Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora
Abstract
:1. Introduction
2. Results
2.1. Identification and Protein Analysis of LPs (<1 kDa)
2.2. Effect of LPs on the Renal Index of Mice
2.3. Effect of LPs on the Renal Function of Mice
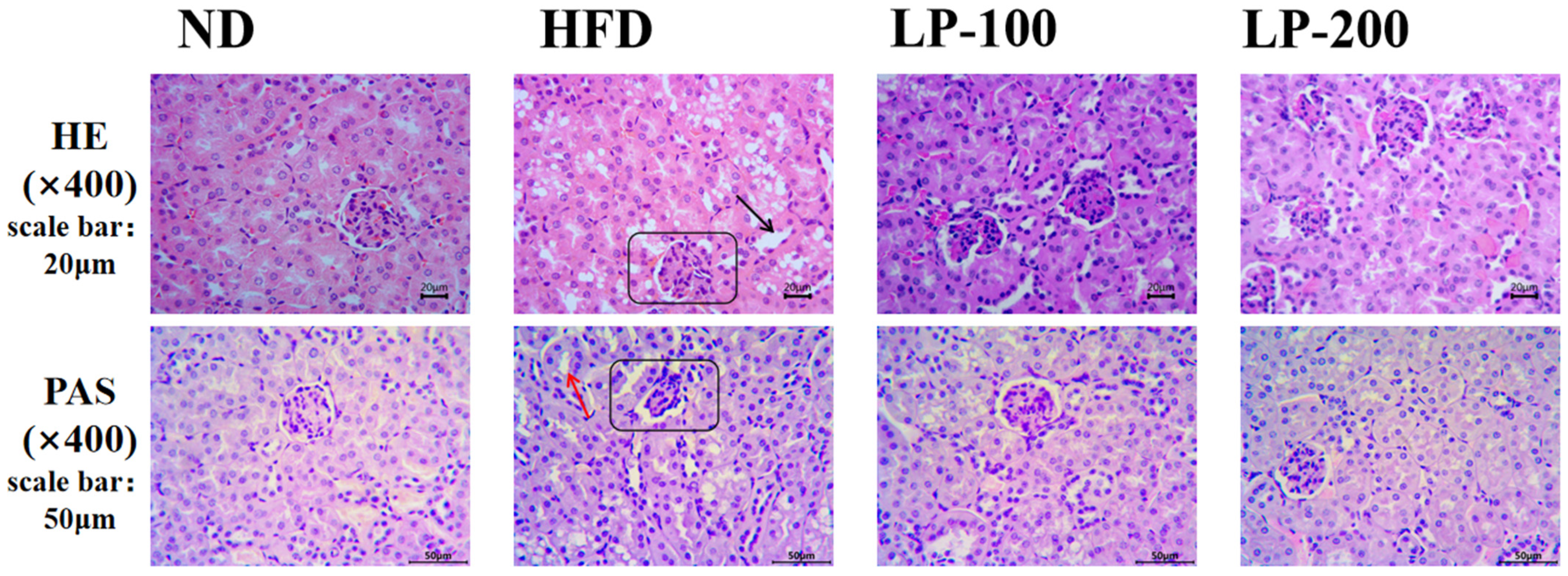
2.4. Effect of LPs on Renal Histomorphology of Mice
2.5. Effect of LPs on Antioxidant Levels in Mouse Kidneys
2.6. Effect of LPs on Inflammatory Factors in the Mice’s Kidneys
2.7. Effect of LPs on Expression of Nrf2-Pathway-Related Proteins
2.8. Effect of LPs on Expression of NF-κB-Pathway-Related Pathway Proteins
2.9. Sequencing Results of Intestinal Microflora
2.10. α-Diversity Analysis of Intestinal Flora
2.11. β-Diversity Analysis and UPGMA Analysis of Intestinal Microbiota in Mice
2.12. Mice Intestinal Microbiota Analysis Using Metastats
2.13. Lda Effect Size (LEfSe) Analysis of Intestinal Microflora in Mice
2.14. Functional Prediction of Intestinal Microbiota in Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Muscle Peptides from Monkfish
4.2. Identification Analysis and Protein Analysis of LPs (<1 kDa)
4.3. Animals and Treatments
4.4. Detection of Renal Function Index
4.5. Indices of Oxidative Stress Detection
4.6. Analysis of Proinflammatory Factors in Renal Tissue
4.7. Histopathological Analysis
4.8. Tissue Protein Extraction and Western Blotting
4.9. Collection of Mice Feces
4.10. Intestinal Microflora Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, L.; Jiang, F.; Zhang, Z.; Wang, X.; Li, H.; Kuang, Y.; Yang, G. Catheter-Based renal denervation attenuates kidney interstitial fibrosis in a canine model of high-fat diet-induced hypertension. Kidney Blood Press. Res. 2019, 44, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J. Chicoric acid ameliorates nonalcoholic fatty liver disease via the AMPK/Nrf2/NF-κB signaling pathway and restores gut microbiota in high-fat-diet-fed mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, J.-G.; Liu, Z.; Gong, X.-J.; Hu, J.-N.; Wang, Y.-P.; Liu, W.-C.; Lin, X.-H.; Wang, Z.; Li, W. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 2021, 267, 113500. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.F.; El-Nahas, M.; Chan, M.K.; Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstital disease. Lancet 1982, 320, 1309–1311. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.J.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Du, X.-G.; Ruan, X.-Z. Lipid Metabolism Disorder and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 525–541. [Google Scholar]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell. Death. Dis. 2020, 11, 914. [Google Scholar] [CrossRef]
- Kim, S.; Indu Viswanath, A.N.; Park, J.-H.; Lee, H.E.; Park, A.Y.; Choi, J.W.; Kim, H.J.; Londhe, A.M.; Jang, B.K.; Lee, J.; et al. Nrf2 activator via interference of Nrf2-Keap1 interaction has antioxidant and anti-inflammatory properties in Parkinson’s disease animal model. Neuropharmacology 2020, 167, 107989. [Google Scholar] [CrossRef]
- Yuan, Y.; Naito, H.; Jia, X.; Kitamori, K.; Nakajima, T. Combination of hypertension along with a high fat and cholesterol diet induces severe hepatic inflammation in rats via a signaling network comprising NF-κB, MAPK, and Nrf2 pathways. Nutrients 2017, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Su, Y. Remdesivir attenuates high fat diet (HFD)-induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem. Biophys. Res. Commun. 2020, 526, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Madduma Hewage, S.; Prashar, S.; Debnath, S.C.; O, K.; Siow, Y.L. Inhibition of inflammatory cytokine expression prevents high-fat diet-induced kidney injury: Role of lingonberry supplementation. Front. Med. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Parks, B.W.; Joo, J.W.J.; Emert, B.; Schwartzman, W.; Kang, E.Y.; Mehrabian, M.; Pan, C.; Knight, R.; Gunsalus, R.; et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. 2015, 25, 1558–1569. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.-L.; Li, Y.; Wang, Y.; Feng, Y. MDG-1, an Ophiopogon polysaccharide, regulate gut microbiota in high-fat diet-induced obese C57BL/6 mice. Int. J. Biol. Macromol. 2015, 81, 576–583. [Google Scholar] [CrossRef]
- Mishima, E.; Ichijo, M.; Kawabe, T.; Kikuchi, K.; Akiyama, Y.; Toyohara, T.; Suzuki, T.; Suzuki, C.; Asao, A.; Ishii, N.; et al. Germ-free conditions modulate host purine metabolism, exacerbating adenine-induced kidney damage. Toxins 2020, 12, 547. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut–kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Nicholson Jeremy, K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Wu Gary, D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh Sue, A.; Bewtra, M.; Knights, D.; Walters William, A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar]
- Evenepoel, P.; Meijers, B.K.I.; Bammens, B.R.M.; Verbeke, K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E. Intestinal-Renal Syndrome: Mirage or Reality? Blood Purif. 2011, 31, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive peptides from marine ascidians and future drug development–A review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-F.; Li, G.-Z.; Peng, H.-B.; Li, Y.; Zhang, F.; Chen, Y. Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am. J. Med. Sci. 2010, 340, 360–366. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Su, X. Analysis of urine composition in type II diabetic mice after intervention therapy using holothurian polypeptides. Front. Chem. 2017, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Zheng, D.; Yang, G.; Li, W.; Yang, H.; Jiang, Q.; Chen, Y.; Zhang, Y.; Xie, X. Tilapia skin peptides ameliorate diabetic nephropathy in STZ-induced diabetic rats and HG-induced GMCs by improving mitochondrial dysfunction. Mar. Drugs 2020, 18, 363. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, J.; Xu, B.; Ye, J.; Yang, Z.; Yuan, F. Optimization of extraction of bioactive peptides from monkfish (Lophius litulon) and characterization of their role in H2O2-induced lesion. Mar. Drugs 2020, 18, 468. [Google Scholar] [CrossRef]
- Ye, J.; Tian, X.; Wang, Q.; Zheng, J.; Yang, Y.; Xu, B.; Zhang, S.; Yuan, F.; Yang, Z. Monkfish peptides mitigate high fat diet-induced hepatic steatosis in mice. Mar. Drugs 2022, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.; Watanabe, E.; Fujimura, L.; Watanabe-Takano, H.; Yoshidome, H.; Swanson, P.E.; Tokuhisa, T.; Oda, S.; Hatano, M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit. Care 2013, 17, R160. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Juncos, L.A. Obesity-related CKD: When kidneys get the munchies. J. Am. Soc. Nephrol. 2017, 28, 3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Liu, Y.; Zhao, Y.; Li, M.; Guo, L. Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1. Free Radic. Biol. Med. 2020, 153, 140–158. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Hu, L.-F.; Lou, D.-S.; Wang, B.-C.; Tan, J. Targeting DUSP16/TAK1 signaling alleviates hepatic dyslipidemia and inflammation in high fat diet (HFD)-challenged mice through suppressing JNK MAPK. Biochem. Biophys. Res. Commun. 2020, 524, 142–149. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Gonzaga-Sánchez, G.; Tapia, E.; Muñoz-Jiménez, I.; Manterola-Romero, L.; Osorio-Alonso, H.; Arellano-Buendía, A.S.; Pedraza-Chaverri, J.; Roncal-Jiménez, C.A.; Lanaspa, M.A.; et al. Osthol ameliorates kidney damage and metabolic syndrome induced by a high-fat/high-sugar diet. Int. J. Mol. Sci. 2021, 22, 2431. [Google Scholar] [CrossRef]
- Xu, S.; Luo, W.; Xu, X.; Qian, Y.; Xu, Z.; Yu, W.; Shan, X.; Guan, X.; Lum, H.; Zhou, H.; et al. MD2 blockade prevents oxLDL-induced renal epithelial cell injury and protects against high-fat-diet-induced kidney dysfunction. J. Nutr. Biochem. 2019, 70, 47–55. [Google Scholar] [CrossRef]
- Selcuk, M.Y.; Aygen, B.; Dogukan, A.; Tuzcu, Z.; Akdemir, F.; Komorowski, J.R.; Atalay, M.; Sahin, K. Chromium picolinate and chromium histidinate protects against renal dysfunction by modulation of NF-κB pathway in high-fat diet fed and Streptozotocin-induced diabetic rats. Nutr. Metab. 2012, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Cheng, Z.; Sun, X.; Si, X.; Gong, E.; Wang, Y.; Tian, J.; Shu, C.; Ma, F.; Li, D.; et al. Lonicera caerulea L. Polyphenols alleviate oxidative stress-induced intestinal environment imbalance and lipopolysaccharide-induced liver injury in HFD-fed rats by regulating the Nrf2/HO-1/NQO1 and MAPK pathways. Mol. Nutr. Food Res. 2020, 64, 1901315. [Google Scholar] [CrossRef]
- Cao, N.; Ma, X.; Guo, Z.; Zheng, Y.; Geng, S.; Meng, M.; Du, Z.; Lin, H.; Duan, Y.; Du, G. Oral kanglaite injection (KLTI) attenuates the lung cancer-promoting effect of high-fat diet (HFD)-induced obesity. Oncotarget 2016, 7, 61093–61106. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet–induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Q.-R.; Ren, Y.-L.; Zhu, J.-J.; Hu, Y.-J.; Zheng, J.-S.; Fan, H.; Xu, Y.; Wang, G.; Liu, W.-X. Resveratrol increases nephrin and podocin expression and alleviates renal damage in rats fed a high-fat diet. Nutrients 2014, 6, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.H.S.; Andrade, J.M.O.; Fernandes, L.R.; Sinisterra, R.D.M.; Sousa, F.B.; Feltenberger, J.D.; Alvarez-Leite, J.I.; Santos, R.A.S. Oral Angiotensin-(1–7) prevented obesity and hepatic inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed with high-fat diet. Peptides 2013, 46, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.-H.; Shan, Q.; Mu, J.-J.; Wang, Y.-J.; Zhang, Z.-F.; Fan, S.-H.; Hu, B.; Li, M.-Q.; Xie, J.; Chen, P.; et al. Purple sweet potato color attenuates kidney damage by blocking VEGFR2/ROS/NLRP3 signaling in high-fat diet-treated mice. Oxidative Med. Cell. Longev. 2019, 2019, 5189819. [Google Scholar] [CrossRef]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Effects of inflammatory responses, apoptosis, and STAT3/NF-κB- and Nrf2-mediated oxidative stress on benign prostatic hyperplasia induced by a high-fat diet. Aging 2019, 11, 5570–5578. [Google Scholar] [CrossRef]
- Marinho, A.D.; Silveira, J.A.M.; Chaves-Filho, A.J.M.; Macedo, D.S.; Carmo, L.D.; Alencar, N.M.N.; Costa, P.H.S.; Lopes, P.L.; Nogueira-Junior, F.A.; Alves, N.T.Q.; et al. Protective effects of A Lipid transfer protein isolated from morinda citrifolia seeds in gentamicin-induced nephrotoxicity in rats. Rev. Bras. Farmacogn. 2020, 30, 568–576. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Ramprasath, T.; Saravanan, B.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Chrysin attenuates high-fat-diet-induced myocardial oxidative stress via upregulating eNOS and Nrf2 target genes in rats. Mol. Cell. Biochem. 2021, 476, 2719–2727. [Google Scholar] [CrossRef]
- Zhang, Y.-K.J.; Wu, K.C.; Liu, J.; Klaassen, C.D. Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol. Appl. Pharmacol. 2012, 264, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yılmaz, I.; Juturu, V. β-Cryptoxanthin ameliorates metabolic risk factors by regulating NF-κB and Nrf2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef]
- Orhan, C.; Er, B.; Deeh, P.B.D.; Bilgic, A.A.; Ojalvo, S.P.; Komorowski, J.R.; Sahin, K. Different sources of dietary magnesium supplementation reduces oxidative stress by regulation Nrf2 and NF-κB signaling pathways in high-fat diet rats. Biol. Trace Elem. Res. 2021, 199, 4162–4170. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef] [PubMed]
- De Minicis, S.; Rychlicki, C.; Agostinelli, L.; Saccomanno, S.; Candelaresi, C.; Trozzi, L.; Mingarelli, E.; Facinelli, B.; Magi, G.; Palmieri, C.; et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 2014, 59, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical properties and biocompatibility evaluation of collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Yu, F.; Zhang, G.; Yang, Z.; Huang, F.; Ding, G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef]









| Amino Acid Species | LPs |
|---|---|
| Asp | 5.82 ± 0.190 |
| Thr | 2.83 ± 0.083 |
| Ser | 2.89 ± 0.080 |
| Glu | 9.75 ± 0.297 |
| Gly | 2.53 ± 0.080 |
| Ala | 3.45 ± 0.107 |
| Cys | 0.00 |
| Val | 3.51 ± 0.096 |
| Met | 2.42 ± 0.051 |
| lle | 2.79 ± 0.079 |
| Leu | 5.57 ± 0.152 |
| Tyr | 1.81 ± 0.014 |
| Phe | 6.19 ± 0.079 |
| Lys | 5.48 ± 0.167 |
| His | 1.55 ± 0.102 |
| Arg | 3.70 ± 0.105 |
| Pro | 0.00 |
| HAA | 25.74 |
| PCAA | 10.73 |
| NCAA | 15.59 |
| EAA | 28.43 |
| ND | HFD | LP-100 | LP-200 | |
|---|---|---|---|---|
| Kidney index (%) | 0.011779 # | 0.012482 * | 0.011972 | 0.011744 # |
| ND | HFD | LP-100 | LP-200 | |
|---|---|---|---|---|
| BUN (mmol/L) | 7.374051 ## | 14.4893 ** | 12.43961 **## | 9.910283 **## |
| CRE (μmol/L) | 8.844319 ## | 15.07347 ** | 12.37732 **## | 10.31556 ## |
| UA (μmol/L) | 8.701216 ## | 18.93794 ** | 15.35509 **## | 9.724888 ## |
| ND | HFD | LP-100 | LP-200 | |
|---|---|---|---|---|
| SOD (U/mgprot) | 142.2944 ## | 41.63757 ** | 83.6647 **## | 124.3602 *## |
| CAT (U/mgprot) | 7.29797 ## | 3.28831 ** | 5.33356 **## | 6.227272 *## |
| T-AOC (U/mgprot) | 64.21501 ## | 33.64305 ** | 47.81102 **## | 54.3458 *## |
| MDA (nmol/mgprot) | 3.475465 ## | 7.23004 ** | 5.129147 **## | 4.09263 ## |
| GSH-Px (U/mgprot) | 28.39727 ## | 9.782904 ** | 17.85859 **## | 26.20776 ## |
| ND | HFD | LP-100 | LP-200 | |
|---|---|---|---|---|
| IL-6 (pg/mL) | 89.78946 ## | 117.9453 ** | 102.73 *# | 93.06537 ## |
| IL-1β (pg/mL) | 64.47391 ## | 99.95587 ** | 85.46253 **# | 72.53305 ## |
| TNF-α (pg/mL) | 26.36301 ## | 78.06664 ** | 64.16955 **# | 46.029 **## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Miao, B.; Cao, H.; Tian, X.; Shen, L.; Yang, Z.; Yuan, F.; Ding, Y. Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora. Molecules 2023, 28, 245. https://doi.org/10.3390/molecules28010245
Ren X, Miao B, Cao H, Tian X, Shen L, Yang Z, Yuan F, Ding Y. Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora. Molecules. 2023; 28(1):245. https://doi.org/10.3390/molecules28010245
Chicago/Turabian StyleRen, Xiangyu, Bingtao Miao, Hongjie Cao, Xiaoxiao Tian, Lujia Shen, Zuisu Yang, Falei Yuan, and Yaping Ding. 2023. "Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora" Molecules 28, no. 1: 245. https://doi.org/10.3390/molecules28010245
APA StyleRen, X., Miao, B., Cao, H., Tian, X., Shen, L., Yang, Z., Yuan, F., & Ding, Y. (2023). Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora. Molecules, 28(1), 245. https://doi.org/10.3390/molecules28010245







