8–17 DNAzyme Silencing Gene Expression in Cells via Cleavage and Antisense
Abstract
:1. Introduction
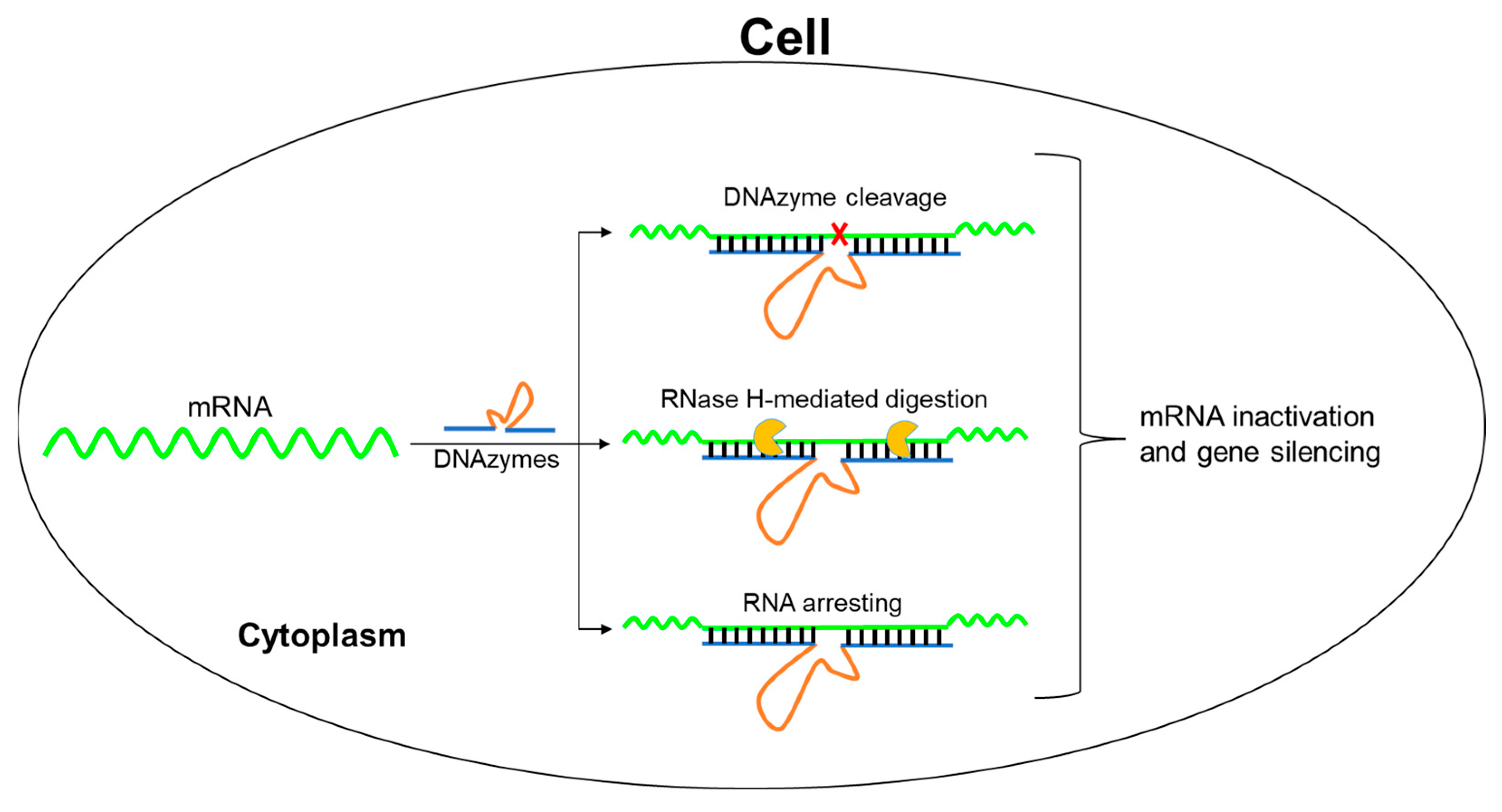
2. Results and Discussion
2.1. The Design of DNAzymes Targeting EGFP mRNA
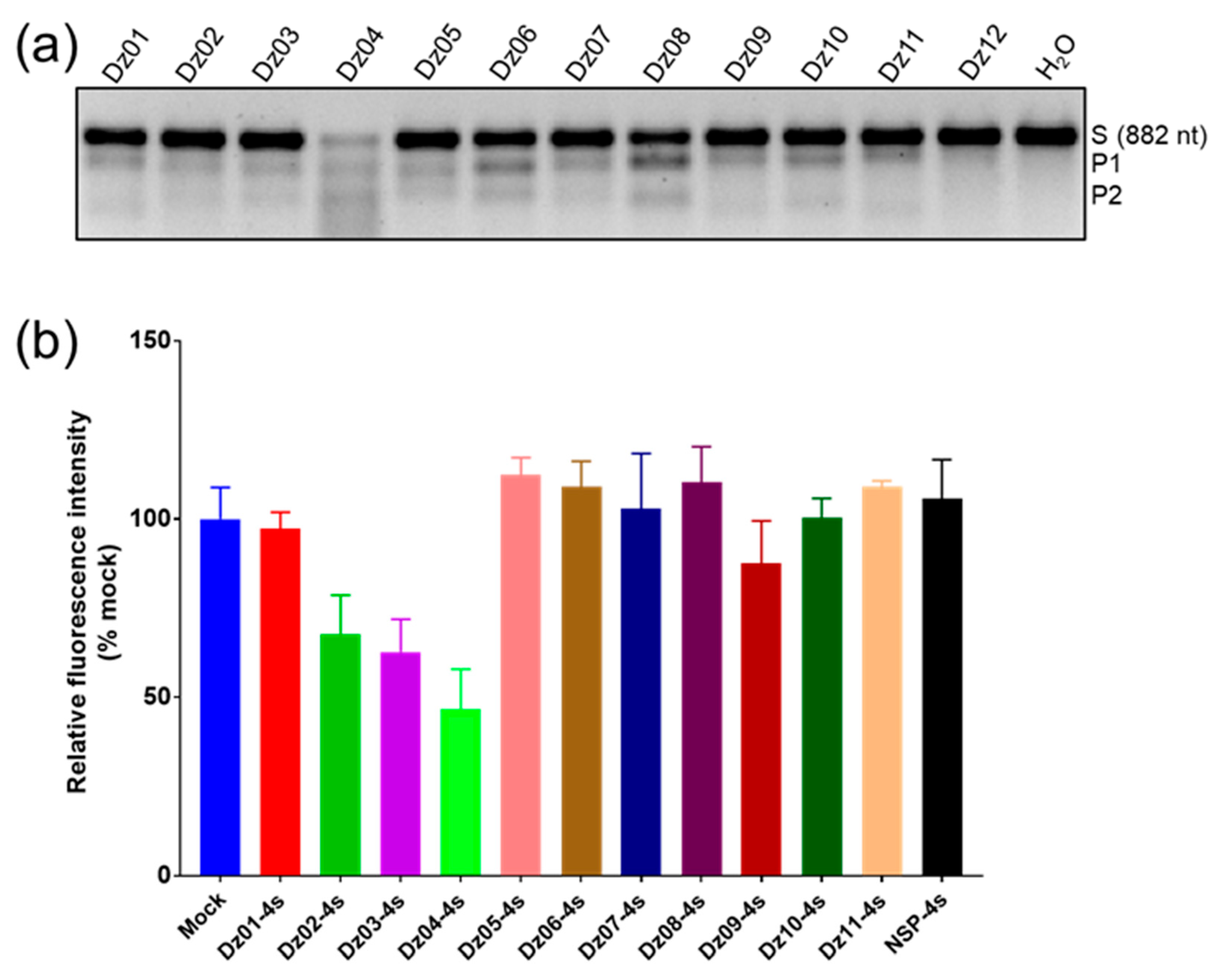
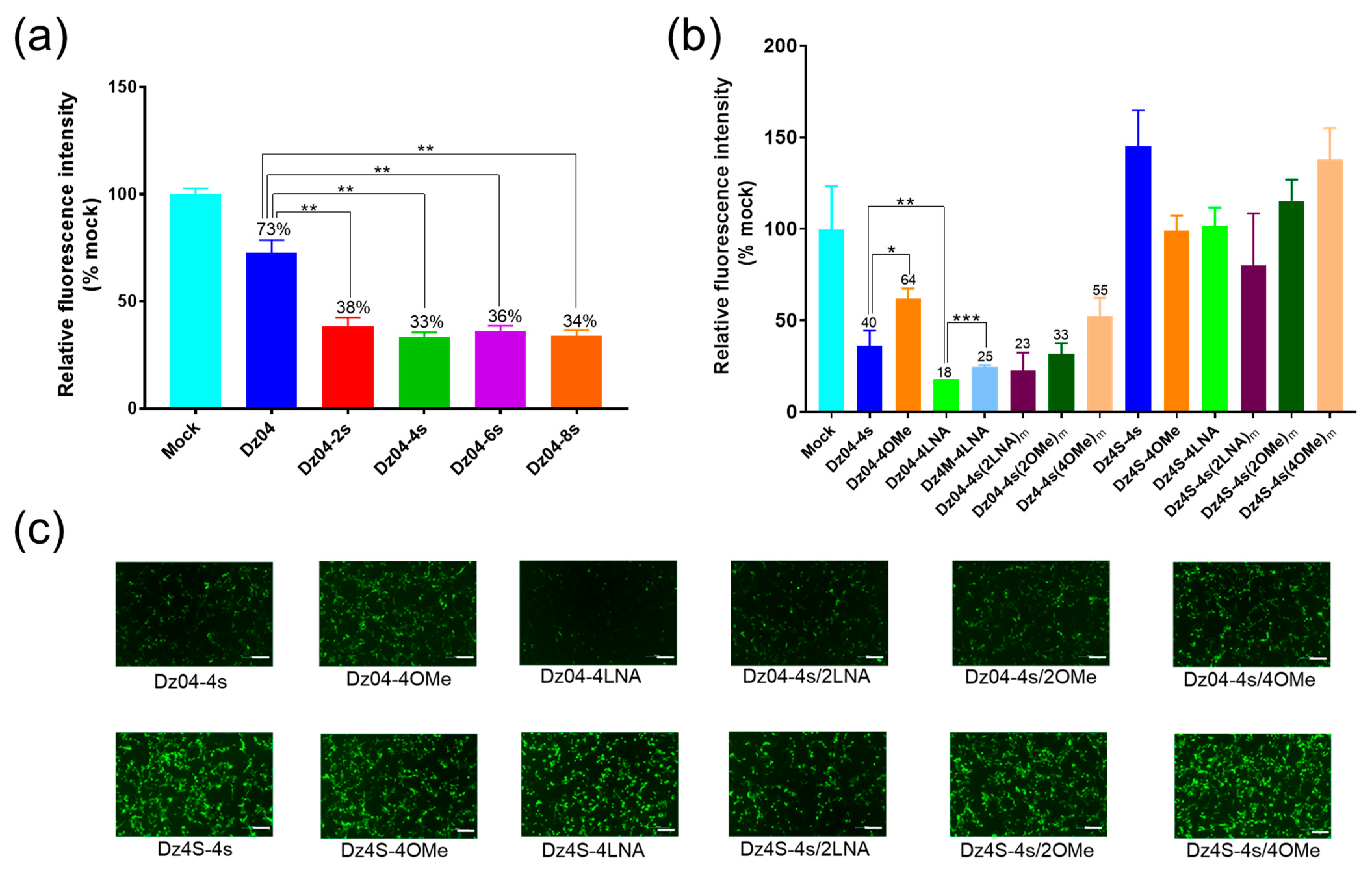
2.2. Identification of the DNAzyme with Gene-Silencing Activity in Cells
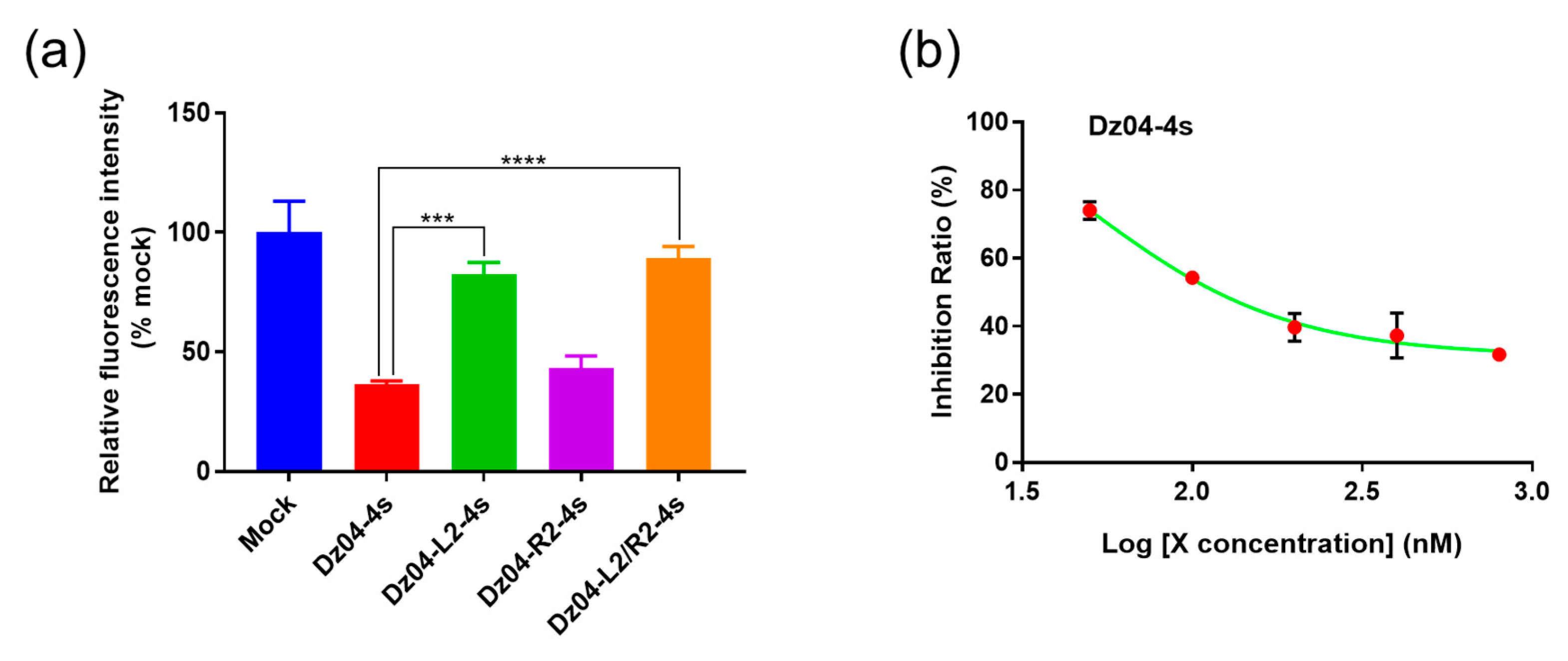
2.3. The DNAzyme with Both Cleavage and Antisense Activities in Cells
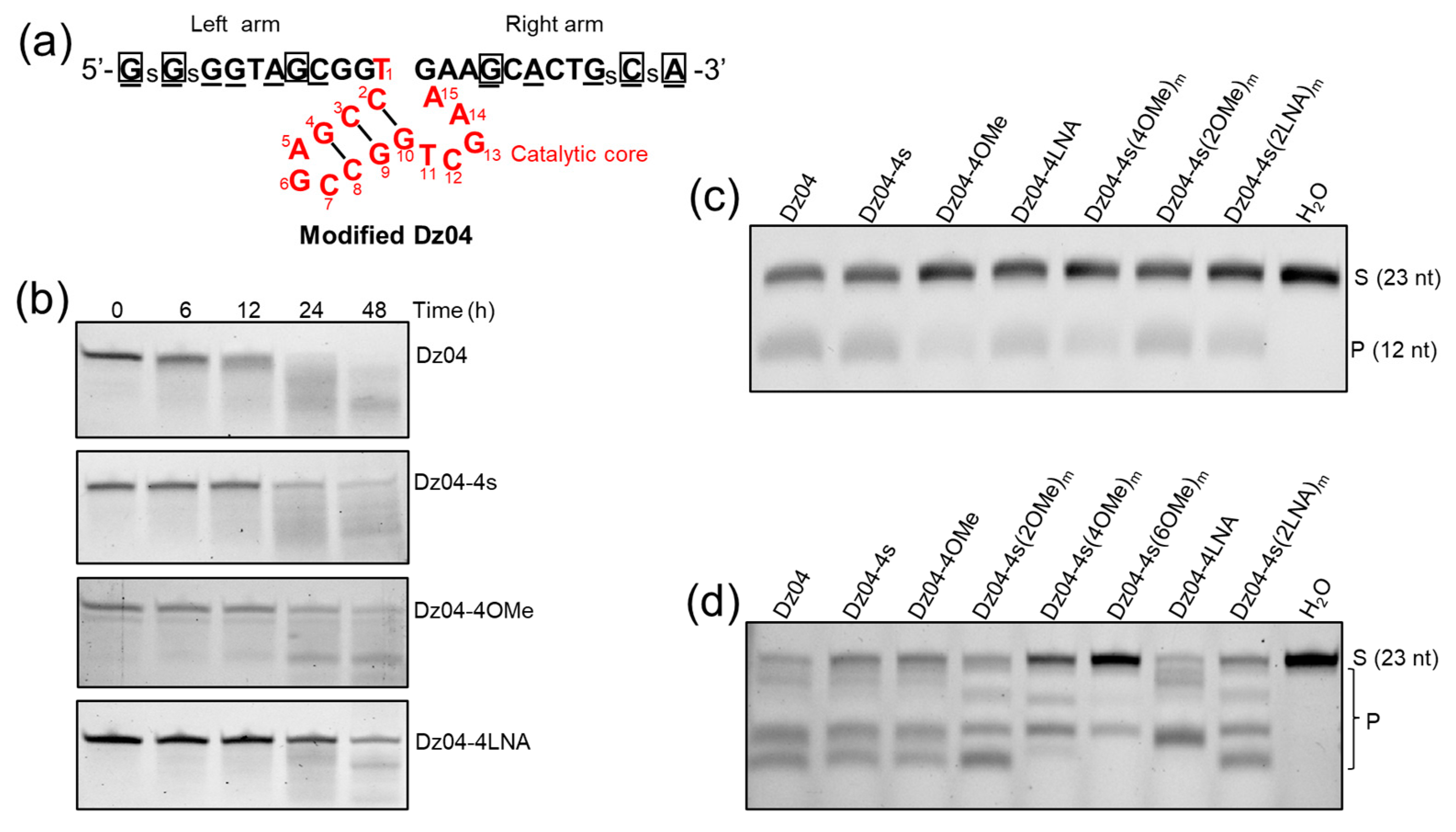
2.4. In Vitro Studies of the Stability, Catalysis and RNase H Compatibility of the DNAzymes Modified on the Arms
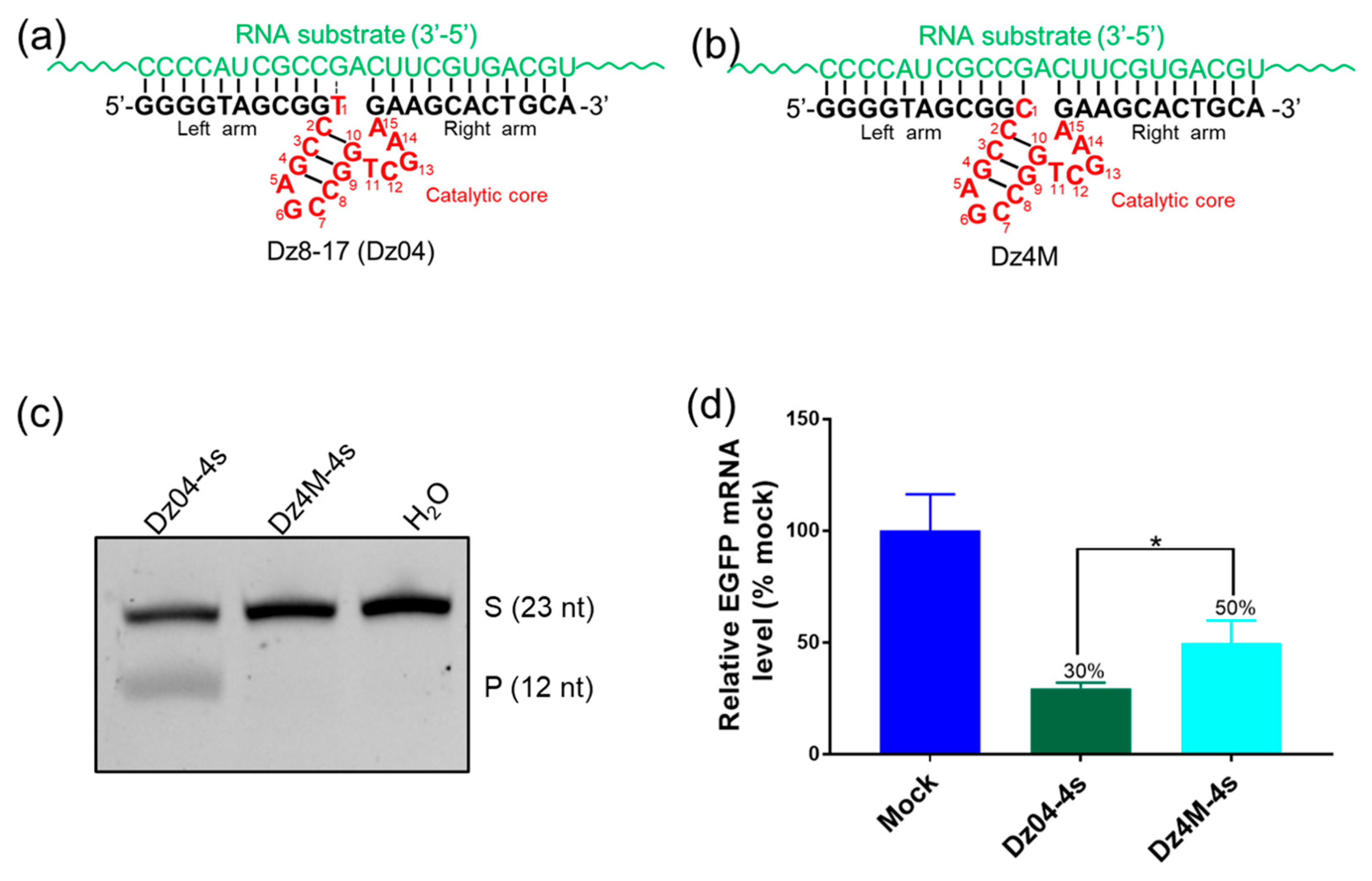
2.5. The Modified DNAzyme with High Activities in Cells
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Bioinformatics Tools for Designing 8–17 DNAzymes
3.2.2. In Vitro Substrate Cleavage
3.2.3. Co-Transfection of 293T Cells with DNAzymes and pEGFP-C1
3.2.4. RNA Quantification (qRT-PCR)
3.2.5. Stability Tests of Modified DNAzymes in FBS
3.2.6. RNase H-Induced Hydrolysis of RNA
3.2.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Sappah, A.H.; Yan, K.; Huang, Q.; Islam, M.M.; Li, Q.; Wang, Y.; Khan, M.S.; Zhao, X.; Mir, R.R.; Li, J.; et al. Comprehensive Mechanism of Gene Silencing and Its Role in Plant Growth and Development. Front. Plant Sci. 2021, 12, 705249. [Google Scholar] [CrossRef]
- de Fougerolles, A.; Vornlocher, H.-P.; Maraganore, J.; Lieberman, J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007, 6, 443–453. [Google Scholar] [CrossRef]
- Felippes, F.F.D.; Wang, J.-W.; Weigel, D. MIGS: miRNA-induced gene silencing. Plant J. 2012, 70, 541–547. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600. [Google Scholar] [CrossRef]
- Watts, J.K.; Corey, D.R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 2012, 226, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Borggräfe, J.; Victor, J.; Rosenbach, H.; Viegas, A.; Gertzen, C.G.W.; Wuebben, C.; Kovacs, H.; Gopalswamy, M.; Riesner, D.; Steger, G.; et al. Time-resolved structural analysis of an RNA-cleaving DNA catalyst. Nature 2022, 601, 144–149. [Google Scholar] [CrossRef]
- Rudeejaroonrung, K.; Hanpanich, O.; Saito, K.; Shimada, N.; Maruyama, A. Cationic copolymer enhances 8-17 DNAzyme and MNAzyme activities. Biomater. Sci. 2020, 8, 3812–3818. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Chen, Y.; Zhang, J.; Wu, B.; Zheng, L.; Haruehanroengra, P.; Wang, R.; Li, S.; Lin, J.; et al. Crystal structure of an RNA-cleaving DNAzyme. Nat. Commun. 2017, 8, 2006. [Google Scholar] [CrossRef]
- Reyes-Gutierrez, P.; Alvarez-Salas, L.M. Cleavage of HPV-16 E6/E7 mRNA mediated by modified 10-23 deoxyribozymes. Oligonucleotides 2009, 19, 233–242. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, K.; Spitale, R.C.; Chaput, J.C. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat. Chem. 2021, 13, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, D.; Nagraj, N.; Kim, H.-K.; Meng, X.; Brown, A.K.; Sun, Q.; Li, W.; Lu, Y. Activity, folding and Z-DNA formation of the 8–17 DNAzyme in the presence of monovalent ions. J. Am. Chem. Soc. 2009, 131, 5506–5515. [Google Scholar] [CrossRef] [Green Version]
- Victor, J.; Steger, G.; Riesner, D. Inability of DNAzymes to cleave RNA in vivo is due to limited Mg(2+) concentration in cells. Eur. Biophys. J. 2018, 47, 333–343. [Google Scholar] [CrossRef]
- Alama, A.; Barbieri, F.; Cagnoli, M.; Schettini, G. Antisense oligonucleotides as therapeutic agents. Pharmacol. Res. 1997, 36, 171–178. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, L.; McMahon, R.; Rossi, J.J.; Forman, S.J.; Snyder, D.S. Inhibition of bcr-abl oncogene expression by novel deoxyribozymes (DNAzymes). Hum. Gene Ther. 1999, 10, 2847–2857. [Google Scholar] [CrossRef]
- Fokina, A.A.; Chelobanov, B.P.; Fujii, M.; Stetsenko, D.A. Delivery of therapeutic RNA-cleaving oligodeoxyribonucleotides (deoxyribozymes): From cell culture studies to clinical trials. Expert Opin. Drug Deliv. 2017, 14, 1077–1089. [Google Scholar] [CrossRef]
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]
- Schubert, S.; Gül, D.C.; Grunert, H.-P.; Zeichhardt, H.; Erdmann, V.A.; Kurreck, J. RNA cleaving ‘10–23′ DNAzymes with enhanced stability and activity. Nucleic Acids Res. 2003, 31, 5982–5992. [Google Scholar] [CrossRef]
- Elmén, J.; Thonberg, H.; Ljungberg, K.; Frieden, M.; Westergaard, M.; Xu, Y.; Wahren, B.; Liang, Z.; Ørum, H.; Koch, T.; et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005, 33, 439–447. [Google Scholar] [CrossRef]
- Shimo, T.; Tachibana, K.; Saito, K.; Yoshida, T.; Tomita, E.; Waki, R.; Yamamoto, T.; Doi, T.; Inoue, T.; Kawakami, J.; et al. Design and evaluation of locked nucleic acid-based splice-switching oligonucleotides in vitro. Nucleic Acids Res. 2014, 42, 8174–8187. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xu, J.; Liu, J.; Yan, X.; Zhu, X.; Xiao, G.; Sun, L.; Tien, P. An efficient RNA-cleaving DNA enzyme can specifically target the 5′-untranslated region of severe acute respiratory syndrome associated coronavirus (SARS-CoV). J. Gene Med. 2007, 9, 1080–1086. [Google Scholar] [CrossRef]
- Wang, F.; Saran, R.; Liu, J. Tandem DNAzymes for mRNA cleavage: Choice of enzyme, metal ions and the antisense effect. Bioorg. Med. Chem. Lett. 2015, 25, 1460–1463. [Google Scholar] [CrossRef] [Green Version]
- Rosenbach, H.; Victor, J.; Etzkorn, M.; Steger, G.; Riesner, D.; Span, I. Molecular Features and Metal Ions That Influence 10–23 DNAzyme Activity. Molecules 2020, 25, 3100. [Google Scholar] [CrossRef]
- Bondensgaard, K.; Petersen, M.; Singh, S.K.; Rajwanshi, V.K.; Kumar, R.; Wengel, J.; Jacobsen, J.P. Structural studies of LNA:RNA duplexes by NMR: Conformations and implications for RNase H activity. Chemistry 2000, 6, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence (5′-3′) 1 | Target Region | Tm (°C) |
|---|---|---|---|
| Dz01 | GGGCAGCTTGTCCGAGCCGGTCGAAGGTGGTGCAGA | 143–165 | 72.5 |
| Dz02 | GCACTGCACGTCCGAGCCGGTCGAAGTAGGTCAGGG | 191–213 | 70.0 |
| Dz03 | GGCTGAAGCATCCGAGCCGGTCGAAGCACGCCGTAG | 198–220 | 70.8 |
| Dz04 | GGGGTAGCGGTCCGAGCCGGTCGAAGAAGCATGCA | 206–228 | 70.1 |
| Dz05 | AGAAGTCGTGTCCGAGCCGGTCGAAGCTTCATGTGG | 231–253 | 65.6 |
| Dz06 | GACGTTGTGGTCCGAGCCGGTCGAAGTTGTAGTTGT | 431–453 | 63.7 |
| Dz07 | CGTTCTTCTGTCCGAGCCGGTCGAATGTCGGCCATG | 459–481 | 66.5 |
| Dz08 | TGCCGTTCTTTCCGAGCCGGTCGAAGCTTGTCGGCC | 462–484 | 70.9 |
| Dz09 | TGCAGATGAATCCGAGCCGGTCGAATCAGGGTCAGC | 126–148 | 65.5 |
| Dz10 | TGAAGTTCACTCCGAGCCGGTCGAATGATGCCGTTC | 477–499 | 63.8 |
| Dz11 | GAGCTGCACGTCCGAGCCGGTCGAAGCCGTCCTCGA | 515–537 | 73.7 |
| Dz12 | ACGCTGCCGTTCCGAGCCGGTCGAATCGATGTTGTG | 508–530 | 68.8 |
| DNAzymes | Inhibition Ratio (%) | DNAzymes | Inhibition Ratio (%) |
|---|---|---|---|
| Dz01 | <5 | Dz07 | 0 |
| Dz02 | 32 | Dz08 | 0 |
| Dz03 | 37 | Dz09 | 12 |
| Dz04 | 53 | Dz10 | 0 |
| Dz05 | 0 | Dz11 | 0 |
| Dz06 | 0 | NSP | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Sun, W.; Huang, Z. 8–17 DNAzyme Silencing Gene Expression in Cells via Cleavage and Antisense. Molecules 2023, 28, 286. https://doi.org/10.3390/molecules28010286
Zhou Z, Sun W, Huang Z. 8–17 DNAzyme Silencing Gene Expression in Cells via Cleavage and Antisense. Molecules. 2023; 28(1):286. https://doi.org/10.3390/molecules28010286
Chicago/Turabian StyleZhou, Zhongchun, Wen Sun, and Zhen Huang. 2023. "8–17 DNAzyme Silencing Gene Expression in Cells via Cleavage and Antisense" Molecules 28, no. 1: 286. https://doi.org/10.3390/molecules28010286
APA StyleZhou, Z., Sun, W., & Huang, Z. (2023). 8–17 DNAzyme Silencing Gene Expression in Cells via Cleavage and Antisense. Molecules, 28(1), 286. https://doi.org/10.3390/molecules28010286







