Locking the GFP Fluorophore to Enhance Its Emission Intensity
Abstract
1. Introduction
2. Fluorescence Enhancement without Structural Modifications
2.1. Crystallization and Aggregation-Induced Emission Enhancement (AIEE)
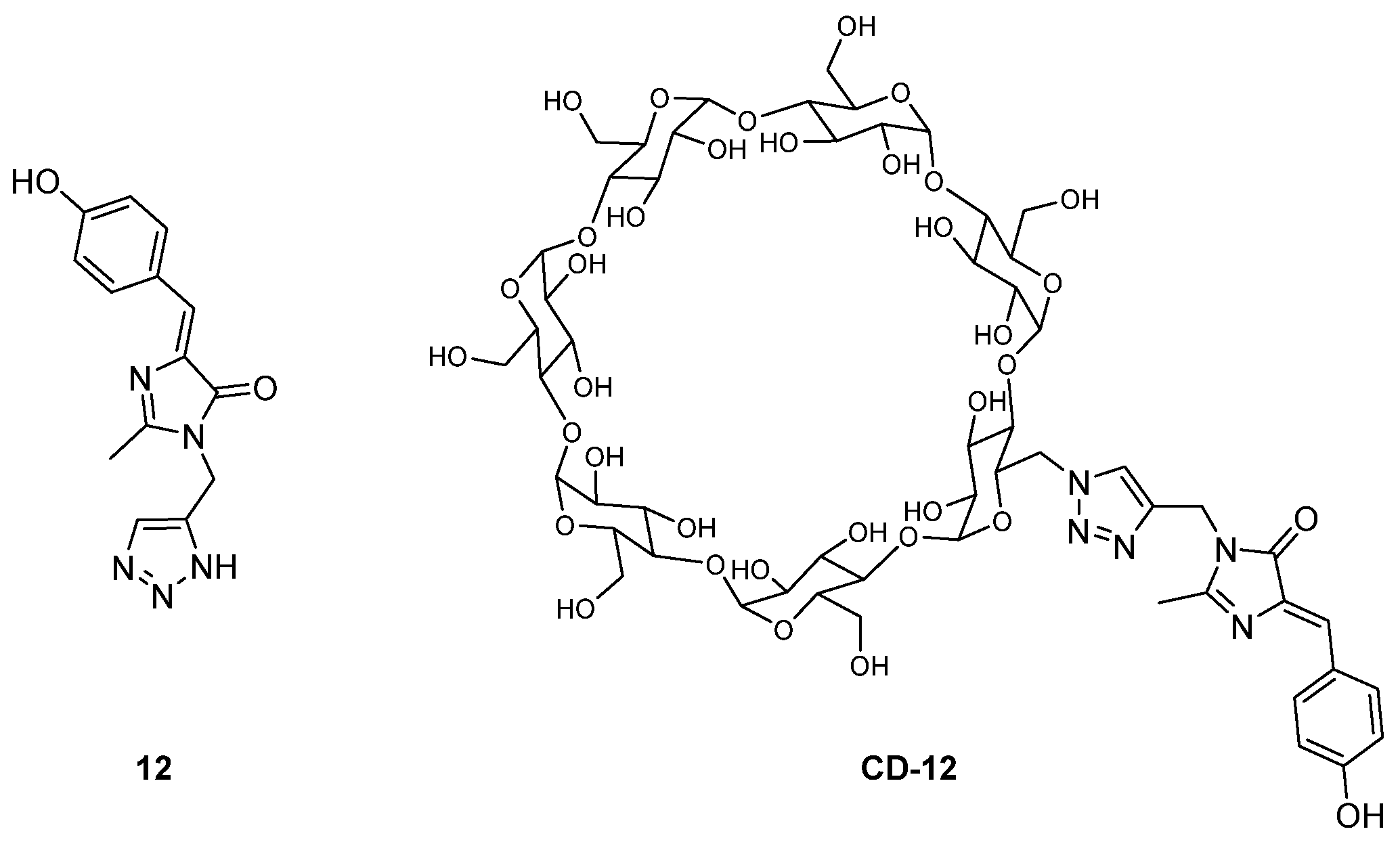
2.2. Supramolecular Hosts
2.3. Polymers
3. Fluorescence Enhancement with Structural Modifications
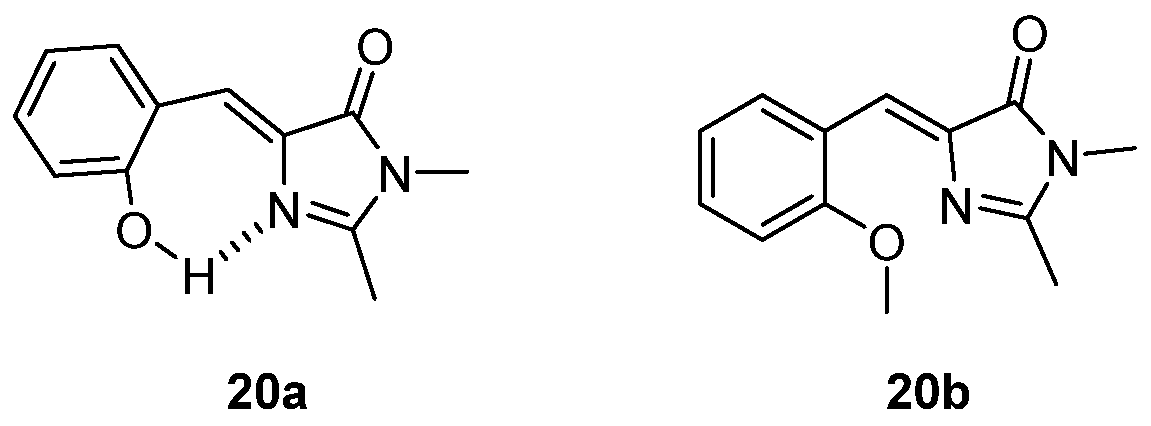
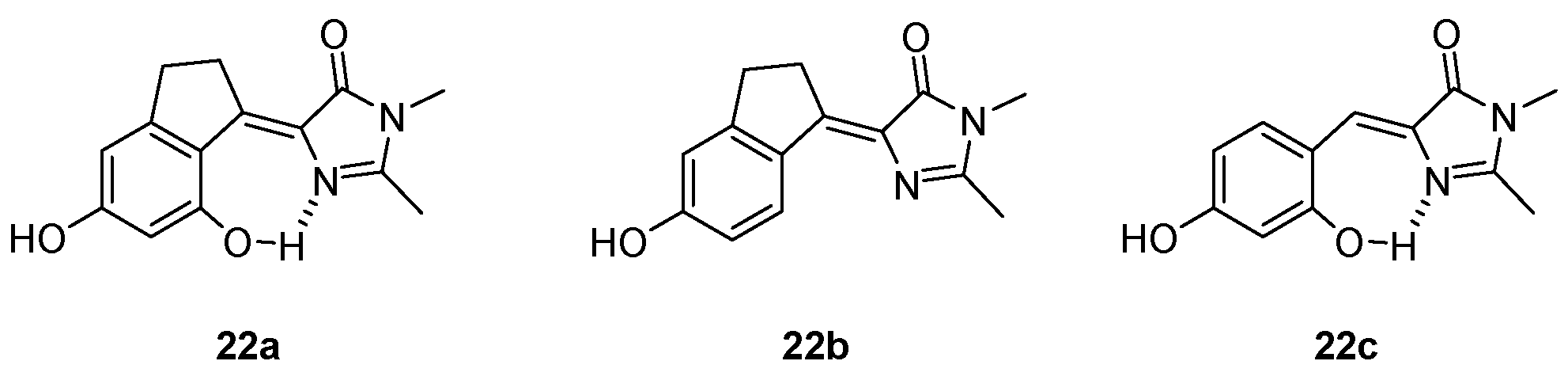
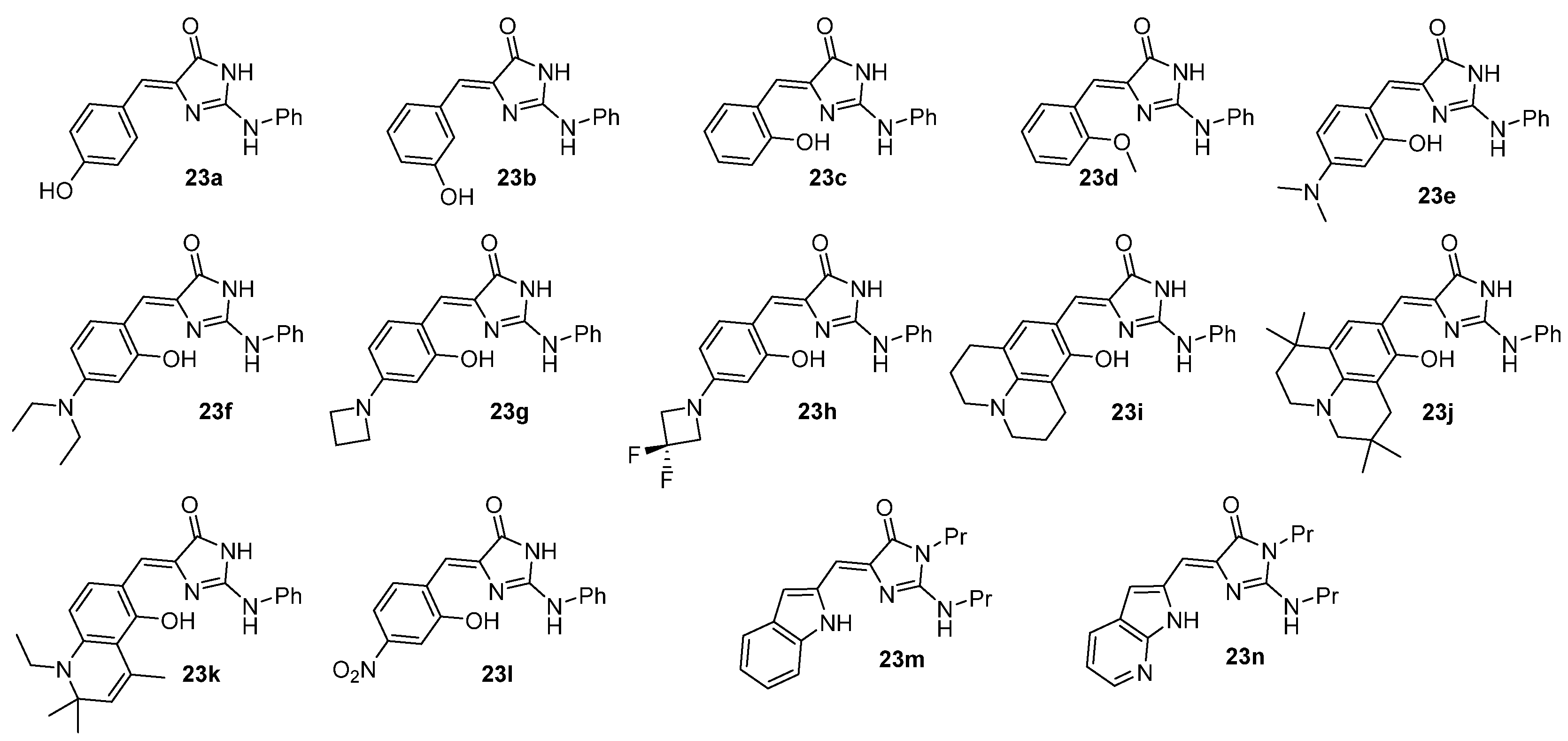
3.1. Intramolecular Hydrogen Bond
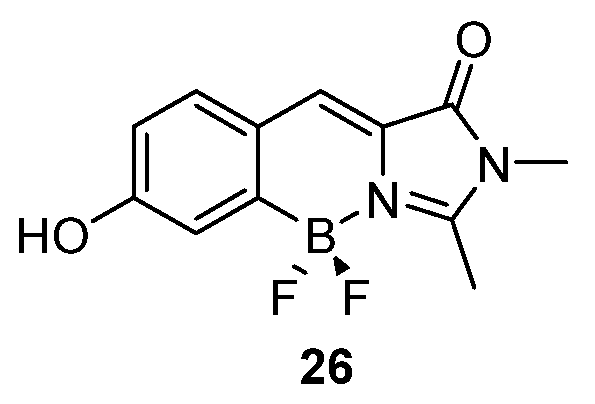
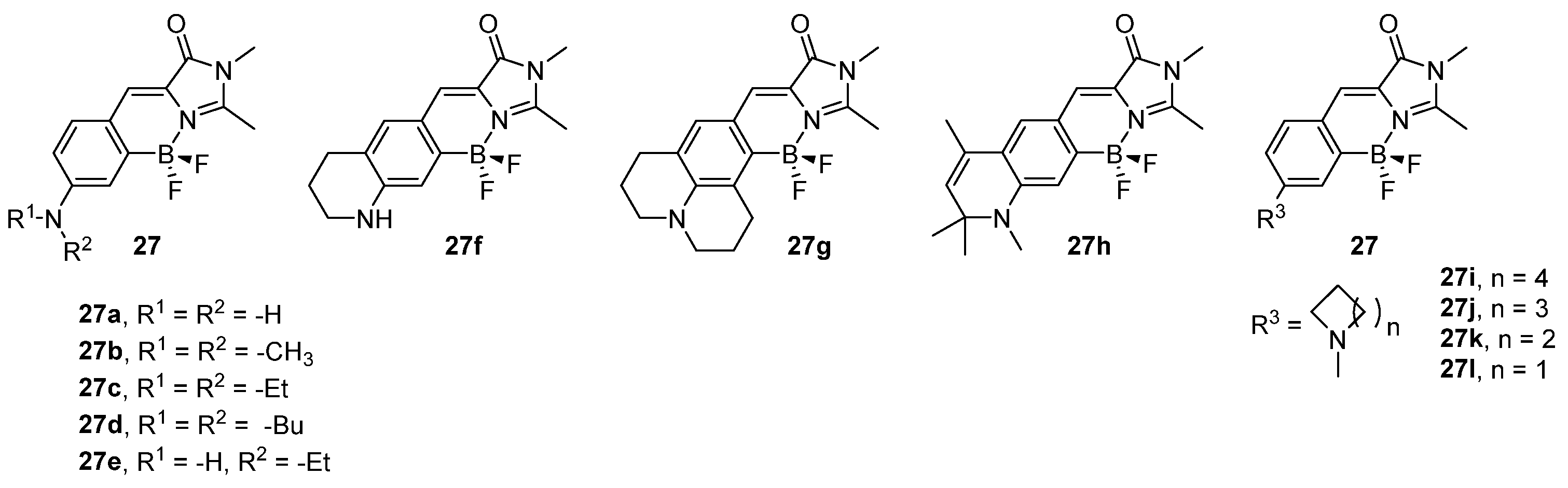
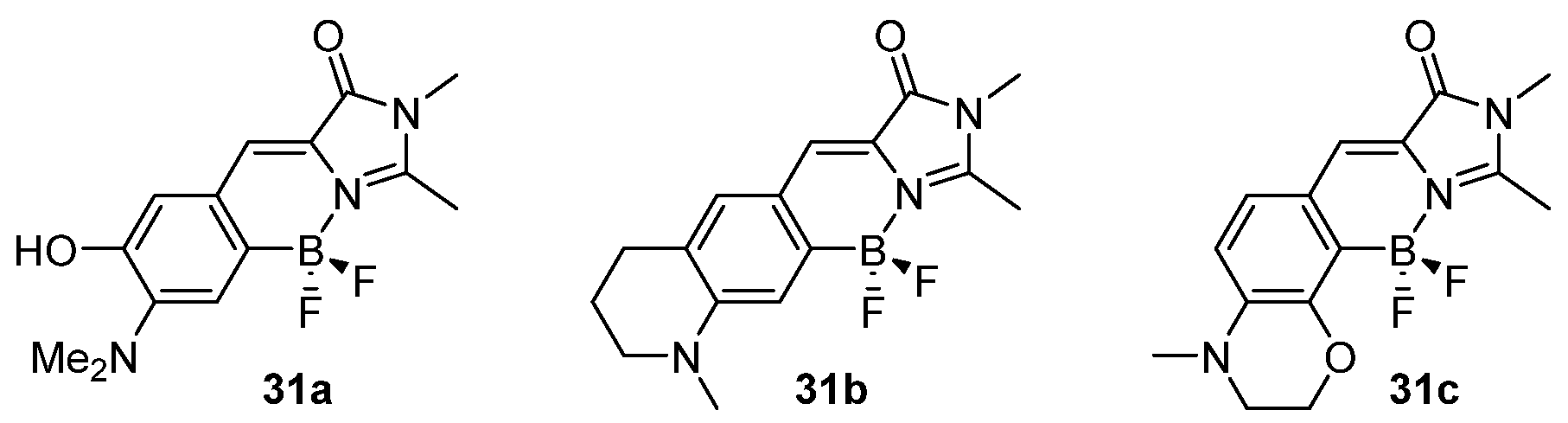
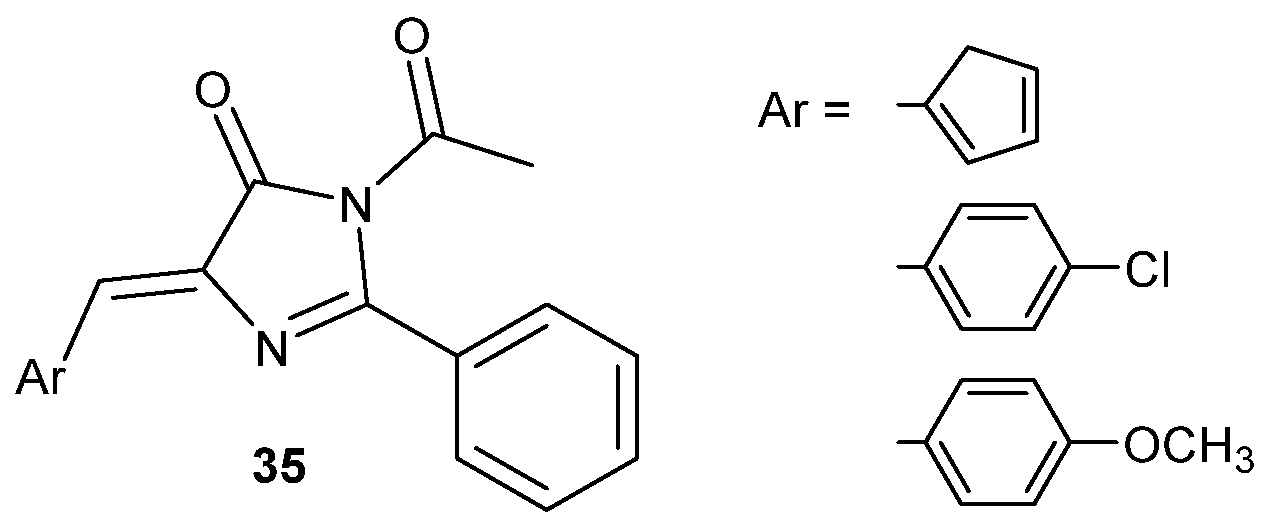
3.2. Boron Complexes
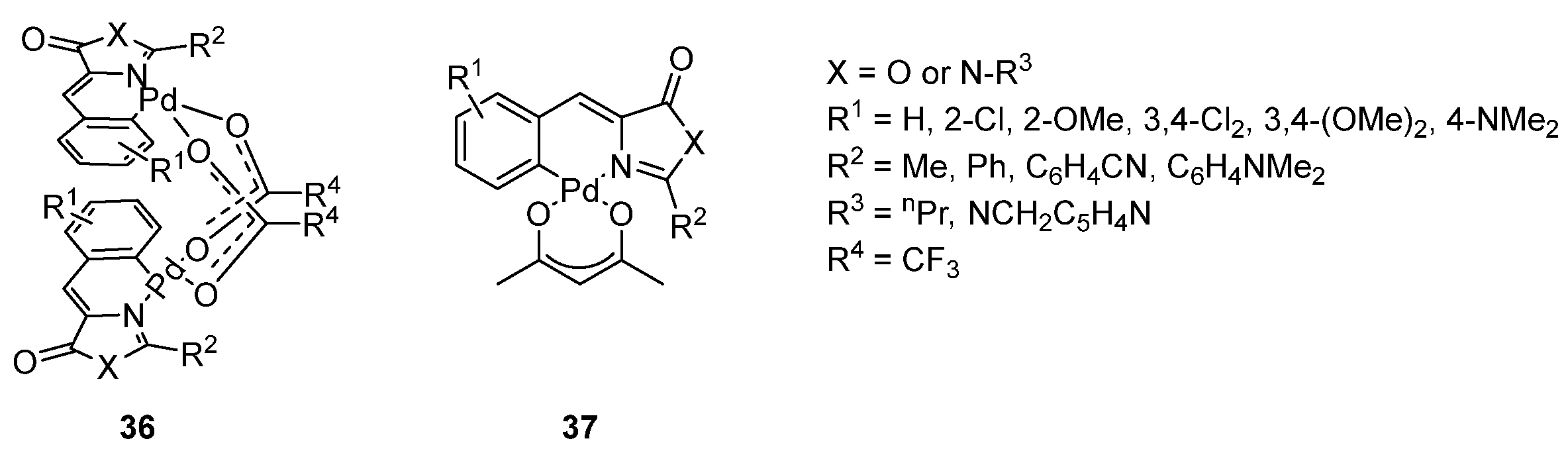
3.3. Metal Complexes
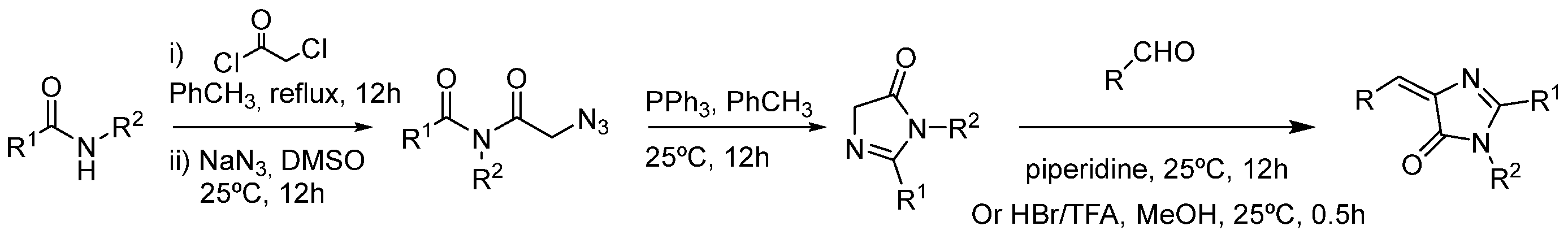
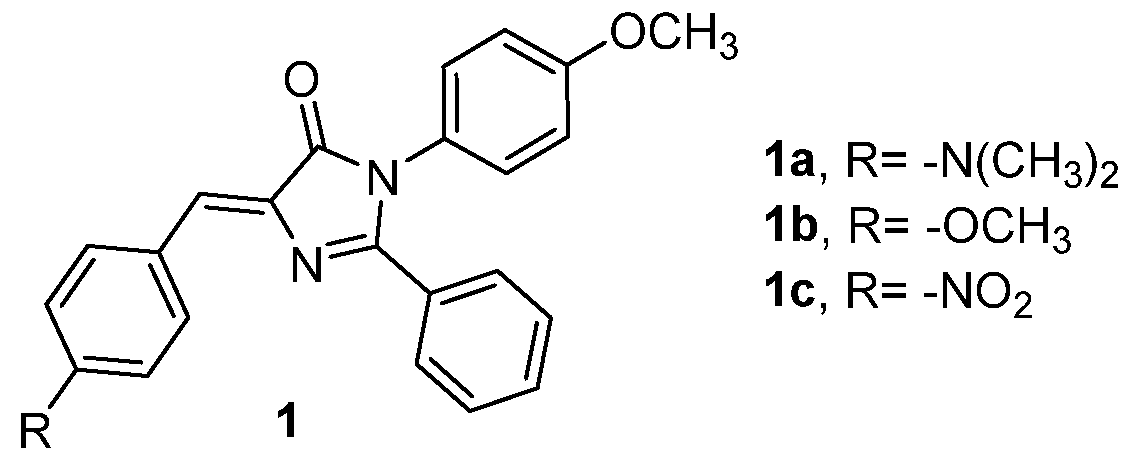
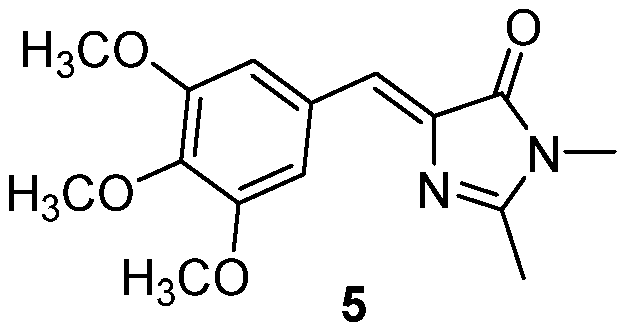
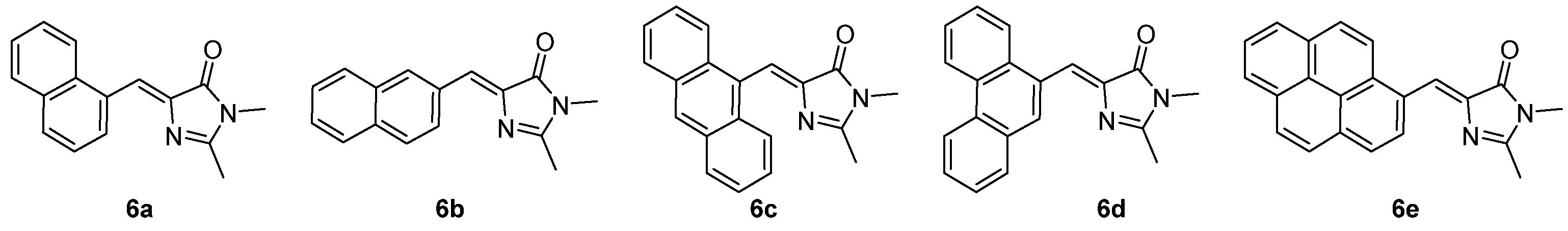
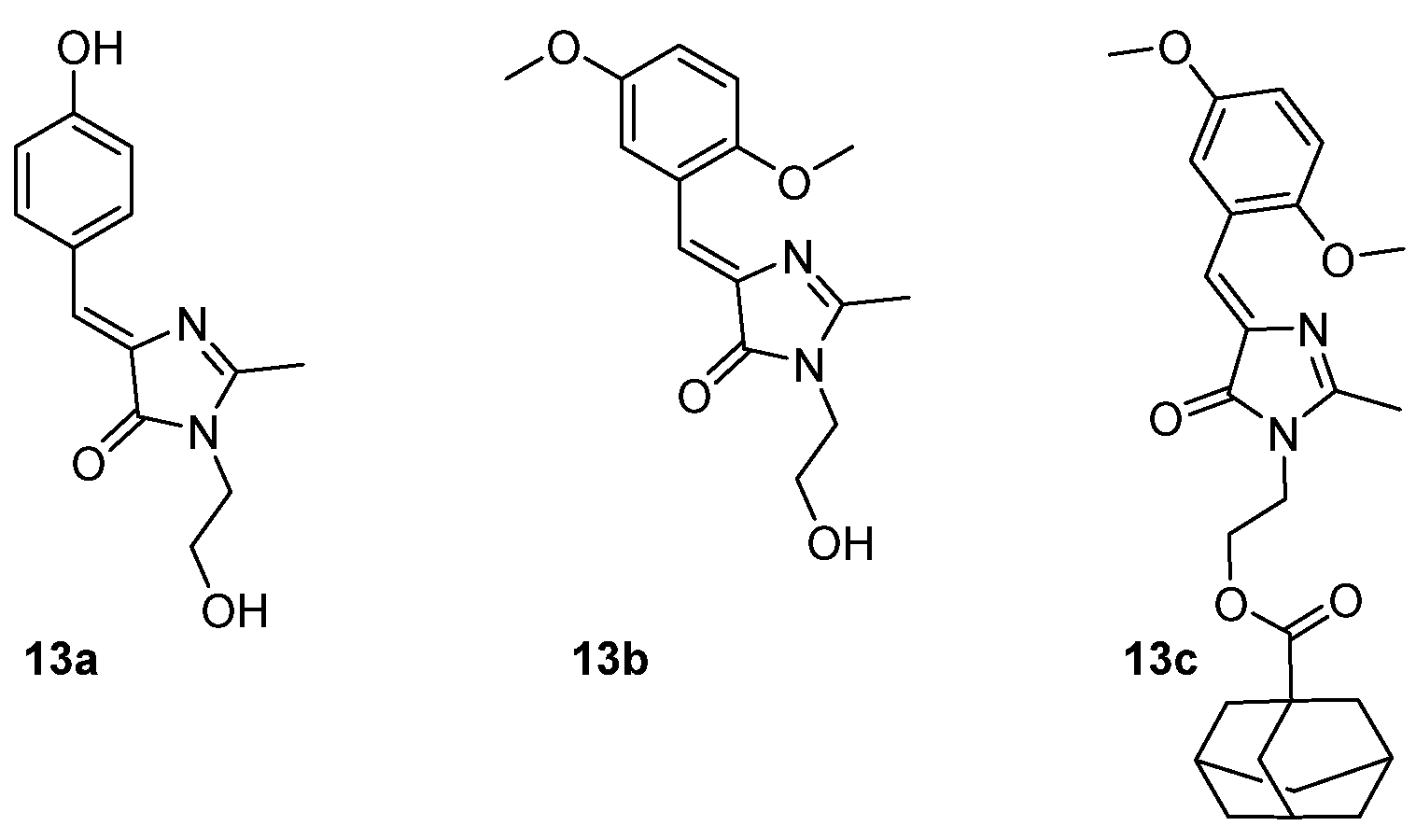
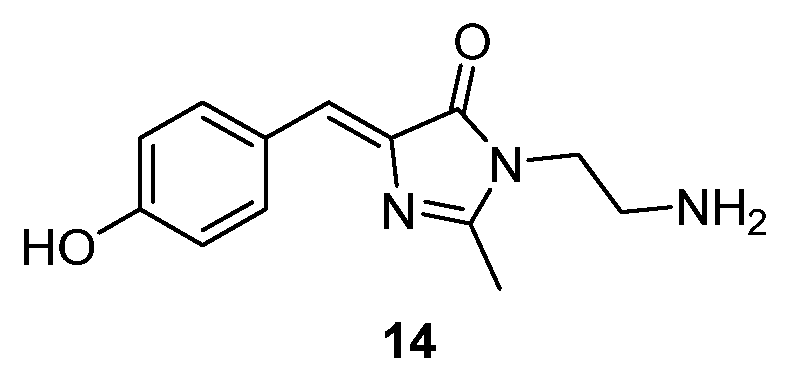
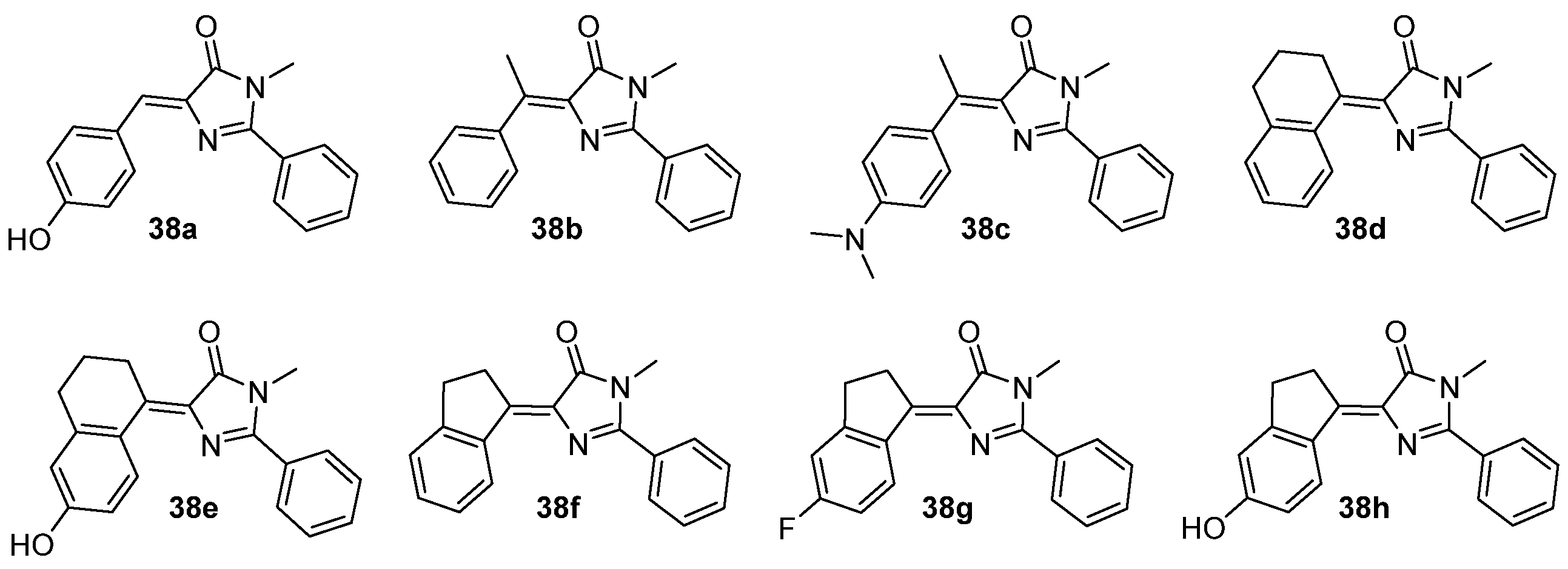
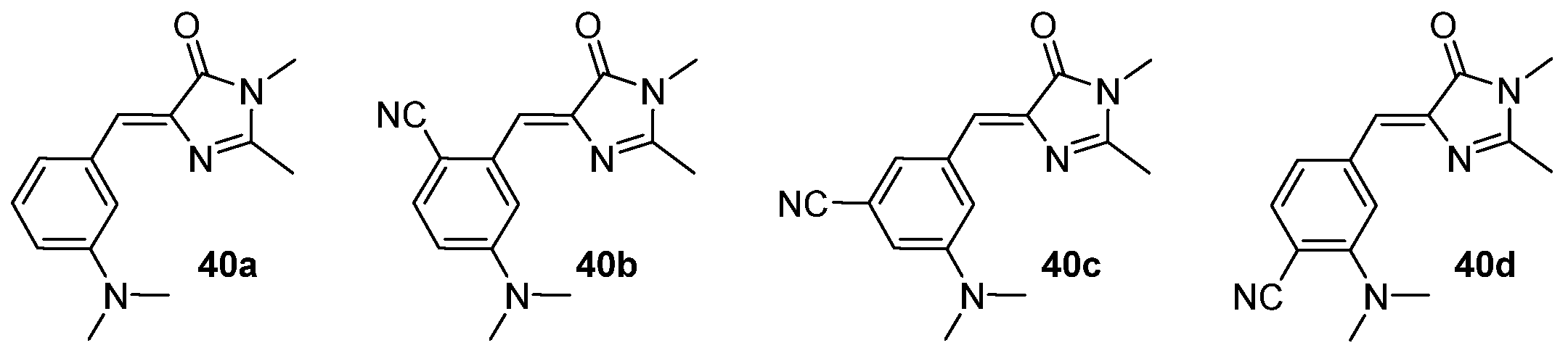
3.4. Covalent Modifications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omary, M.A.; Patterson, H.H. Luminescence, Theory. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 636–653. ISBN 9780128032244. [Google Scholar]
- Jabłoński, A. Über Den Mechanismus Der Photolumineszenz von Farbstoffphosphoren. Z. Für Phys. 1935, 94, 38–46. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem.-A Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.; Verkhusha, V.; Kuznetsova, I.; Uversky, V.; Turoverov, K. Fluorescent Proteins as Biomarkers and Biosensors: Throwing Color Lights on Molecular and Cellular Processes. Curr. Protein Pept. Sci. 2008, 9, 338–369. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, B.; Azab, M.M.; Gamil, M.; El-Feky, H.H. Enhancement for the Fluorescent Properties of New Synthesized GFPs Chromophore. Egypt. J. Chem. 2021, 64, 3717–3727. [Google Scholar] [CrossRef]
- Remington, S.J. Green Fluorescent Protein: A Perspective. Protein Sci. 2011, 20, 1509–1519. [Google Scholar] [CrossRef]
- Yu, C.; Hao, E.; Fang, X.; Wu, Q.; Wang, L.; Li, J.; Xu, L.; Jiao, L.; Wong, W.Y. AIE-Active Difluoroboronated Acylhydrozone Dyes (BOAHY) Emitting across the Entire Visible Region and Their Photo-Switching Properties. J. Mater. Chem. C 2019, 7, 3269–3277. [Google Scholar] [CrossRef]
- Wu, L.; Burgess, K. Syntheses of Highly Fluorescent GFP-Chromophore Analogues. J. Am. Chem. Soc. 2008, 130, 4089–4096. [Google Scholar] [CrossRef]
- Shen, X.; Huang, G.; Li, K.; Zhang, G.; Zhang, D. Tuning the Solid-State Emission of the Analogous GFP Chromophore by Varying Alkyl Chains in the Imidazolinone Ring. Sci. China Chem. 2013, 56, 1197–1203. [Google Scholar] [CrossRef]
- Dong, J.; Solntsevn, K.M.; Tolbert, L.M. Activation and Tuning of Green Fluorescent Protein Chromophore Emission by Alkyl Substituent-Mediated Crystal Packing. J. Am. Chem. Soc. 2009, 131, 662–670. [Google Scholar] [CrossRef]
- Cacciarini, M.; Nielsen, M.B.; de Castro, E.M.; Marinescu, L.; Bols, M. β-Cyclodextrin as a Mimetic of the Natural GFP-Chromophore Environment. Tetrahedron Lett. 2012, 53, 973–976. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Liu, Z.P.; James, T.D.; Long, Y.T. Simultaneous Determination of Hg(II) and Zn(II) Using a GFP Inspired Chromophore. Talanta 2012, 100, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Erlenmeyer-Plöchl Azlactone Synthesis. Compr. Org. Name React. Reag. 2010, 217, 997–1000. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; da Silva Fonseca, I.; Rocha, J.; Silva, A.M.S.; Guieu, S. Synthesis and Luminescence Properties of Analogues of the Green Fluorescent Protein Chromophore. Dye. Pigment. 2020, 177, 108267. [Google Scholar] [CrossRef]
- Ghodbane, A.; Brett Fellows, W.; Bright, J.R.; Ghosh, D.; Saffon, N.; Tolbert, L.M.; Fery-Forgues, S.; Solntsev, K.M. Effects of the Benzoxazole Group on Green Fluorescent Protein Chromophore Crystal Structure and Solid State Photophysics. J. Mater. Chem. C 2016, 4, 2793–2801. [Google Scholar] [CrossRef]
- Carayon, C.; Ghodbane, A.; Gibot, L.; Dumur, R.; Wang, J.; Saffon, N.; Rols, M.P.; Solntsev, K.M.; Fery-Forgues, S. Conjugates of Benzoxazole and GFP Chromophore with Aggregation-Induced Enhanced Emission: Influence of the Chain Length on the Formation of Particles and on the Dye Uptake by Living Cells. Small 2016, 12, 6602–6612. [Google Scholar] [CrossRef]
- Carayon, C.; André-Barrès, C.; Leygue, N.; Saffon-Merceron, N.; Boggio-Pasqua, M.; Fery-Forgues, S. The Role of the Synthetic Chromophore of GFP in Generating Polymorphism-Dependent on/off Photoluminescence. Dye. Pigment. 2021, 187, 109119. [Google Scholar] [CrossRef]
- Mali, B.P.; Dash, S.R.; Nikam, S.B.; Puthuvakkal, A.; Vanka, K.; Manoj, K.; Gonnade, R.G. Five Concomitant Polymorphs of a Green Fluorescent Protein Chromophore (GFPc) Analogue: Understanding Variations in Photoluminescence with π-Stacking Interactions. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 850–864. [Google Scholar] [CrossRef]
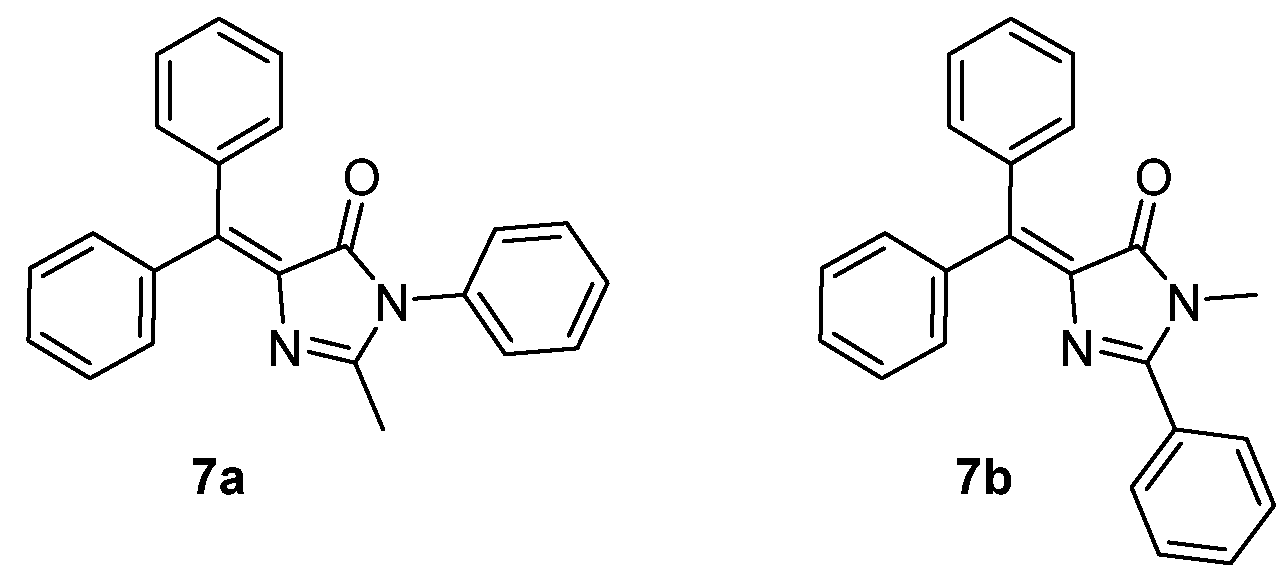
- Huang, G.; Zhang, G.; Wu, Y.; Liao, Q.; Fu, H.; Zhang, D. Modification of the Green Fluorescent Protein Chromophore with Large Aromatic Moieties: Photophysical Study and Solid-State Emission. Asian J. Org. Chem. 2012, 1, 352–358. [Google Scholar] [CrossRef]
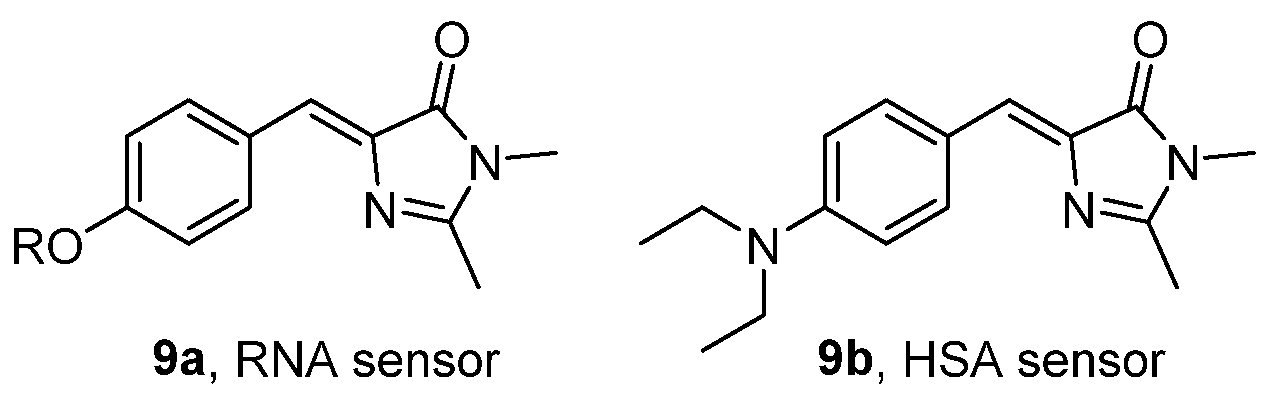
- Ikejiri, M.; Matsumoto, K.; Hasegawa, H.; Yamaguchi, D.; Tsuchino, M.; Chihara, Y.; Yamaguchi, T.; Mori, K.; Imanishi, T.; Obika, S.; et al. Synthesis and Fluorescence Properties of 4-Diarylmethylene Analogues of the Green Fluorescent Protein Chromophore. Tetrahedron 2015, 71, 4987–4998. [Google Scholar] [CrossRef]
- Baldridge, A.; Samanta, S.R.; Jayaraj, N.; Ramamurthy, V.; Tolbert, L.M. Activation of Fluorescent Protein Chromophores by Encapsulation. J. Am. Chem. Soc. 2010, 132, 1498–1499. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Baldridge, A.; Feng, S.; SiQiang, Y.; Kim, Y.K.; Tolbert, L.M.; Chang, Y.-T. Fluorescence Response Profiling for Small Molecule Sensors Utilizing the Green Fluorescent Protein Chromophore and Its Derivatives. ACS Comb. Sci. 2011, 13, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, A.; Feng, S.; Chang, Y.-T.; Tolbert, L.M. Recapture of GFP Chromophore Fluorescence in a Protein Host. ACS Comb. Sci. 2011, 13, 214–217. [Google Scholar] [CrossRef]
- Baldridge, A.; Amador, A.; Tolbert, L.M. Fluorescence Turn On by Cholate Aggregates. Langmuir 2011, 27, 3271–3274. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, C.; Gong, L.; Zhu, X. Self-Restricted Green Fluorescent Protein Chromophore Analogues: Dramatic Emission Enhancement and Remarkable Solvatofluorochromism. J. Phys. Chem. Lett. 2016, 7, 2935–2944. [Google Scholar] [CrossRef]
- Ge, S.; Deng, H.; Su, Y.; Zhu, X. Emission Enhancement of GFP Chromophore in Aggregated State via Combination of Self-Restricted Effect and Supramolecular Host–Guest Complexation. RSC Adv. 2017, 7, 17980–17987. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, F.; Wu, M.; Tian, Y.; Niu, Z. Confined Chromophores in Tobacco Mosaic Virus to Mimic Green Fluorescent Protein. Chem. Commun. 2015, 51, 15122–15124. [Google Scholar] [CrossRef]
- Sun, H.; Leng, H.X.; Liu, J.S.; Roy, G.; Yan, J.W.; Zhang, L. A Novel Fluorescent Protein Chromophore Analogue to Simultaneously Probe Lysosome Viscosity and β-Amyloid Fibrils. Sens. Actuators B Chem. 2020, 305, 127509. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, G.; Deng, H.; Su, Y.; Liu, J.; Zhu, X. Temperature-Induced Fluorescence Enhancement of GFP Chromophore Containing Copolymers for Detection of Bacillus Thermophilus. Polym. Chem. 2014, 5, 2521–2529. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, Q.; Wang, D.; Tu, C.; Zhu, B.; Zhu, X. GFP-Inspired Fluorescent Polymer. Polym. Chem. 2012, 3, 1975. [Google Scholar] [CrossRef]
- Deng, H.; Su, Y.; Hu, M.; Jin, X.; He, L.; Pang, Y.; Dong, R.; Zhu, X. Multicolor Fluorescent Polymers Inspired from Green Fluorescent Protein. Macromolecules 2015, 48, 5969–5979. [Google Scholar] [CrossRef]
- Singh, A.; Samanta, D.; Boro, M.; Maji, T.K. Gfp Chromophore Integrated Conjugated Microporous Polymers: Topological and ESPT Effects on Emission Properties. Chem. Commun. 2019, 55, 2837–2840. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Dolgopolova, E.A.; Pellechia, P.J.; Palukoshka, A.; Wilson, T.J.; Tan, R.; Maier, J.M.; Greytak, A.B.; Smith, M.D.; Krause, J.A.; et al. Mimic of the Green Fluorescent Protein β-Barrel: Photophysics and Dynamics of Confined Chromophores Defined by a Rigid Porous Scaffold. J. Am. Chem. Soc. 2015, 137, 2223–2226. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Cheng, Y.-M.; Lai, C.-H.; Hsu, C.-C.; Ho, M.-L.; Lee, G.-H.; Chou, P.-T. Ortho Green Fluorescence Protein Synthetic Chromophore; Excited-State Intramolecular Proton Transfer via a Seven-Membered-Ring Hydrogen-Bonding System. J. Am. Chem. Soc. 2007, 129, 4534–4535. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-T.; Hsieh, C.-C.; Lai, C.-H.; Lai, C.-H.; Shih, C.-W.; Chen, K.-Y.; Hung, W.-Y.; Hsu, Y.-H.; Chou, P.-T. Excited-State Intramolecular Proton Transfer Molecules Bearing o -Hydroxy Analogues of Green Fluorescent Protein Chromophore. J. Org. Chem. 2011, 76, 8189–8202. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.A.; Tseng, H.W.; Zhang, Z.; Shen, J.Y.; Chuang, W.T.; Lin, T.C.; Lee, C.S.; Hung, W.Y.; Hong, B.C.; et al. Locked Ortho- and Para -Core Chromophores of Green Fluorescent Protein; Dramatic Emission Enhancement via Structural Constraint. J. Am. Chem. Soc. 2014, 136, 11805–11812. [Google Scholar] [CrossRef]
- Kovács, E.; Cseri, L.; Jancsó, A.; Terényi, F.; Fülöp, A.; Rózsa, B.; Galbács, G.; Mucsi, Z. Synthesis and Fluorescence Mechanism of the Aminoimidazolone Analogues of the Green Fluorescent Protein: Towards Advanced Dyes with Enhanced Stokes Shift, Quantum Yield and Two-Photon Absorption. Eur. J. Org. Chem. 2021, 2021, 5649–5660. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Martínez-Lõpez, D.; Morõn, M.; Sucunza, D.; Sampedro, D.; Domingo, A.; Salgado, A.; Vaquero, J.J. Highly Fluorescent Green Fluorescent Protein Chromophore Analogues Made by Decorating the Imidazolone Ring. Chem.-A Eur. J. 2015, 21, 18758–18763. [Google Scholar] [CrossRef]
- Baleeva, N.S.; Zaitseva, S.O.; Gorbachev, D.A.; Smirnov, A.Y.; Zagudaylova, M.B.; Baranov, M.S. The Role of N-Substituents in Radiationless Deactivation of Aminated Derivatives of a Locked GFP Chromophore. Eur. J. Org. Chem. 2017, 2017, 5219–5224. [Google Scholar] [CrossRef]
- Baranov, M.S.; Lukyanov, K.A.; Borissova, A.O.; Shamir, J.; Kosenkov, D.; Slipchenko, L.V.; Tolbert, L.M.; Yampolsky, I.V.; Solntsev, K.M. Conformationally Locked Chromophores as Models of Excited-State Proton Transfer in Fluorescent Proteins. J. Am. Chem. Soc. 2012, 134, 6025–6032. [Google Scholar] [CrossRef]
- Baleeva, N.S.; Tsarkova, A.S.; Baranov, M.S. Conformationally Locked Chromophores of CFP and Sirius Protein. Tetrahedron Lett. 2016, 57, 3043–3045. [Google Scholar] [CrossRef]
- Baleeva, N.S.; Myannik, K.A.; Yampolsky, I.V.; Baranov, M.S. Bioinspired Fluorescent Dyes Based on a Conformationally Locked Chromophore of the Fluorescent Protein Kaede. Eur. J. Org. Chem. 2015, 2015, 5716–5721. [Google Scholar] [CrossRef]
- Baranov, M.S.; Solntsev, K.M.; Baleeva, N.S.; Mishin, A.S.; Lukyanov, S.A.; Lukyanov, K.A.; Yampolsky, I.V. Red-Shifted Fluorescent Aminated Derivatives of a Conformationally Locked GFP Chromophore. Chem.-A Eur. J. 2014, 20, 13234–13241. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, S.O.; Farkhutdinova, D.A.; Baleeva, N.S.; Smirnov, A.Y.; Zagudaylova, M.B.; Shakhov, A.M.; Astafiev, A.A.; Baranov, M.S.; Bochenkova, A.V. Excited-State Locked Amino Analogues of the Green Fluorescent Protein Chromophore with a Giant Stokes Shift. RSC Adv. 2019, 9, 38730–38734. [Google Scholar] [CrossRef]
- Baleeva, N.S.; Khavroshechkina, A.V.; Zaitseva, E.R.; Myasnyanko, I.N.; Zagudaylova, M.B.; Baranov, M.S. Naphthalene Derivatives of a Conformationally Locked GFP Chromophore with Large Stokes Shifts. Tetrahedron Lett. 2019, 60, 150963. [Google Scholar] [CrossRef]
- Chen, C.; Boulanger, S.A.; Sokolov, A.I.; Baranov, M.S.; Fang, C. A Novel Dialkylamino GFP Chromophore as an Environment-Polarity Sensor Reveals the Role of Twisted Intramolecular Charge Transfer. Chemosensors 2021, 9, 234. [Google Scholar] [CrossRef]
- Baldridge, A.; Solntsev, K.M.; Song, C.; Tanioka, T.; Kowalik, J.; Hardcastle, K.; Tolbert, L.M. Inhibition of Twisting of a Green Fluorescent Protein-like Chromophore by Metal Complexation. Chem. Commun. 2010, 46, 5686–5688. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, L.; Qin, L.-X.; Qu, L.-L.; Jing, C.; Lan, M.; James, T.D.; Long, Y.-T. An OFF–ON Fluorescent Probe for Zn2+ Based on a GFP-Inspired Imidazolone Derivative Attached to a 1,10-Phenanthroline Moiety. Chem. Commun. 2011, 47, 4361. [Google Scholar] [CrossRef]
- Collado, S.; Pueyo, A.; Baudequin, C.; Bischoff, L.; Jiménez, A.I.; Cativiela, C.; Hoarau, C.; Urriolabeitia, E.P. Orthopalladation of GFP-Like Fluorophores Through C-H Bond Activation: Scope and Photophysical Properties. Eur. J. Org. Chem. 2018, 2018, 6158–6166. [Google Scholar] [CrossRef]
- Chatterjee, S.; Karuso, P. An Efficient and Concise Method to Synthesize Locked GFP Chromophore Analogues. Tetrahedron Lett. 2016, 57, 5197–5200. [Google Scholar] [CrossRef]
- Tsai, M.S.; Ou, C.L.; Tsai, C.J.; Huang, Y.C.; Cheng, Y.C.; Sun, S.S.; Yang, J.S. Fluorescence Enhancement of Unconstrained GFP Chromophore Analogues Based on the Push-Pull Substituent Effect. J. Org. Chem. 2017, 82, 8031–8039. [Google Scholar] [CrossRef] [PubMed]


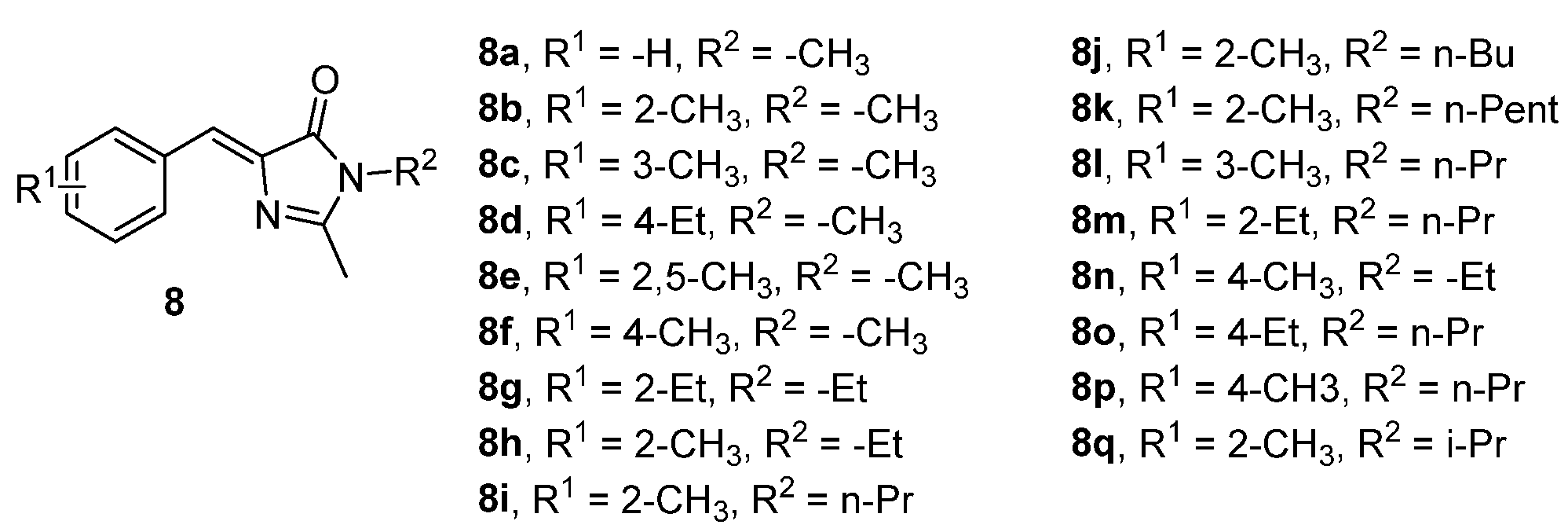
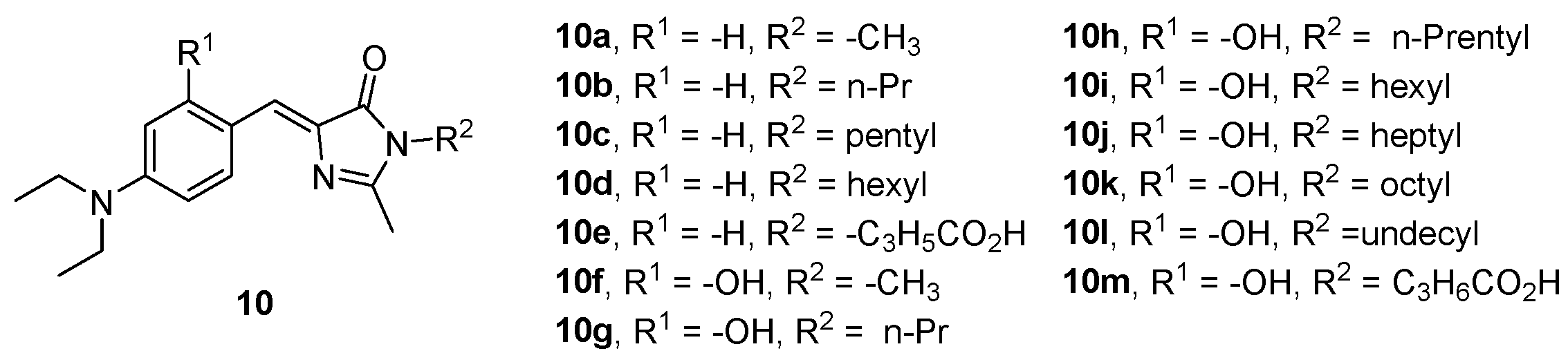
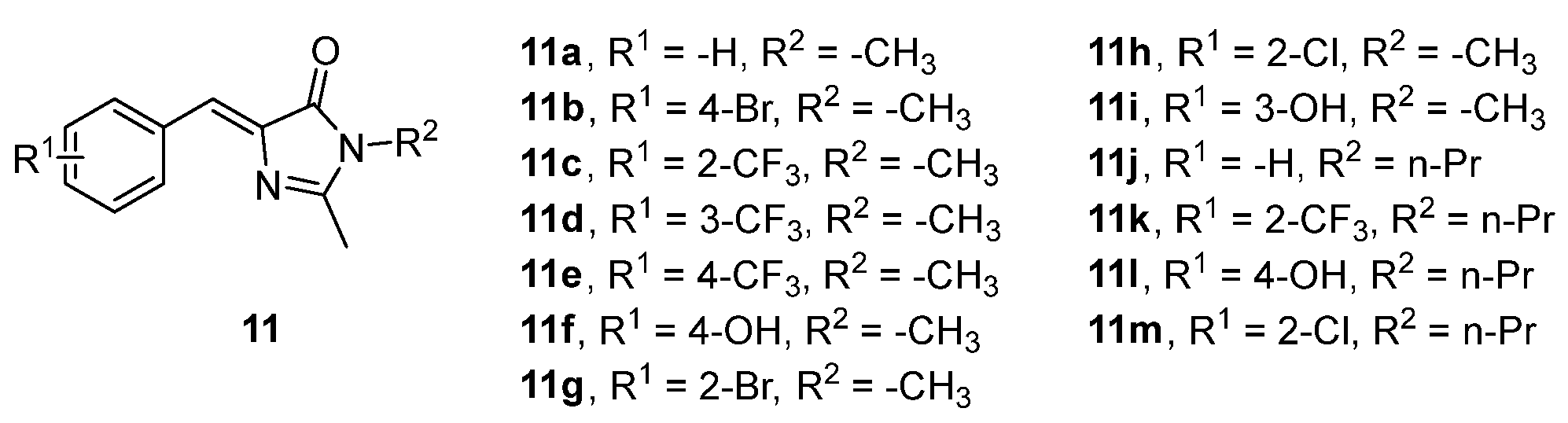




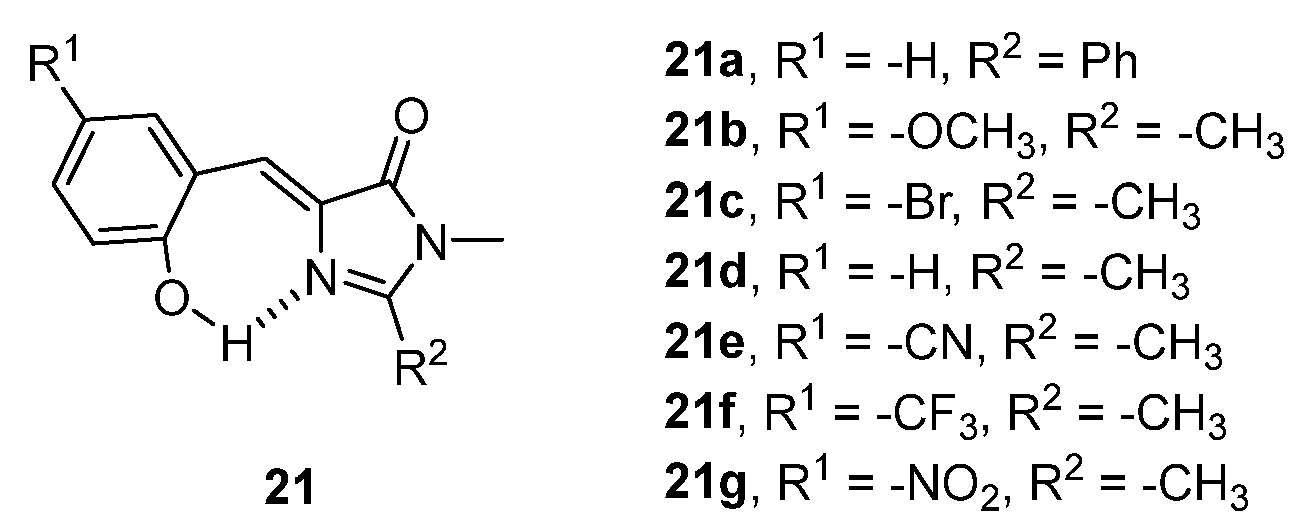




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.R.M.; Esteves, C.I.C.; Marques, M.M.B.; Guieu, S. Locking the GFP Fluorophore to Enhance Its Emission Intensity. Molecules 2023, 28, 234. https://doi.org/10.3390/molecules28010234
Ferreira JRM, Esteves CIC, Marques MMB, Guieu S. Locking the GFP Fluorophore to Enhance Its Emission Intensity. Molecules. 2023; 28(1):234. https://doi.org/10.3390/molecules28010234
Chicago/Turabian StyleFerreira, Joana R. M., Cátia I. C. Esteves, Maria Manuel B. Marques, and Samuel Guieu. 2023. "Locking the GFP Fluorophore to Enhance Its Emission Intensity" Molecules 28, no. 1: 234. https://doi.org/10.3390/molecules28010234
APA StyleFerreira, J. R. M., Esteves, C. I. C., Marques, M. M. B., & Guieu, S. (2023). Locking the GFP Fluorophore to Enhance Its Emission Intensity. Molecules, 28(1), 234. https://doi.org/10.3390/molecules28010234














