Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine
Abstract
:1. Introduction
2. Historical Perspective
3. General Characterization of Nomilin
3.1. Structure and Chemistry
3.2. Natural Plant Extraction and Biosynthesis
3.2.1. Natural Plant Extraction
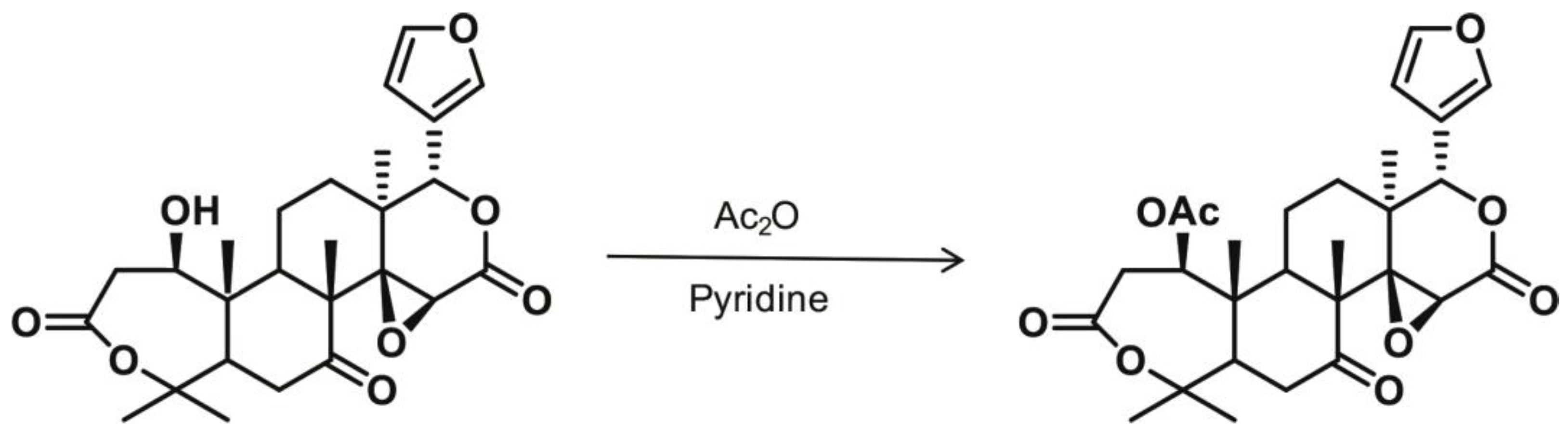
3.2.2. Biosynthesis and Chemical Synthesis
3.3. Bioavailability and Absorption
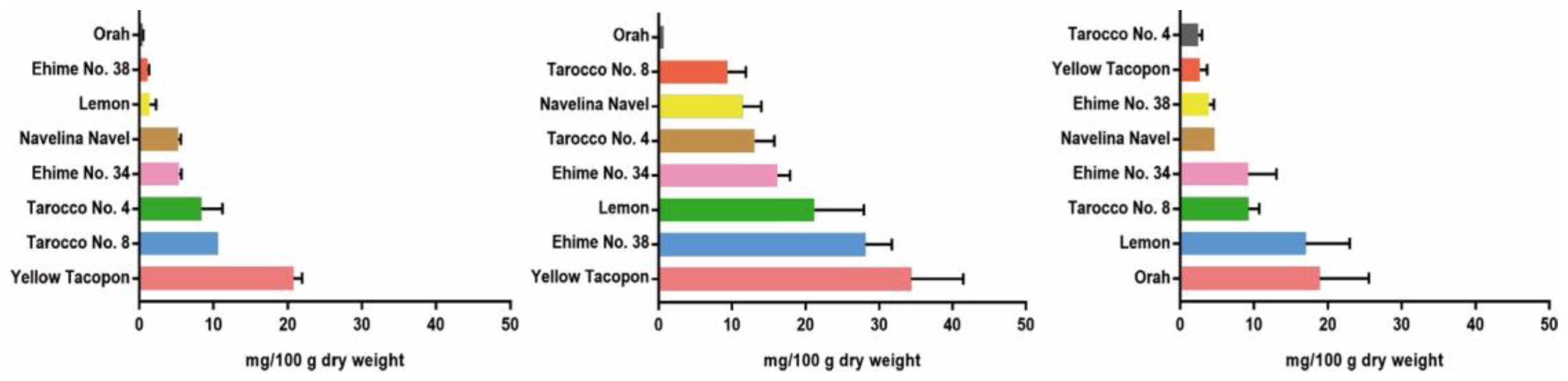
4. Variation in Nomilin Content in the Fruits of Several Citrus Varieties
5. Bioactivities and Health Beneficial Effects of Nomilin
5.1. The Detoxification Effects of Nomilin
5.2. Anti-Oxidant
5.3. Anti-Cancer Activity
5.4. Immunomodulation
5.5. Anti-Inflammation
5.6. Anti-Obesity Effect
5.7. Anti-Viral
5.8. Anti-Osteoclastogenesis
5.9. Neuro-Protective function
6. The Pharmacological Properties of Nomilin Analogous
6.1. Obacunone and Cancers
6.2. Obacunone and Digestive Diseases
6.3. Obacunone and Metabolic Disorders
6.4. Obacunone and Vascular Diseases
6.5. Obacunone and Wound Healing
6.6. Obacunone and Neurodegenerative Diseases
6.7. Anti-Bacterial Effects of Obacunone
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manners, G.D. Citrus Limonoids: Analysis, Bioactivity, and Biomedical Prospects. J. Agric. Food Chem. 2007, 55, 8285–8294. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Miyake, M. Biochemistry and biological functions of citrus limonoids. Food Rev. Int. 1996, 12, 413–415. [Google Scholar] [CrossRef]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Antiproliferative effects of citrus limonoids against human neuroblastoma and colonic adenocarcinoma cells. Nutr. Cancer 2006, 56, 103–112. [Google Scholar] [CrossRef]
- Hasegawa, S.; Bennett, R.D.; Verdon, C.P. Limonoids in Citrus Seeds: Origin and Relative Concentration. J. Agric. Food Chem. 1980, 28, 922–925. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Maier, V.P.; Turnbaugh, J.G. Effect of some Citrus Juice Constituents on Taste Thresholds for Limonin and Naringin Bitterness. J. Sci. Fd. Agric. 1973, 24, 1277–1288. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Matthews, R.F. Nomilin, Taste Threshold and Relative Bitterness. J. Food Sci. 1984, 49, 777–779. [Google Scholar] [CrossRef]
- Hashinaga, F.; Ejima, H.; Nagahama, H.; Itoo, S. Limonoids in Citrus Fruits. I. Seasonal Changes of Limonoid Components in Ponkan, Tonkan, Early Satsuma Mandarin and Natsudaidai Fruits. Bull. Fac. Agric. Kagoshima Univ. 1977, 27, 171–180. [Google Scholar]
- Rouseff, R.L. Nomilin, a New Bitter Component in Grapefruit Juice. J. Agric. Food Chem. 1982, 30, 504–507. [Google Scholar] [CrossRef]
- Lam, L.K.T.; Hasegawa, S. Inhibition of benzo[a]pyrene-induced forestomach neoplasia in mice by citrus limonoids. Nutr. Cancer 1989, 12, 43–47. [Google Scholar] [CrossRef]
- Tian, Q.; Miller, E.G.; Ahmad, H.; Tang, L.; Patil, B.S. Differential inhibition of human cancer cell proliferation by citrus limonoids. Nutr. Cancer. 2001, 40, 180–184. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Nomilin inhibits tumor-specific angiogenesis by downregulating VEGF, NO and proinflammatory cytokine profile and also by inhibiting the activation of MMP-2 and MMP-9. Eur. J. Pharmacol. 2011, 668, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Raphael, T.J.; Kuttan, G. Nomilin Inhibits Metastasis via Induction of Apoptosis and Regulates the Activation of Transcription Factors and the Cytokine Profile in B16F-10 Cells. Integr. Cancer Ther. 2012, II, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013, 4, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Raphael, T.J.; Kuttan, G. Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine 2003, 10, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Chakraborty, S.; Jayaprakasha, G.K.; Muthuchamy, M.; Patil, B.S. Citrus nomilin down-regulates TNF-α-induced proliferation of aortic smooth muscle cells via apoptosis and inhibition of IκB. Eur. J. Pharmacol. 2017, 811, 93–100. [Google Scholar] [CrossRef]
- Ono, E.I.J.; Hashidume, T.; Shimizu, M.; Sato, R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2011, 410, 677–681. [Google Scholar] [CrossRef]
- Battinelli, L.; Mengoni, F.; Lichtner, M.; Mazzanti, G.; Saija, A.; Mastroianni, C.M.; Vullo, V. Effect of limonin and nomilin on HIV-1 replication on infected human mononuclear cells. Planta Med. 2003, 69, 910–913. [Google Scholar]
- Kimira, Y.; Taniuchi, Y.; Nakatani, S.; Sekiguchi, Y.; Kim, H.J.; Shimizu, J.; Ebata, M.; Wada, M.; Matsumoto, A.; Mano, H. Citrus limonoid nomilin inhibits osteoclastogenesis in vitro by suppression of NFATc1 and MAPK signaling pathways. Phytomedicine 2015, 22, 1120–1124. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.S.; Zhang, Y.; Liu, B.; Li, C.B.; Wu, J.; Li, Y. Nomilin protects against cerebral ischemia-reperfusion induced neurological deficits and blood-brain barrier disruption via the Nrf2 pathway. Food Funct 2019, 10, 5323–5332. [Google Scholar] [CrossRef]
- Manners, G.D.; Jacob, R.A.; Breksa, A.P.; Schoch, T.K.; Hasegawa, S. Bioavailability of Citrus limonoids in humans. J. Agric. Food Chem. 2003, 51, 4156–4161. [Google Scholar] [CrossRef]
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, J.R.; Jayaprakasha, G.K.; Murthy, K.N.C.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Bartholomew, E.T.; Sinclair, W. The Lemon Fruit; University of California: Berkeley, CA, USA, 1952. [Google Scholar]
- Sinclair, W.B. The Orange; University of California: Berkeley, CA, USA, 1961. [Google Scholar]
- Scora, R.W. On the history and origin of citrus. Bull Torrey Bot Club 1975, 102, 369–375. [Google Scholar] [CrossRef]
- Ramana, K.R.; Govindarajan, V.S.; Ranganna, S. Citrus fruits—Varieties, chemistry, technology, and quality evaluation. Part I: Varieties, production, handling, and storage. Crit. Rev. Food Sci. Nutr. 1981, 15, 353–431. [Google Scholar] [CrossRef] [PubMed]
- Gmitter, F.G.; Hu, X. The Possible Role of Yunnan, China, in the Origin of Contemporary Citrus Species (Rutaceae). Econ. Bot. 1990, 44, 267–277. [Google Scholar] [CrossRef]
- Reuther, W.; Batchelor, L.D.; Webber, H.J. History and Development of the Citrus Industry. The Citrus Industry. Vol. 1. History, World Distribution, Botany and Varieties; University of California, Berkeley, Division of Agricultural Sciences: Berkeley, CA, USA, 1967; pp. 1–39. [Google Scholar]
- Nagy, S.A.J. Citrus Nutrition and Quality; American Chemical Society: Washington, DC, USA, 1980. [Google Scholar]
- Anitei, S. Where Did Citrus Fruits Originate from? Softpedia. 2007. Available online: http://news.softpedia.com/news/Where-Did-Citrus-Fruits-Originate-From-67365.shtml (accessed on 10 November 2022).
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Safety 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Hasegawa, S.; Maier, V. The biochemistry of limonoid citrus juice bitter principles and biochemical debittering processes. Dev. Food Sci. 1990, 25, 281–287. [Google Scholar]
- Hasegawa, S.; Berhow, M.A.; Fong, C.H. Analysis of bitter principles in Citrus. Mod. Methods Plant Anal. 1996, 18, 60–80. [Google Scholar]
- Pettit, G.R.; Barton, D.H.; Herald, C.L. Evaluation of Limonoids Against the Murine P388 Lymphocytic Leukemia Cell Line. J. Nat. Prod. 1983, 46, 379–390. [Google Scholar] [CrossRef]
- Ozaki, Y.; Ayano, S.; Inaba, N. Limonoid glucosides in fruit, juice and processing byproducts of Satsuma mandarin (Citrus unshiu Marcov.). J. Food Sci. 1995, 60, 186–189. [Google Scholar] [CrossRef]
- Schechter, M.S.; Haller, H.L. The Identity of Obaculactone, Evodin and Dictamnolactone with Limonin. J. Am. Chem. Soc. 1940, 62, 1307–1309. [Google Scholar] [CrossRef]
- Emerson, O.H. The Bitter Principles of Citrus Fruit. I. Isolation of Nomilin, a New Bitter Principle from the Seeds of Oranges and Lemons. J. Am. Chem. Soc. 1948, 70, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. An Overview on Chemical Aspects and Potential Health Benefits of Limonoids and Their Derivatives. Crit. Rev. Food Sci. Nutri. 2014, 54, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ni, H.; Wu, Y.L.; Cai, H.N.; Wu, G.B. Study on Extraction Process of Bitterness from Guanxi Pomelo. Food Sci. 2007, 128, 152–155. [Google Scholar]
- Sun, C.D.; Chen, K.S.; Chen, Q.J.; Ren, Y.; Zhang, S. Extraction, Identification and Determination of Natural Limonin and Nomilin from Citrus Fruits. J. Chin. Inst. Food Sci. Technol. 2004, 4, 6–11. [Google Scholar]
- Emerson, O.E. Bitter Principles of Citrus. II. Relation of Nomilin and Obacunone. J. Am. Chem. Soc. 1951, 73, 2621–2623. [Google Scholar] [CrossRef]
- Luo, X.R.; Fan, J.D.; Mo, B.B.; Yang, J. Response Surface Optimization of Supercritical Carbon Dioxide Extraction of Nomilin from Citrus Seeds. Food Sci. 2010, 31, 74–76. [Google Scholar]
- Tian, Q.G.; Ding, X.L. Isolation of limonoids from seeds of Citrus sinensis. Chem. Indust. Forest Prod. 1999, 19, 71–74. [Google Scholar]
- Li, H.; Peng, Y.; Zheng, J. Metabolic Activation and Toxicities of Furanoterpenoids. Adv. Mol. Toxicol. 2016, 10, 55–97. [Google Scholar]
- Hasegawa, S.; Herman, Z. Biosynthesis of limonoids: Conversion of deacetylnomilinate to nomilin in Citrus limon. Phytochemistry 1986, 25, 2523–2524. [Google Scholar] [CrossRef]
- Dreyer, D.L. Citrus Bitter Principles. III. Isolation of Deacetylnomilin and Deoxylimonin. J. Org. Chem. 1965, 30, 749–750. [Google Scholar] [CrossRef]
- Cai, Y.P.; Zhang, S.; Wang, Q.Q.; Sun, H.; Zhou, S. A Rapid, Selective and Sensitive UPLC-MS/MS Method for Quantification of Nomilin in Rat Plasma and Its Application in a Pharmacokinetic Study. Planta Med. 2016, 82, 224–229. [Google Scholar] [CrossRef]
- Shanmugam, H.; Acharya, P.; Jayaprakasha, G.K.; Patil, B.S. Nanoformulation and characterization of nomilin with different poly (lactic-co-glycolic acid) resomers and surfactants for the enhanced inhibition of alpha-amylase and angiotensin-converting-enzyme. J. Funct. Foods 2017, 37, 564–573. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Bo, X.; Xia, Q.; Wang, Z. Variation in limonin and nomilin content in citrus fruits of eight varieties determined by modified HPLC. Food Sci. Biotechnol. 2019, 28, 641–647. [Google Scholar] [CrossRef]
- Lam, L.K.T.; Li, Y.; Hasegawa, S. Effects of Citrus Limonoids on Glutathione S-Transferase Activity in Mice. J. Agric. Food Chem. 1989, 37, 878–880. [Google Scholar] [CrossRef]
- Kelly, C.; Jewell, C.; O’Brien, N.M. The effect of dietary supplementation with the citrus limonoids, limonin and nomilin on xenobiotic-metabolizing enzymes in the liver and small intestine of the rat. Nutri. Res. 2003, 23, 681–690. [Google Scholar] [CrossRef]
- Perez, J.L.; Jayaprakasha, G.K.; Cadena, A.; Martinez, E.; Ahmad, H.; Patil, B.S. In vivo induction of phase II detoxifying enzymes, glutathione transferase and quinone reductase by citrus triterpenoids. BMC Complem. Med. Therap. 2010, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piyapolrungroj, N.; Phattanawasin, P.; Sotanaphun, U.; Maw, M.P. Role of citrus limonoid as a possible bioavailability enhancer. Key Eng. Mater. 2020, 859, 132–138. [Google Scholar]
- Fan, Y.; Chen, Y.; Chen, J.; Zhang, F.; Ondieki, G.; Li, Y.; He, X. Inhibition of Cytochrome P450 by Nomilin and Obacunone and Potential Mechanism in Human Liver Microsomes. Chin. Herb. Med. 2017, 5, 295–298. [Google Scholar] [CrossRef]
- Hosoi, S.; Shimizu, E.; Usami, N.; Yamamoto, I.; Arimori, K.; Okumura, M.; Hidaka, M.; Yamada, M.; Sakushima, A. Isolation of cytochrome P450 3A (CYP3A) inhibitors from Hyuganatsu, Citrus tamurana Hort. J. Nat. Med. 2006, 60, 240–242. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Suganuma, T. Antioxidant and antimicrobial activities of the methanol extracts from pummelo (Citrus grandis Osbeck) fruit albedo tissues. Eur. Food Res. Technol. 2006, 224, 39–47. [Google Scholar] [CrossRef]
- Kuljarachanan, T.; Devahastin, S.; Chiewchan, N. Evolution of antioxidant compounds in lime residues during drying. Food Chem. 2009, 113, 944–949. [Google Scholar] [CrossRef]
- Sun, C.D.; Chen, K.S.; Chen, Y.; Chen, Q.J. Contents and antioxidant capacity of limonin and nomilin in different tissues of citrus fruit of four cultivars during fruit growth and maturation. Food Chem. 2005, 93, 599–605. [Google Scholar] [CrossRef]
- Breksa, A.P.; Manners, G.D. Evaluation of the antioxidant capacity of limonin, nomilin, and limonin glucoside. J. Agric. Food Chem. 2006, 54, 3827–3831. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, N.; Manners, G.D.; Hasegawa, S.; Freeman, D. Inhibition of human breast cancer cells by citrus limonoids. In Citrus Limonoids: Functional Chemicals in Agriculture and Foods; American Chemical Society: Washington, DC, USA, 2000; Volume 758, pp. 164–174. [Google Scholar]
- Murthy, K.N.C.; Jayaprakasha, G.K.; Safe, S.; Patil, B.S. Citrus limonoids induce apoptosis and inhibit the proliferation of pancreatic cancer cells. Food Funct. 2021, 12, 1111–1120. [Google Scholar] [CrossRef]
- Kim, J.; Jayaprakasha, G.K.; Muthuchamy, M.; Patil, B.S. Structure-function relationships of citrus limonoids on p38 MAP kinase activity in human aortic smooth muscle cells. Eur. J. Pharmacol. 2011, 670, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Song, H.Y.; Lee, M. Sudachinoid- and Ichangensin-Type Limonoids from Citrus junos Downregulate Pro-Inflammatory Cytokines. Int. J. Mol. Sci. 2020, 21, 6963. [Google Scholar] [CrossRef]
- Sasaki, T.; Mita, M.; Ikari, N.; Kuboyama, A.; Hashimoto, S.; Kaneko, T.; Ishiguro, M.; Shimizu, M.; Inoue, J.; Sato, R. Identification of key amino acid residues in the hTGR5-nomilin interaction and construction of its binding model. PLoS ONE 2017, 12, e0179226. [Google Scholar] [CrossRef] [Green Version]
- Magurano, F.; Sucameli, M.; Picone, P.; Micucci, M.; Baggieri, M.; Marchi, A.; Bucci, P.; Gioacchini, S.; Catinella, G.; Borgonovo, G.; et al. Antioxidant Activity of Citrus Limonoids and Investigation of Their Virucidal Potential against SARS-CoV-2 in Cellular Models. Antioxidants 2021, 10, 1794. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Xue, X.H.; Xue, J.X.; Hu, W.; Shi, F.L.; Yang, Y. Nomilin targets the Keap1-Nrf2 signalling and ameliorates the development of osteoarthritis. J. Cell Mol. Med. 2020, 24, 8579–8588. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Obacunone and obacunone glucoside inhibit human colon cancer (SW480) cells by the induction of apoptosis. Food Chem. Toxicol. 2011, 49, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Cytotoxicity of obacunone and obacunone glucoside in human prostate cancer cells involves Akt-mediated programmed cell death. Toxicology 2015, 329, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Apoptosis mediated cytotoxicity of citrus obacunone in human pancreatic cancer cells. Toxicol Vitr. 2011, 25, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, A.H.; Qiu, S.; Zhang, T.L.; Li, X.N.; Yan, G.L.; Sun, H.; Liu, L.; Wang, X.J. Identification of the perturbed metabolic pathways associating with prostate cancer cells and anticancer affects of obacunone. J. Proteomics 2019, 206, 103447. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Tsukio, Y.; Honjo, S.; Tanino, M.; Miyake, M.; Wada, K. Citrus limonoids obacunone and limonin inhibit azoxymethane-induced colon carcinogenesis in rats. Biofactors 2000, 13, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Maeda, M.; Kohno, H.; Murakami, M.; Kagami, S.; Miyake, M.; Wada, K. Inhibition of azoxymethane-induced colon carcinogenesis in male F344 rats by the citrus limonoids obacunone and limonin. Carcinogenesis 2001, 22, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Z.; Yue, B.; Ren, J.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Obacunone reduces inflammatory signalling and tumour occurrence in mice with chronic inflammation-induced colorectal cancer. Pharm. Biol. 2020, 58, 886–897. [Google Scholar] [CrossRef]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Obacunone exhibits anti-proliferative and anti-aromatase activity in vitro by inhibiting the p38 MAPK signaling pathway in MCF-7 human breast adenocarcinoma cells. Biochimie 2014, 105, 36–44. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, R.; Liu, F.; Liu, H.; Fei, Q.; Han, Y.; Cai, R.; Peng, C.; Qi, Y. Obacunone causes sustained expression of MKP-1 thus inactivating p38 MAPK to suppress pro-inflammatory mediators through intracellular MIF. J. Cell Biochem. 2018, 119, 837–849. [Google Scholar] [CrossRef]
- Poulose, S.M.; Harris, E.D.; Patil, B.S. Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. J. Nutr. 2005, 135, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Yue, B.; Yu, Z.; Ren, Y.; Zhang, J.; Ren, J.; Wang, Z.; Dou, W. Obacunone Protects Against Ulcerative Colitis in Mice by Modulating Gut Microbiota, Attenuating TLR4/NF-kappaB Signaling Cascades, and Improving Disrupted Epithelial Barriers. Front. Microbiol. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, W.; Wang, L.; Ma, L.; Zhai, D.; Wang, F.; Shi, R.; Liu, C.; Xu, Q.; Chen, G.; et al. Obacunone Attenuates Liver Fibrosis with Enhancing Anti-Oxidant Effects of GPx-4 and Inhibition of EMT. Molecules 2021, 26, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, T.; Wang, H.; Jiang, Y.; Peng, S. Obacunone attenuates high glucose-induced oxidative damage in NRK-52E cells by inhibiting the activity of GSK-3beta. Biochem. Biophys. Res. Commun. 2019, 513, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Horiba, T.; Katsukawa, M.; Mita, M.; Sato, R. Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARgamma pathway. Biochem. Biophys. Res. Commun. 2015, 463, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Park, M.; Lee, J.; Min, B.S.; Ryoo, S. Endothelial nitric oxide synthase activation through obacunone-dependent arginase inhibition restored impaired endothelial function in ApoE-null mice. Vascul. Pharmacol. 2014, 60, 102–109. [Google Scholar] [CrossRef]
- Park, K.R.; Kim, S.; Cho, M.; Yun, H.M. Limonoid Triterpene, Obacunone Increases Runt-Related Transcription Factor 2 to Promote Osteoblast Differentiation and Function. Int. J. Mol. Sci. 2021, 22, 2483. [Google Scholar] [CrossRef]
- Jeong, G.S.; Byun, E.; Li, B.; Lee, D.S.; Kim, Y.C.; An, R.B. Neuroprotective effects of constituents of the root bark of Dictamnus dasycarpus in mouse hippocampal cells. Arch. Pharm. Res. 2010, 33, 1269–1275. [Google Scholar] [CrossRef]
- Huang, D.R.; Dai, C.M.; Li, S.Y.; Li, X.F. Obacunone protects retinal pigment epithelium cells from ultra-violet radiation-induced oxidative injury. Aging 2021, 13, 11010–11025. [Google Scholar] [CrossRef]
- Vikram, A.; Jesudhasan, P.R.; Jayaprakasha, G.K.; Pillai, B.S.; Patil, B.S. Grapefruit bioactive limonoids modulate E. coli O157:H7 TTSS and biofilm. Int. J. Food Microbiol. 2010, 140, 109–116. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Obacunone represses Salmonella pathogenicity islands 1 and 2 in an envZ-dependent fashion. Appl. Environ. Microbiol. 2012, 78, 7012–7022. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yan, Y.; Li, H.; Feng, Y.; Huang, C.; Fan, S. Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine. Molecules 2023, 28, 269. https://doi.org/10.3390/molecules28010269
Zhou Z, Yan Y, Li H, Feng Y, Huang C, Fan S. Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine. Molecules. 2023; 28(1):269. https://doi.org/10.3390/molecules28010269
Chicago/Turabian StyleZhou, Zhenyu, Yingxuan Yan, Hongli Li, Yaru Feng, Cheng Huang, and Shengjie Fan. 2023. "Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine" Molecules 28, no. 1: 269. https://doi.org/10.3390/molecules28010269




