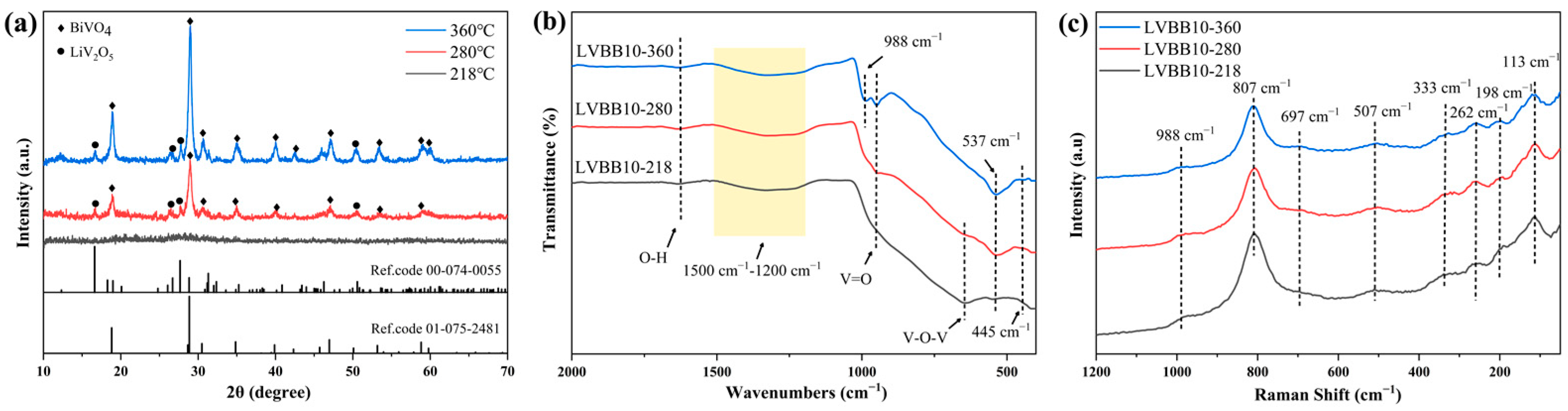
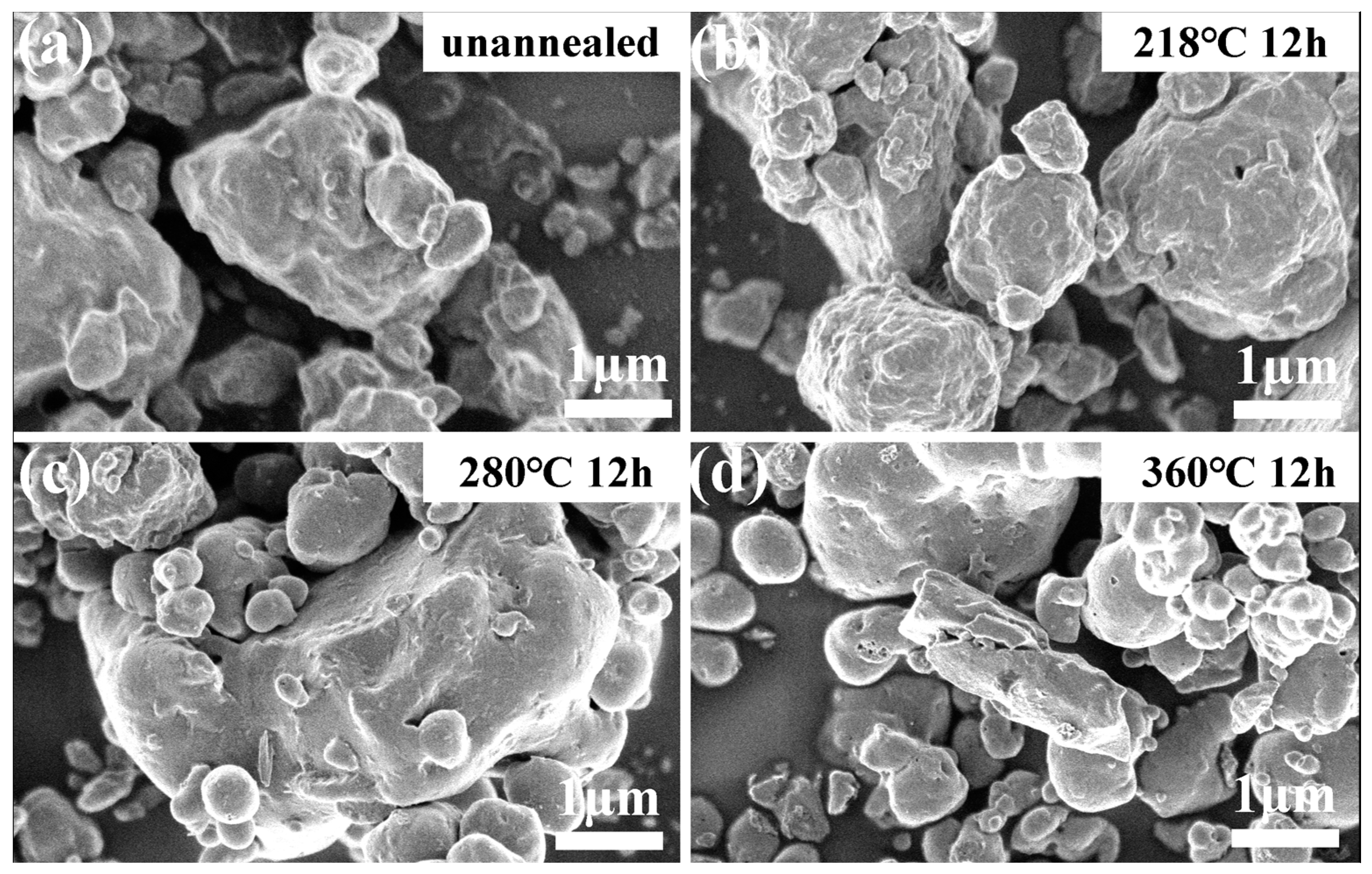
2.3. Structural and Morphological Characterization of Li2O-V2O5-B2O3-Bi2O3 Glass-Ceramic Materials
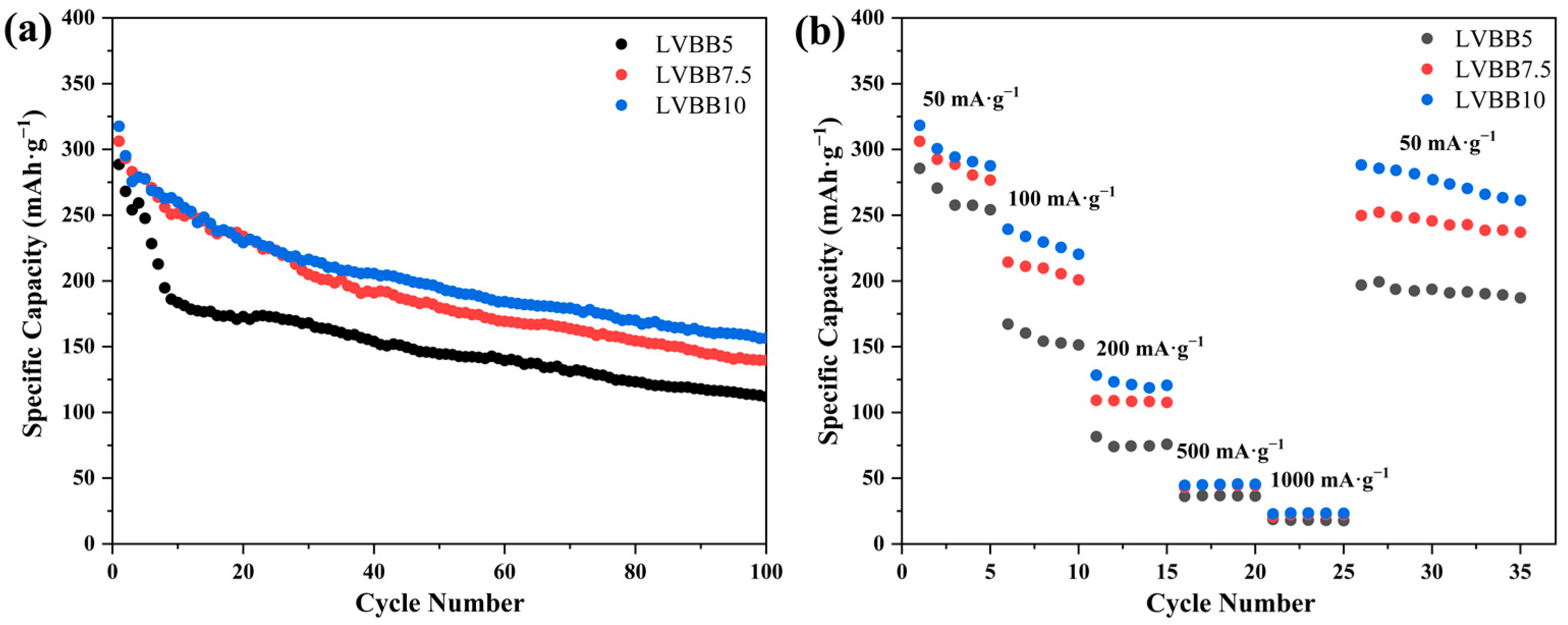
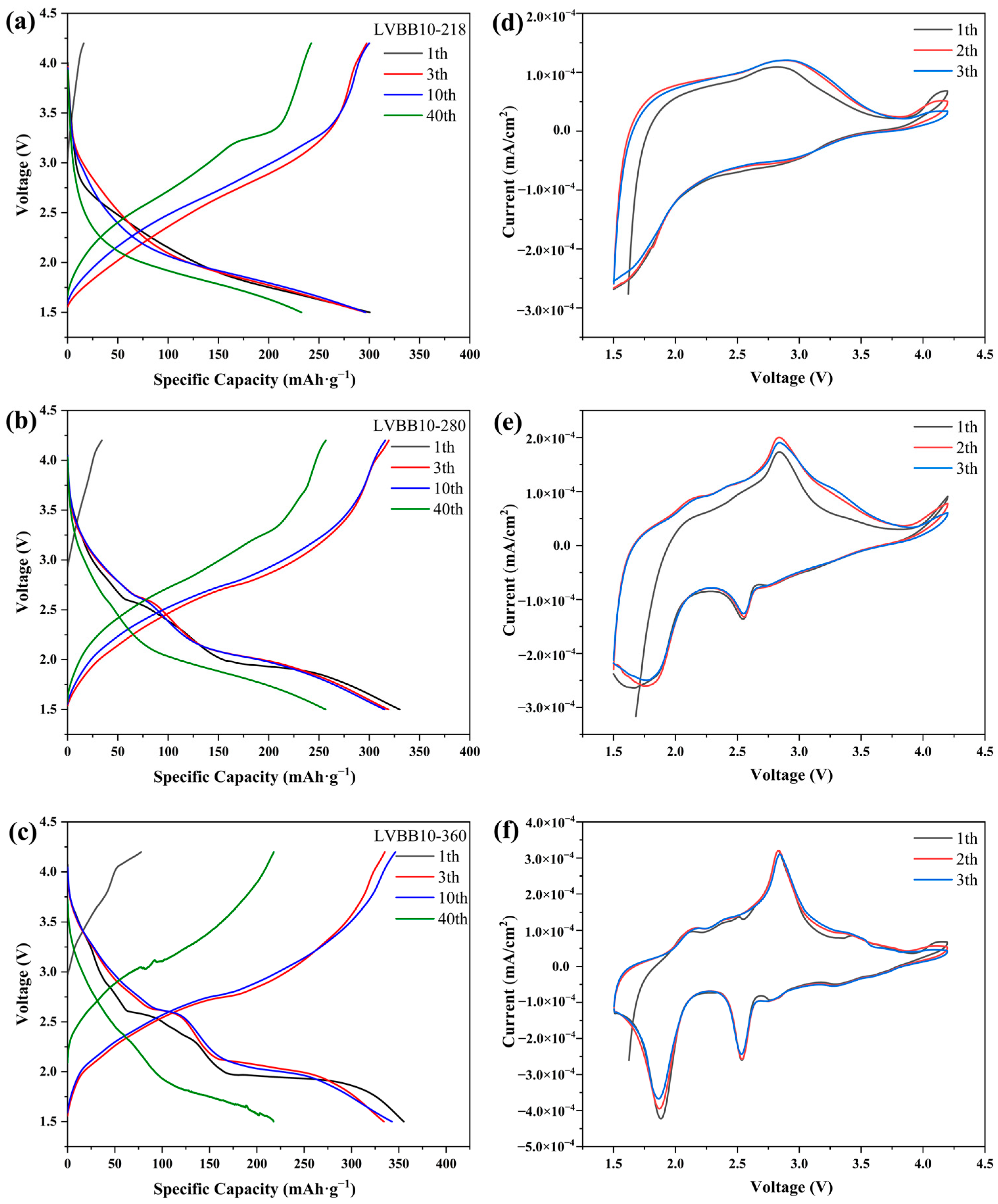
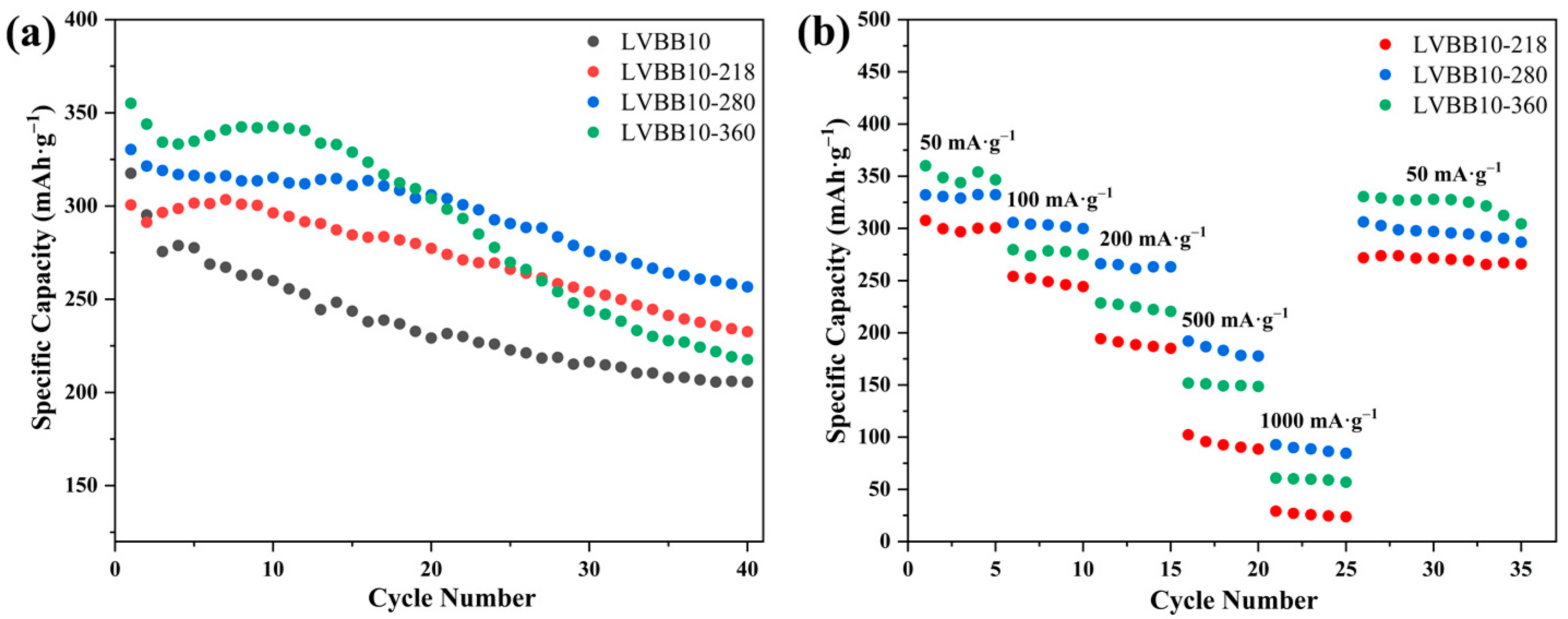
2.4. Electrochemical Properties of Li2O-V2O5-B2O3-Bi2O3 Glass-Ceramic Cathode
3. Materials and Methods
3.1. Preparation of Glass Electrode Materials
3.2. Characterization of the Glass Materials
3.3. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bates, A.M.; Preger, Y.; Torres-Castro, L.; Harrison, K.L.; Harris, S.J.; Hewson, J. Are Solid-State Batteries Safer than Lithium-Ion Batteries? Joule 2022, 6, 742–755. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.S.; Kanagavalli, P.; Sriram, G.; B, R.P.; John, N.S.; Veerapandian, M.; Kurkuri, M.; Hegde, G. Low Cost, Catalyst Free, High Performance Supercapacitors Based on Porous Nano Carbon Derived from Agriculture Waste. J. Energy Storage 2020, 32, 101829. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) Cathode Material for Lithium-Ion Batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Jin, S.; Lee, S.; Noda, I.; Jung, Y. Investigation of the Phase Transition Mechanism in LiFePO4 Cathode Using In Situ Raman Spectroscopy and 2D Correlation Spectroscopy during Initial Cycle. Molecules 2019, 24, 291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-M.; Li, F.; Zhu, X.-Q.; Yu, J.-G. Triallyl Isocyanurate as an Efficient Electrolyte Additive for Layered Oxide Cathode Material-Based Lithium-Ion Batteries with Improved Stability under High-Voltage. Molecules 2022, 27, 3107. [Google Scholar] [CrossRef]
- Uchaker, E.; Zheng, Y.Z.; Li, S.; Candelaria, S.L.; Hu, S.; Cao, G.Z. Better than Crystalline: Amorphous Vanadium Oxide for Sodium-Ion Batteries. J. Mater. Chem. A 2014, 2, 18208–18214. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ikejiri, J.; Tsunoda, K.; Tanaka, A.; Sato, F.; Honma, T.; Komatsu, T. Enhanced Rate Capabilities in a Glass-Ceramic-Derived Sodium All-Solid-State Battery. Sci. Rep. 2020, 10, 9453. [Google Scholar] [CrossRef]
- Afyon, S.; Krumeich, F.; Mensing, C.; Borgschulte, A.; Nesper, R. New High Capacity Cathode Materials for Rechargeable Li-Ion Batteries: Vanadate-Borate Glasses. Sci. Rep. 2015, 4, 7113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernardou, D.; Drosos, C.; Kafizas, A.; Pemble, M.E.; Koudoumas, E. Towards High Performance Chemical Vapour Deposition V2O5 Cathodes for Batteries Employing Aqueous Media. Molecules 2020, 25, 5558. [Google Scholar] [CrossRef] [PubMed]
- Shreenivasa, L.; Yogeeshwari, R.T.; Viswanatha, R.; Sriram, G.; Kalegowda, Y.; Kurkuri, M.D.; Ashoka, S. An Introduction of New Nanostructured Zn0.29V2O5 Cathode Material for Lithium Ion Battery: A Detailed Studies on Synthesis, Characterization and Lithium Uptake. Mater. Res. Express 2019, 6, 115035. [Google Scholar] [CrossRef]
- Shreenivasa, L.; Viswanatha, R.; Ganesan, S.; Kalegowda, Y.; Kurkuri, M.D.; Ashoka, S. Scalable Chemical Approach to Prepare Crystalline Mn2V2O7 Nanoparticles: Introducing a New Long-Term Cycling Cathode Material for Lithium-Ion Battery. J. Mater. Sci. Mater. Electron. 2020, 31, 19638–19646. [Google Scholar] [CrossRef]
- Lee, Y. Li-Ion Conductivity in Li2O-B2O3-V2O5 Glass System. Solid State Ion. 2004, 175, 687–690. [Google Scholar] [CrossRef]
- Jozwiak, P.; Garbarczyk, J. Mixed Electronic–Ionic Conductivity in the Glasses of the Li2O-V2O5-P2O5 System. Solid State Ion. 2005, 176, 2163–2166. [Google Scholar] [CrossRef]
- Shao, Y.; Lu, Z.; Li, L.; Liu, Y.; Yang, L.; Shu, T.; Li, X.; Liao, S. Significant Enhancement of the Capacity and Cycling Stability of Lithium-Rich Manganese-Based Layered Cathode Materials via Molybdenum Surface Modification. Molecules 2022, 27, 2100. [Google Scholar] [CrossRef]
- Kindle, M.; Cha, Y.; McCloy, J.S.; Song, M.-K. Alternatives to Cobalt: Vanadate Glass and Glass-Ceramic Structures as Cathode Materials for Rechargeable Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 629–638. [Google Scholar] [CrossRef]
- Fu, X.; Pu, X.; Wang, H.; Zhao, D.; Liu, G.; Zhao, D.; Chen, Z. Understanding Capacity Fading of the LiVO3 Cathode Material by Limiting the Cutoff Voltage. Phys. Chem. Chem. Phys. 2019, 21, 7009–7015. [Google Scholar] [CrossRef]
- Du, M.; Huang, K.; Guo, Y.; Xie, Z.; Jiang, H.; Li, C.; Chen, Y. High Specific Capacity Lithium Ion Battery Cathode Material Prepared by Synthesizing Vanadate–Phosphate Glass in Reducing Atmosphere. J. Power Sources 2019, 424, 91–99. [Google Scholar] [CrossRef]
- Kindle, M.; Kmiec, S.; d’Anciães Almeida Silva, I.; Eckert, H.; Martin, S.W.; Song, M.-K.; McCloy, J.S. Structural Properties of Alumina-Doped Lithium Borovanadate Glasses and Glass-Ceramics. J. Non-Cryst. Solids 2019, 521, 119551. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Yin, Y.; Chen, J.; Wang, L.; Zhang, P.; Lai, X.; Yue, B.; Hu, X.; He, D. Effects of V2O5 and Fe2O3 on the Structures and Electrochemical Performances of Li2O-V2O5-B2O3 Glass Materials in Lithium-Ion Batteries. J. Alloys Compd. 2021, 879, 160293. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K.J.; Kurudirek, M.; Thakur, S. Study of Environment Friendly Bismuth Incorporated Lithium Borate Glass System for Structural, Gamma-Ray and Fast Neutron Shielding Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117309. [Google Scholar] [CrossRef] [PubMed]
- Shapaan, M. Structure and Electric Conductivity of Mixed Electronic-Ionic Bi2O3-Li2O-V2O5-B2O3 Glass System. Int. J. Thin Fil. Sci. Tec. 2017, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Margha, F.H.; El-Bassyouni, G.T.; Turky, G.M. Enhancing the Electrical Conductivity of Vanadate Glass System (Fe2O3, B2O3, V2O5) via Doping with Sodium or Strontium Cations. Ceram. Int. 2019, 45, 11838–11843. [Google Scholar] [CrossRef]
- Dantas, N.O.; Ayta, W.E.F.; Silva, A.C.A.; Cano, N.F.; Silva, S.W.; Morais, P.C. Effect of Fe2O3 Concentration on the Structure of the SiO2-Na2O-Al2O3-B2O3 Glass System. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 140–143. [Google Scholar] [CrossRef]
- Abdel-Hameed, S.A.M.; Fathi, A.M.; Elwan, R.L.; Margha, F.H. Effect of F− and B3+ Ions and Heat Treatment on the Enhancement of Electrochemical and Electrical Properties of Nanosized LiTi2(PO4)3 Glass-Ceramic for Lithium-Ion Batteries. J. Alloys Compd. 2020, 832, 154943. [Google Scholar] [CrossRef]
- Laorodphan, N.; Pooddee, P.; Kidkhunthod, P.; Kunthadee, P.; Tapala, W.; Puntharod, R. Boron and Pentavalent Vanadium Local Environments in Binary Vanadium Borate Glasses. J. Non-Cryst. Solids 2016, 453, 118–124. [Google Scholar] [CrossRef]
- Saetova, N.S.; Raskovalov, A.A.; Antonov, B.D.; Yaroslavtseva, T.V.; Reznitskikh, O.G.; Zabolotskaya, E.V.; Kadyrova, N.I.; Telyatnikova, A.A. Conductivity and Spectroscopic Studies of Li2O-V2O5-B2O3 Glasses. Ionics 2018, 24, 1929–1938. [Google Scholar] [CrossRef]
- Mandal, S.; Hazra, S.; Das, D.; Ghosh, A. Structural Studies of Binary Iron Vanadate Glass. J. Non-Cryst. Solids 1995, 183, 315–319. [Google Scholar] [CrossRef]
- Baia, L.; Stefan, R.; Kiefer, W.; Popp, J.; Simon, S. Structural Investigations of Copper Doped B2O3–Bi2O3 Glasses with High Bismuth Oxide Content. J. Non-Cryst. Solids 2002, 303, 379–386. [Google Scholar] [CrossRef]
- ElBatal, F.H.; Abdelghany, A.M.; Ezz ElDin, F.M.; ElBatal, H.A. Vanadium Structural Role in Binary Fluoride Borate Glasses and Effects of Gamma Irradiation. Radiat. Phys. Chem. 2020, 170, 108659. [Google Scholar] [CrossRef]
- Garbarczyk, J. Studies of Silver-Vanadate-Phosphate Glasses by Raman, EPR and Impedance Spectroscopy Methods. Solid State Ion. 2000, 136–137, 1077–1083. [Google Scholar] [CrossRef]
- Galembeck, A.; Alves, O.L. BiVO4 Thin Film Preparation by Metalorganic Decomposition. Thin Solid Films 2000, 365, 90–93. [Google Scholar] [CrossRef]
- Bale, S.; Rao, N.S.; Rahman, S. Spectroscopic Studies of Bi2O3-Li2O-ZnO-B2O3 Glasses. Solid State Sci. 2008, 10, 326–331. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E.; Eckert, H.; Jefferson, D.A. Vanadium(V) Environments in Bismuth Vanadates: A Structural Investigation Using Raman Spectroscopy and Solid State 51V NMR. J. Solid State Chem. 1991, 90, 194–210. [Google Scholar] [CrossRef]
- Kong, F.; Yi, L.; Huang, S.; Liang, X.; Rao, Y.; Su, Z.; Li, C.; Jiang, H. Using Glass Defect Engineering to Obtain Order-Disorder Transformation in Cathode for High Specific Capacity Lithium Ion Battery. Appl. Surf. Sci. 2021, 552, 149495. [Google Scholar] [CrossRef]
- Esper, J.D.; Zhuo, Y.; Barr, M.K.S.; Yokosawa, T.; Spiecker, E.; de Ligny, D.; Bachmann, J.; Peukert, W.; Romeis, S. Shape-Anisotropic Cobalt-Germanium-Borate Glass Flakes as Novel Li-Ion Battery Anodes. Powder Technol. 2020, 363, 218–231. [Google Scholar] [CrossRef]
- Singh, K. Electrical Conductivity of Li2O-B2O3-Bi2O3: A Mixed Conductor. Solid State Ion. 1996, 93, 147–158. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, S.; Yan, H. Hydrothermal Preparation of BiVO4 Powders. Mater. Sci. Eng. B 2003, 104, 36–39. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, W.; Wu, Z.; He, F.; Zhang, J.; Xiao, Y.; Zhong, B.; Guo, X. Effect of Niobium Doping on the Structure and Electrochemical Performance of LiNi0.5Co0.2Mn0.3O2 Cathode Materials for Lithium Ion Batteries. Ceram. Int. 2017, 43, 3866–3872. [Google Scholar] [CrossRef]
- Liu, P.; Wang, B.; Sun, X.; Gentle, I.; Zhao, X.S. A Comparative Study of V2O5 Modified with Multi-Walled Carbon Nanotubes and Poly(3,4-Ethylenedioxythiophene) for Lithium-Ion Batteries. Electrochim. Acta 2016, 213, 557–564. [Google Scholar] [CrossRef]


| Cathode Material | Cycle Number | Current Density (mA·g−1) | Specific Capacity (mAh·g−1) | Capacity Retention | Reference |
|---|---|---|---|---|---|
| Crystalline Zn0.29V2O5 | 100 | 63.2 | 316 | 104 | [13] |
| Crystalline Mn2V2O7 | 1142 | 344 | 242 | 82 | [14] |
| Glass V2O5-P2O5 | 100 | 17 | 270 | 90 | [20] |
| Glass V2O5-LiBO2 | 10 | 50 | 327 | 88 | [21] |
| Glass RGO/V2O5-LiBO2 | 100 | 50 | 400 | 75 | [21] |
| Glass Al2O3-V2O5-LiBO2 | 100 | 50 | 250 (after rate capability testing) | 50 | [18] |
| Glass Li2O-V2O5-B2O3 | 100 | 100 | 170 | 81.7 | [22] |
| Glass Li2O-V2O5-B2O3-Fe2O3 | 100 | 100 | 306.2 | 39.3 | [22] |
| Glass Li2O-V2O5-B2O3-Bi2O3 | 100 | 50 | 317.6 | 49.2 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhao, Y.; Liu, F.; Ding, M.; Wang, J.; Jiang, J.; Boulet, P.; Record, M.-C. Structural and Electrochemical Properties of Li2O-V2O5-B2O3-Bi2O3 Glass and Glass-Ceramic Cathodes for Lithium-Ion Batteries. Molecules 2023, 28, 229. https://doi.org/10.3390/molecules28010229
Chen Y, Zhao Y, Liu F, Ding M, Wang J, Jiang J, Boulet P, Record M-C. Structural and Electrochemical Properties of Li2O-V2O5-B2O3-Bi2O3 Glass and Glass-Ceramic Cathodes for Lithium-Ion Batteries. Molecules. 2023; 28(1):229. https://doi.org/10.3390/molecules28010229
Chicago/Turabian StyleChen, Yuan, Yufei Zhao, Feihong Liu, Mengdie Ding, Juan Wang, Jiuxin Jiang, Pascal Boulet, and Marie-Christine Record. 2023. "Structural and Electrochemical Properties of Li2O-V2O5-B2O3-Bi2O3 Glass and Glass-Ceramic Cathodes for Lithium-Ion Batteries" Molecules 28, no. 1: 229. https://doi.org/10.3390/molecules28010229





