Treatment of High-Polyphenol-Content Waters Using Biotechnological Approaches: The Latest Update
Abstract
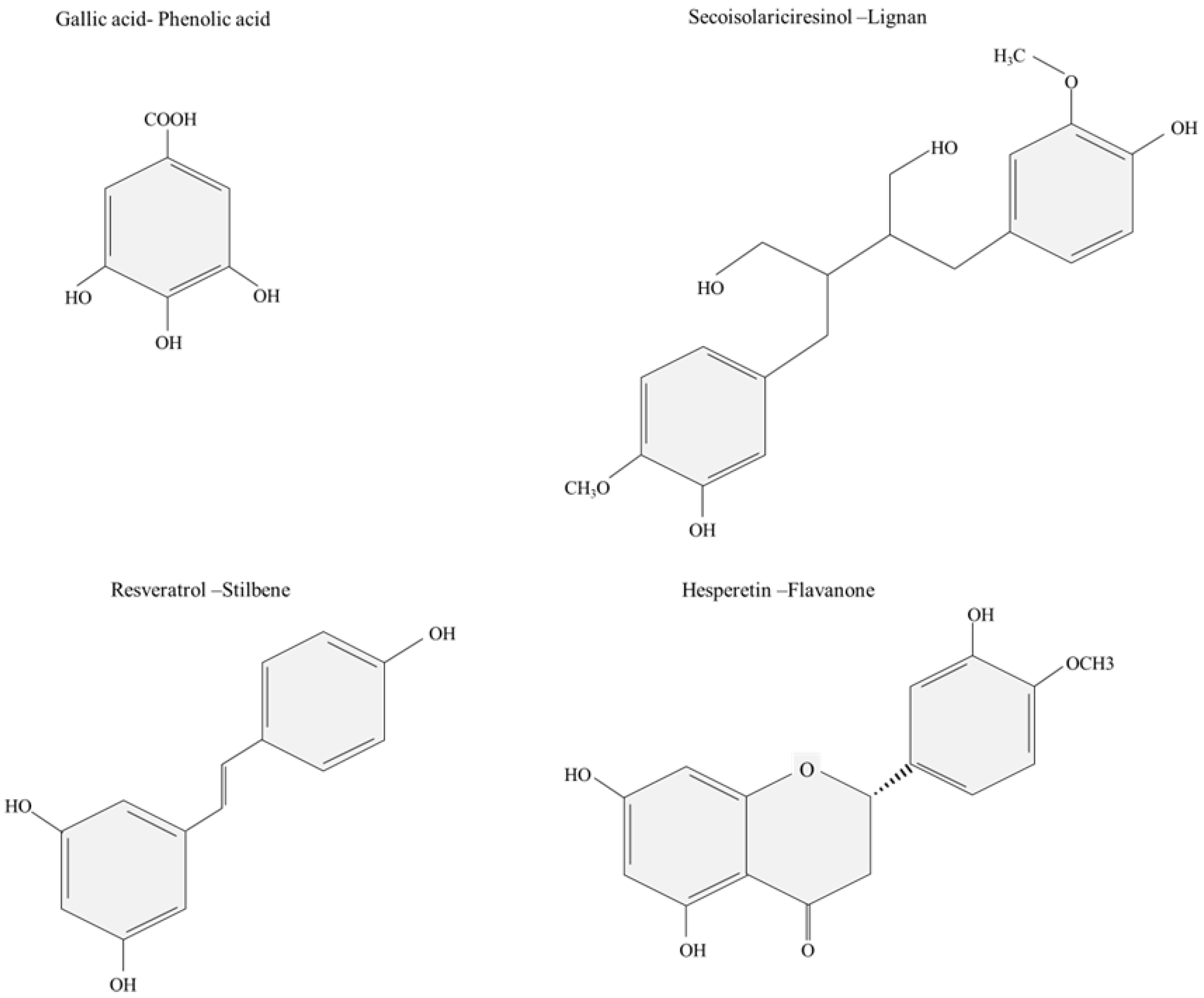
:1. Introduction
2. Polluted Waters as Sources of Phenolic Compounds: The Challenge of Their Profitability in the Circular Economy
3. Recovery of Polyphenols from Wastewater
4. Technologies to Remove Phenolic Compounds from Water Sources
5. Feasible Biological Technologies for the Removal of Phenolic Compounds from Waters
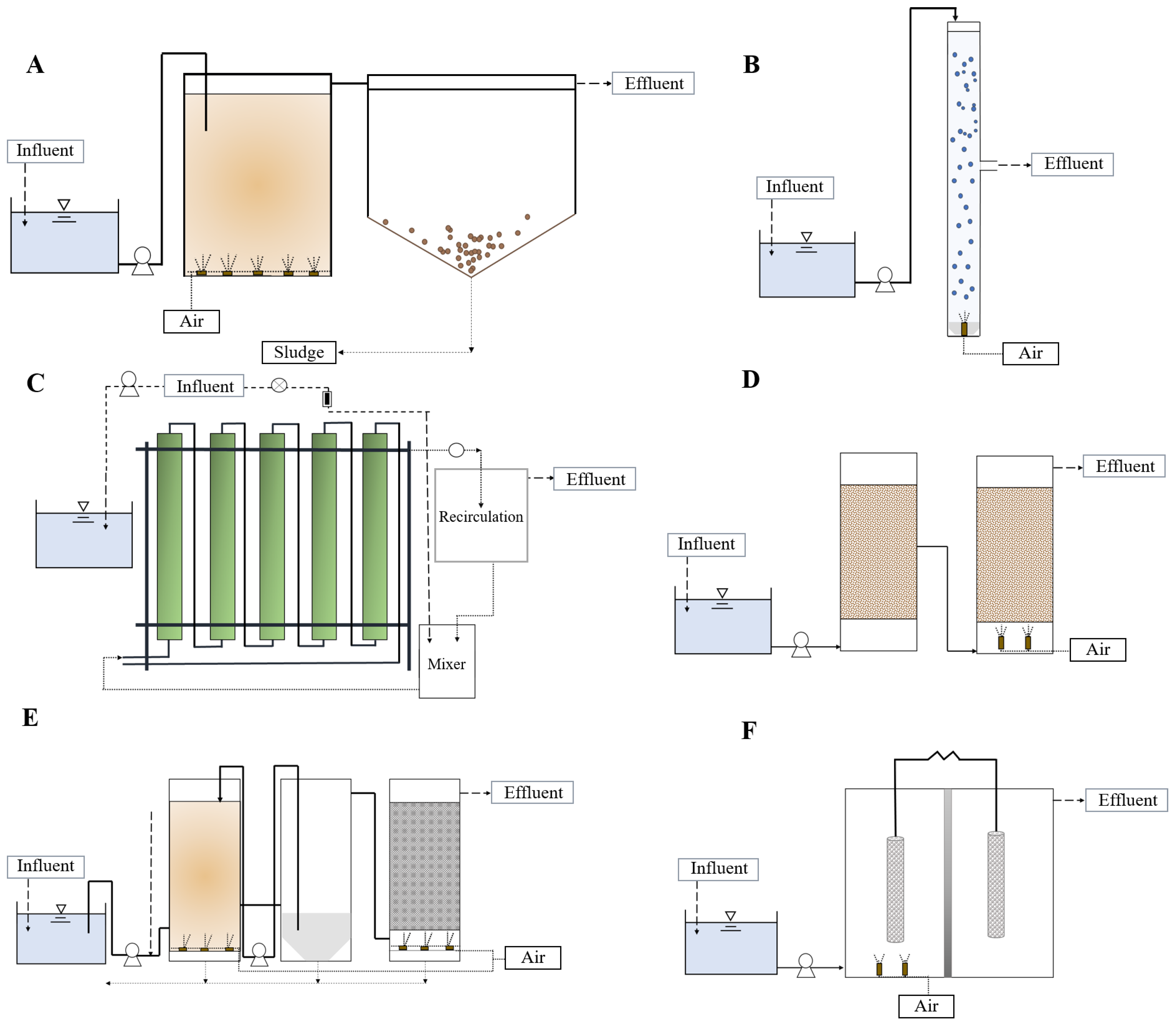
5.1. Conventional Activated Sludge
5.2. Aerobic Granular Sludge
5.3. Photobioreactors
5.4. Biofilters
5.5. Various Treatments Combined with Biofilters
5.5.1. Conventional Activated Sludge Coupled with an Immobilized Biological Filter
5.5.2. Expanded Granular Sludge Bed Coupled with Biofilter
5.5.3. Membrane Bioreactor Technology
5.6. Microbial Bio-electrochemical Technology
6. Microorganisms and Their Products Involved in Phenolic Biodegradation
7. Conclusions
- (a)
- The concentration and nature of the polyphenols (variability of composition);
- (b)
- The presence of other toxic and recalcitrant compounds in the raw water matrix;
- (c)
- The carbon, nitrogen, and phosphorous concentration;
- (d)
- The water quality requirements for the treated water;
- (e)
- Logistics: the operational and economic possibilities for its implementation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohd, A. Presence of Phenol in Wastewater Effluent and Its Removal: An Overview. Int. J. Environ. Anal. Chem. 2022, 102, 1362–1384. [Google Scholar] [CrossRef]
- Peeters, K.; Višnjevec, A.M.; Esakkimuthu, E.S.; Schwarzkopf, M.; Tavzes, Č. The Valorisation of Olive Mill Wastewater from Slovenian Istria by Fe3 O4 Particles to Recover Polyphenolic Compounds for the Chemical Specialties Sector. Molecules 2021, 26, 6946. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128132784. [Google Scholar]
- Cladis, D.P.; Weaver, C.M.; Ferruzzi, M.G. (Poly)Phenol Toxicity in Vivo Following Oral Administration: A Targeted Narrative Review of (Poly)Phenols from Green Tea, Grape, and Anthocyanin-Rich Extracts. Phytother. Res. 2022, 36, 323–335. [Google Scholar] [CrossRef]
- Bernini, R.; Pasqualetti, M.; Provenzano, G.; Tempesta, S. Ecofriendly Synthesis of Halogenated Flavonoids and Evaluation of Their Antifungal Activity. New J. Chem. 2015, 39, 2980–2987. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Provenzano, G.; Fabrizi, G.; Tempesta, S.; Pasqualetti, M. Obtaining New Flavanones Exhibiting Antifungal Activities by Methyltrioxorhenium-Catalyzed Epoxidation-Methanolysis of Flavones. Tetrahedron 2008, 64, 7561–7566. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary Lignans: Definition, Description and Research Trends in Databases Development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum Indicum l.): A Comprehensive Review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V.; Srivastana, J. Assessment of Bioremediation Ofoil and Phenol Contents in Refinery Waste Water via Bacterial Con-Sortium. J. Pet. Environ. Biotechnol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Raza, W.; Lee, J.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J. Removal of Phenolic Compounds from Industrial Waste Water Based on Membrane-Based Technologies. J. Ind. Eng. Chem. 2019, 71, 1–18. [Google Scholar] [CrossRef]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial Degradation of Phenol and Phenolic Derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Hydrophobic Eutectic Solvents for Extraction of Natural Phenolic Antioxidants from Winery Wastewater. Sep. Purif. Technol. 2021, 254, 117590. [Google Scholar] [CrossRef]
- Froldi, G.; Ragazzi, E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef]
- Santos-Buelga, C. Polyphenols and Human Beings: From Epidemiology to Molecular Targets. Molecules 2021, 26, 4218. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Sahiner, M.; Sanem Yilmaz, A.; Gungor, B.; Ayoubi, Y.; Sahiner, N. Therapeutic and Nutraceutical Effects of Polyphenolics from Natural Sources. Molecules 2022, 27, 6225. [Google Scholar] [CrossRef]
- Gorrasi, S.; Pasqualetti, M.; Muñoz-palazon, B.; Novello, G.; Mazzucato, A.; Campiglia, E.; Fenice, M. Comparison of the Peel-Associated Epiphytic Bacteria of Anthocyanin-Rich “Sun Black” and Wild-Type Tomatoes under Organic and Conventional Farming. Microorganisms 2022, 10, 2240. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Laganà, P.; Anastasi, G.; Marano, F.; Piccione, S.; Singla, R.K.; Dubey, A.K.; Delia, S.; Coniglio, M.A.; Facciolà, A.; di Pietro, A.; et al. Phenolic Substances in Foods: Health Effects as Anti-Inflammatory and Antimicrobial Agents. J. AOAC Int. 2019, 102, 1378–1387. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kargari, A.; Sanaeepur, H.; Abbassian, K.; Najafi, A.; Mofarrah, E. Phenol Removal from Industrial Wastewaters: A Short Review. Desalination Water Treat 2015, 53, 2215–2234. [Google Scholar] [CrossRef]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; de Castro, I.M.; Juarez-Jimenez, B.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J. Performance and Microbial Community Structure of an Aerobic Granular Sludge System at Different Phenolic Acid Concentrations. J. Hazard. Mater. 2019, 376, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, X.; Gong, Z.; Yang, F. Removal of COD, Phenols and Ammonium from Lurgi Coal Gasification Wastewater Using A2O-MBR System. J. Hazard. Mater. 2012, 235–236, 78–84. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Martínez-Toledo, M.V.; González-López, J.; Rodelas, B.; Juárez-Jiménez, B.; Fenice, M. Biodegradation of Olive Washing Wastewater Pollutants by Highly Efficient Phenol-Degrading Strains Selected from Adapted Bacterial Community. Int. Biodeterior. Biodegrad. 2013, 82, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of Different Ultrafiltration Membranes as First Step for the Recovery of Phenolic Compounds from Olive-Oil Washing Wastewater. Process Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of Bioactive Phenolic Compounds in Olive Mill Wastewater by Direct Contact Membrane Distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of High Added Value Natural Polyphenols from Actual Olive Mill Wastewater through Solid Phase Extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Nabavi, S.F.; di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; Rosa, M.J.F.; de Pinho, M.N. Concentration Polarization in Ultrafiltration/Nanofiltration for the Recovery of Polyphenols from Winery Wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Yangui, A.; Abderrabba, M. Towards a High Yield Recovery of Polyphenols from Olive Mill Wastewater on Activated Carbon Coated with Milk Proteins: Experimental Design and Antioxidant Activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Chen, B.; Yang, S.; Qian, Y. Optimal Design of an Efficient Polyphenols Extraction Process for High Concentrated Phenols Wastewater. J. Clean. Prod. 2017, 165, 1395–1406. [Google Scholar] [CrossRef]
- Mudimu, O.A.; Peters, M.; Brauner, F.; Braun, G. Overview of Membrane Processes for the Recovery of Polyphenols from Olive Mill Wastewater Olive Mill Wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Hellwig, V.; Gasser, J. Polyphenols from Waste Streams of Food Industry: Valorisation of Blanch Water from Marzipan Production. Phytochem. Rev. 2020, 19, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Thue, P.S.; Adebayo, M.A.; Lima, E.C.; Sieliechi, J.M.; Machado, F.M.; Dotto, G.L.; Vaghetti, J.C.P.; Dias, S.L.P. Preparation, Characterization and Application of Microwave-Assisted Activated Carbons from Wood Chips for Removal of Phenol from Aqueous Solution. J. Mol. Liq. 2016, 223, 1067–1080. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Removal of Methylene Blue and Phenol onto Prepared Activated Carbon from Fox Nutshell by Chemical Activation in Batch and Fixed-Bed Column. J. Clean. Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, L.; Wu, X.; Tang, Y.; Wu, Y. Activated Carbon Coated Palygorskite as Adsorbent by Activation and Its Adsorption for Methylene Blue. J. Environ. Sci. 2015, 33, 97–105. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W.; Lee, C.Y.; Kang, S. Removal of Pb and Cu Ions from Aqueous Solution by Mn3O4-Coated Activated Carbon. J. Ind. Eng. Chem. 2015, 21, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Hakim, A.; Iqbal, A.; Bhuiyan, F.R.; Begum, M.K.; Sharmin, S.; Abir, R.A. Computational Study and Homology Modeling of Phenol Hydroxylase: Key Enzyme for Phenol Degradation. Int. J. Comput. Bioinform. Silico Model. 2015, 4, 691–698. [Google Scholar]
- Xiao, J.; Xie, Y.; Han, Q.; Cao, H.; Wang, Y.; Nawaz, F.; Duan, F. Superoxide Radical-Mediated Photocatalytic Oxidation of Phenolic Compounds over Ag+/TiO2: Influence of Electron Donating and Withdrawing Substituents. J. Hazard. Mater. 2016, 304, 126–133. [Google Scholar] [CrossRef]
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Aerobic Granulation: A Recent Development on the Biological Treatment of Pulp and Paper Wastewater. Environ. Technol. Innov. 2018, 9, 265–274. [Google Scholar] [CrossRef]
- Jemaat, Z.; Suárez-Ojeda, M.E.; Pérez, J.; Carrera, J. Sequentially Alternating Pollutant Scenarios of Phenolic Compounds in a Continuous Aerobic Granular Sludge Reactor Performing Simultaneous Partial Nitritation and O-Cresol Biodegradation. Bioresour. Technol. 2014, 161, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Leong, K.Y.; Ng, H.Y. Anaerobic Treatment of Pharmaceutical Wastewater: A Critical Review. Bioresour. Technol. 2017, 245, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Comparative Performance of Upflow Anaerobic Sludge Blanket Reactor and Anaerobic Membrane Bioreactor Treating Phenolic Wastewater: Overcoming High Salinity. Chem. Eng. J. 2019, 366, 480–490. [Google Scholar] [CrossRef]
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Impact of Long-Term Salinity Exposure in Anaerobic Membrane Bioreactors Treating Phenolic Wastewater: Performance Robustness and Endured Microbial Community. Water Res. 2018, 141, 172–184. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Huang, Y.; Hou, X.; Liu, S.; Ni, J. Correspondence Analysis of Bio-Refractory Compounds Degradation and Microbiological Community Distribution in Anaerobic Filter for Coking Wastewater Treatment. Chem. Eng. J. 2016, 304, 864–872. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular Polymeric Substances of Bacteria and Their Potential Environmental Applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Vashi, H.; Iorhemen, O.T.; Tay, J.H. Extensive Studies on the Treatment of Pulp Mill Wastewater Using Aerobic Granular Sludge (AGS) Technology. Chem. Eng. J. 2019, 359, 1175–1194. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, A.; Roy, C.; Yadav, A.K. An Algal Assisted Constructed Wetland-Microbial Fuel Cell Integrated with Sand Filter for Efficient Wastewater Treatment and Electricity Production. Chemosphere 2021, 263, 128132. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, S.; Moon, S.H.; Sivabalan, R.; Arabindoo, B.; Murugesan, V. Agricultural Solid Waste for the Removal of Organics: Adsorption of Phenol from Water and Wastewater by Palm Seed Coat Activated Carbon. Waste Manag. 2002, 22, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Edward, A.; William, T.L. Experiments on the Oxidation of Sewage without the Aid of Filters. J. Soc. Chem. Ind. 1914, 33, 523–539. [Google Scholar]
- Koivuranta, E.; Stoor, T.; Hattuniemi, J.; Niinimäki, J. On-Line Optical Monitoring of Activated Sludge Floc Morphology. J. Water Process. Eng. 2015, 5, 28–34. [Google Scholar] [CrossRef]
- Abdel Wahaab, R.; Mahmoud, M.; van Lier, J.B. Toward Achieving Sustainable Management of Municipal Wastewater Sludge in Egypt: The Current Status and Future Prospective. Renew. Sustain. Energy Rev. 2020, 127, 109880. [Google Scholar] [CrossRef]
- Siripattanakul-Ratpukdi, S. Phenolic Based Pharmaceutical Contaminated Wastewater Treatment Kinetics by Activated Sludge Process. J. Clean Energy Technol. 2014, 2, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A.; Das, S.K. Phenol Adsorption from Wastewater Using Clarified Sludge from Basic Oxygen Furnace. J. Environ. Chem. Eng. 2019, 7, 103259. [Google Scholar] [CrossRef]
- Elmansour, T.E.; Mandi, L.; Ahmali, A.; Elghadraoui, A.; Aziz, F.; Hejjaj, A.; Del Bubba, M.; Ouazzani, N. Effect of Polyphenols on Activated Sludge Biomass during the Treatment of Highly Diluted Olive Mill Wastewaters: Biomass Dynamics and Purifying Performances. Water Sci. Technol. 2020, 82, 1416–1429. [Google Scholar] [CrossRef]
- Schellenberg, T.; Subramanian, V.; Ganeshan, G.; Tompkins, D.; Pradeep, R. Wastewater Discharge Standards in the Evolving Context of Urban Sustainability–The Case of India. Front. Environ. Sci. 2020, 8, 30. [Google Scholar] [CrossRef]
- Center, Regional Environmental. Regional Environmental Center Section 5 Water Protection Legislation. In Handbook on the Implementation of EC Environmental Legislation; Publication office: European Commission: Brussels, Belgium, 2008; pp. 613–733. [Google Scholar]
- Fang, F.; Qiao, L.L.; Ni, B.J.; Cao, J.S.; Yu, H.Q. Quantitative Evaluation on the Characteristics of Activated Sludge Granules and Flocs Using a Fuzzy Entropy-Based Approach. Sci. Rep. 2017, 7, srep42910. [Google Scholar] [CrossRef] [Green Version]
- Rosa-Masegosa, A.; Muñoz-Palazon, B.; Gonzalez-Martinez, A.; Fenice, M.; Gorrasi, S.; Gonzalez-Lopez, J. New Advances in Aerobic Granular Sludge Technology Using Continuous Flow Reactors: Engineering and Microbiological Aspects. Water 2021, 13, 1792. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.; Wibisono, Y.; Jaafar, J.; Indra Mahlia, T.M.; Khan, A.L.; Aslam, M. Recent Progress in Integrated Fixed-Film Activated Sludge Process for Wastewater Treatment: A Review. J. Environ. Manag. 2020, 268, 110718. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.E.; Abusam, A.; Mydlarczyk, A. Kinetic Modeling of GAC-IFAS Chemostat for Petrochemical Wastewater Treatment. J. Water Resour. Hydraul. Eng. 2017, 6, 27–33. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsien, T.Y. Kinetics of Biodegradation of Phenolic Wastewater in a Biofilm Reactor. Water Sci. Technol. 2009, 59, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Chen, Y.Y.; Lin, B.; Lee, D.J. Degrading High-Strength Phenol Using Aerobic Granular Sludge. Appl. Microbiol. Biotechnol. 2010, 85, 2009–2015. [Google Scholar] [CrossRef]
- Suja, E.; Nancharaiah, Y.V.; Venugopalan, V.P. P-Nitrophenol Biodegradation by Aerobic Microbial Granules. Appl. Biochem. Biotechnol. 2012, 167, 1569–1577. [Google Scholar] [CrossRef]
- Wang, S.G.; Liu, X.W.; Zhang, H.Y.; Gong, W.X.; Sun, X.F.; Gao, B.Y. Aerobic Granulation for 2,4-Dichlorophenol Biodegradation in a Sequencing Batch Reactor. Chemosphere 2007, 69, 769–775. [Google Scholar] [CrossRef]
- Duque, A.F.; Bessa, V.S.; Carvalho, M.F.; de Kreuk, M.K.; van Loosdrecht, M.C.M.; Castro, P.M.L. 2-Fluorophenol Degradation by Aerobic Granular Sludge in a Sequencing Batch Reactor. Water Res. 2011, 45, 6745–6752. [Google Scholar] [CrossRef]
- Liu, Y.; Woon, K.H.; Yang, S.F.; Tay, J.H. Influence of Phenol on Cultures of Acetate-Fed Aerobic Granular Sludge. Lett. Appl. Microbiol. 2002, 35, 162–165. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.L.; Tay, J.H.; Tay, S.T.L. Aggregation of Immobilized Activated Sludge Cells into Aerobically Grown Microbial Granules for the Aerobic Biodegradation of Phenol. Lett. Appl. Microbiol. 2002, 35, 439–445. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.-Z.; Liu, M.; Cai, H.W. Continuous Microalgae Cultivation in Aquaculture Wastewater by a Membrane Photobioreactor for Biomass Production and Nutrients Removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous Microalgae Cultivation and Wastewater Treatment in Submerged Membrane Photobioreactors: A Review. Algal. Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Muñoz, R.; Köllner, C.; Guieysse, B. Biofilm Photobioreactors for the Treatment of Industrial Wastewaters. J. Hazard. Mater. 2009, 161, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; González-Martínez, A.; Martínez-Toledo, M.V.; Fenice, M.; Lasserrot, A.; González-López, J. Biotreatment of Industrial Olive Washing Water by Synergetic Association of Microalgal-Bacterial Consortia in a Photobioreactor. Environ. Sci. Pollut. Res. 2017, 24, 527–538. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; González-Martínez, A.; Rodelas, B.; González-López, J. Full-Scale Photobioreactor for Biotreatment of Olive Washing Water: Structure and Diversity of the Microalgae-Bacteria Consortium. Bioresour. Technol. 2017, 238, 389–398. [Google Scholar] [CrossRef]
- Kirisits, M.J.; Emelko, M.B.; Pinto, A.J. Applying Biotechnology for Drinking Water Biofiltration: Advancing Science and Practice. Curr. Opin. Biotechnol. 2019, 57, 197–204. [Google Scholar] [CrossRef]
- Lewandowski, Z.; Boltz, J.P. Biofilms in Water and Wastewater Treatment. Treatise Water Sci. 2011, 4, 529–570. [Google Scholar] [CrossRef]
- González-Martínez, A.; Muñoz-Palazon, B.; Kruglova, A.; Vilpanen, M.; Kuokkanen, A.; Mikola, A.; Heinonen, M. Performance and Microbial Community Structure of a Full-Scale ANITATMMox Bioreactor for Treating Reject Water Located in Finland. Chemosphere 2021, 271, 129526. [Google Scholar] [CrossRef]
- Garcia-Ruiz, M.J.; Muñoz-Palazon, B.; Gonzalez-Lopez, J.; Osorio, F. Performance and Microbial Community Structure of an Anammox Biofilter Treating Real Wastewater from a Sludge Return. J. Environ. Chem. Eng. 2021, 9, 105211. [Google Scholar] [CrossRef]
- Hellal, M.S.; Abou-Elela, S.I.; Aly, O.H. Potential of Using Nonwoven Polyester Fabric (NWPF) as a Packing Media in Multistage Passively Aerated Biological Filter for Municipal Wastewater Treatment. Water Environ. J. 2020, 34, 247–258. [Google Scholar] [CrossRef]
- Zhu, I.X.; Getting, T.; Bruce, D. Review of Biologically Active Filters in Drinking Water Applications. J. Am. Water Work. Assoc. 2010, 102, 67–77. [Google Scholar] [CrossRef]
- Orhon, D.; Sözen, S. Reshaping the Activated Sludge Process: Has the Time Come or Passed? J. Chem. Technol. Biotechnol. 2020, 95, 1632–1639. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Behera, S.S.; Patra, J.K.; Thatoi, H.; Parhi, P.K. Potential Application of Bacterial Biofilm for Bioremediation of Toxic Heavy Metals and Dye-Contaminated Environments; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444642790. [Google Scholar]
- Muffler, K.; Ulber, R. Productive Biofilms; Springer: Cham, Switzerland, 2014; Volume 146, ISBN 978-3-319-09694-0. [Google Scholar]
- Hou, J.; Yu, C.; Meng, F.; He, X.; Wang, Y.; Chen, W.; Li, M. Succession of the Microbial Community during the Process of Mechanical and Biological Pretreatment Coupled with a Bio-Filter for Removal of VOCs Derived from Domestic Waste: A Field Study. RSC Adv. 2021, 11, 39924–39933. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P.K. Treatment of Heavy Oil Wastewater by a Conventional Activated Sludge Process Coupled with an Immobilized Biological Filter. Int. Biodeterior. Biodegrad. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Hasan, S.A.; Jabeen, S. Degradation Kinetics and Pathway of Phenol by Pseudomonas and Bacillus Species. Biotechnol. Biotechnol. Equip. 2015, 29, 45–53. [Google Scholar] [CrossRef]
- Prasad, R.; Aranda, E. Approaches in Bioremediation: The New Era of Environmental Microbiology and Nanobiotechnology; Springer: Cham, Switzerland, 2018; ISBN 978-3-030-02368-3. [Google Scholar]
- Wang, J.; Wang, X.; Yu, Z.; Huang, S.; Yao, D.; Xiao, J.; Chen, W.; Wang, Z.; Zan, F. Using Algae Bacteria Consortia to Effectively Treat Coking Wastewater: Performance, Microbial Community, and Mechanism. J. Clean. Prod. 2022, 334, 130269. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, G.; Wang, Q.; Chen, C.; Li, M.; Guo, S. Refining Wastewater Treatment Using EGSB-BAF System. Desalination Water Treat 2015, 53, 2808–2815. [Google Scholar] [CrossRef]
- Collins, G.; Foy, C.; McHugh, S.; O’Flaherty, V. Anaerobic Treatment of 2,4,6-Trichlorophenol in an Expanded Granular Sludge Bed-Anaerobic Filter (EGSB-AF) Bioreactor at 15 °C. FEMS Microbiol. Ecol. 2005, 53, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Rintala, J.A.; Puhakka, J.A. Anaerobic Treatment in Pulp- and Paper-Mill Waste Management: A Review. Bioresour. Technol. 1994, 47, 1–18. [Google Scholar] [CrossRef]
- Song, J.; Zhao, Q.; Guo, J.; Yan, N.; Chen, H.; Sheng, F.; Lin, Y.; An, D. The Microbial Community Responsible for Dechlorination and Benzene Ring Opening during Anaerobic Degradation of 2,4,6-trichlorophenol. Sci. Total Environ. 2019, 651, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane Bioreactor Technology for Wastewater Treatment and Reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Koyuncu, I.; Sengur, R.; Turken, T.; Guclu, S.; Pasaoglu, M.E. Advances in Water Treatment by Microfiltration, Ultrafiltration, and Nanofiltration; Woodhead Publishing: Oxford, UK, 2015; Volume 2019, ISBN 9781782421269. [Google Scholar]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Muñoz-Palazon, B.; Rivadeneyra, M.A.; Hurtado-Martinez, M.; Martin-Ramos, D.; Gonzalez-Martinez, A.; Poyatos, J.M.; Gonzalez-Lopez, J. Biofouling Formation and Bacterial Community Structure in Hybrid Moving Bed Biofilm Reactor-Membrane Bioreactors: Influence of Salinity Concentration. Water 2018, 10, 1133. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Olivares, C.I.; Pinto, A.J.; Lauderdale, C.V.; Brown, J.; Selbes, M.; Karanfil, T. The Control of Disinfection Byproducts and Their Precursors in Biologically Active Filtration Processes. Water Res. 2017, 124, 630–653. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Loh, K.C. Phenolic Wastewater Treatment through Extractive Recovery Coupled with Biodegradation in a Two-Phase Partitioning Membrane Bioreactor. Chemosphere 2015, 141, 176–182. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Borghei, S.M. The Treatment of Phenolic Wastewater Using a Moving Bed Bio-Reactor. Process. Biochem. 2005, 40, 1027–1031. [Google Scholar] [CrossRef]
- Mancuso, A.; Morante, N.; De Carluccio, M.; Sacco, O.; Rizzo, L.; Fontana, M.; Esposito, S.; Vaiano, V.; Sannino, D. Solar Driven Photocatalysis Using Iron and Chromium Doped TiO2 Coupled to Moving Bed Biofilm Process for Olive Mill Wastewater Treatment. Chem. Eng. J. 2022, 450, 138107. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, A.; Gonzalez-Lopez, J.; Ruiz, L.M.; Gómez-Nieto, M.Á.; Muñoz-Palazon, B. Effect of Ultrasonic Frequency on the Bacterial Community Structure during Biofouling Formation in Microfiltration Membrane Bioreactors for Wastewater Treatment. Int. Biodeterior. Biodegrad. 2020, 155, 105102. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Muñoz-Palazon, B.; Gonzalez-Lopez, J.; Poyatos, J.M. Effect of Variable Salinity Wastewater on Performance and Kinetics of Membrane-Based Bioreactors. J. Chem. Technol. Biotechnol. 2019, 94, 3236–3250. [Google Scholar] [CrossRef]
- Muñoz Sierra, J.D.; Oosterkamp, M.J.; Spanjers, H.; van Lier, J.B. Effects of Large Salinity Fluctuations on an Anaerobic Membrane Bioreactor Treating Phenolic Wastewater. Chem. Eng. J. 2021, 417, 129263. [Google Scholar] [CrossRef]
- Mosca Angelucci, D.; Donati, E.; Tomei, M.C. Extractive Membrane Bioreactor to Detoxify Industrial/Hazardous Landfill Leachate and Facilitate Resource Recovery. Sci. Total Environ. 2022, 806, 150892. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A.; Febbraio, F.; Andreescu, S. Microbial Electrochemical Systems: Principles, Construction and Biosensing Applications. Sensors 2021, 21, 1279. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial Fuel Cell as New Technol Ogy for Bioelectricity Generation: A Review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, M.; Wan Daud, W.R.; Ismail, M.; Rahimnejad, M.; Ismail, A.F.; Leong, J.X.; Miskan, M.; Ben Liew, K. Effect of Pre-Treatment and Biofouling of Proton Exchange Membrane on Microbial Fuel Cell Performance. Int. J. Hydrogen Energy 2013, 38, 5480–5484. [Google Scholar] [CrossRef]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of Hydrogen from Domestic Wastewater Using a Bioelectrochemically Assisted Microbial Reactor (BEAMR). Int. J. Hydrogen Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Wang, X.; Aulenta, F.; Puig, S.; Esteve-Núñez, A.; He, Y.; Mu, Y.; Rabaey, K. Microbial Electrochemistry for Bioremediation. Environ. Sci. Ecotechnology 2020, 1, 100013. [Google Scholar] [CrossRef]
- Maddalwar, S.; Kumar Nayak, K.; Kumar, M.; Singh, L. Plant Microbial Fuel Cell: Opportunities, Challenges, and Prospects. Bioresour. Technol. 2021, 341, 125772. [Google Scholar] [CrossRef]
- Friman, H.; Schechter, A.; Nitzan, Y.; Cahan, R. Phenol Degradation in Bio-Electrochemical Cells. Int. Biodeterior. Biodegrad. 2013, 84, 155–160. [Google Scholar] [CrossRef]
- Hedbavna, P.; Rolfe, S.A.; Huang, W.E.; Thornton, S.F. Biodegradation of Phenolic Compounds and Their Metabolites in Contaminated Groundwater Using Microbial Fuel Cells. Bioresour. Technol. 2016, 200, 426–434. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.N.; Tang, C.; Zhou, J.; Wu, X.Y.; Xie, X.X.; Wei, P.; Jia, H.H.; Yong, X.Y. Degradation of Phenolic Compounds with Simultaneous Bioelectricity Generation in Microbial Fuel Cells: Influence of the Dynamic Shift in Anode Microbial Community. Bioresour. Technol. 2019, 291, 121862. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Collins, M.A.; Borole, A.P.; Pavlostathis, S.G. The Extent of Fermentative Transformation of Phenolic Compounds in the Bioanode Controls Exoelectrogenic Activity in a Microbial Electrolysis Cell. Water Res. 2017, 109, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Jiménez, B.; Manzanera, M.; Rodelas, B.; Martínez-Toledo, M.V.; Gonzalez-López, J.; Crognale, S.; Pesciaroli, C.; Fenice, M. Metabolic characterization of a strain (BM90) of Delftia tsuruhatensis showing highly diversified capacity to degrade low molecular weight phenols. Biodegradation 2010, 21, 475–489. [Google Scholar] [CrossRef]
- Juarez Jimenez, B.; Reboleiro Rivas, P.; Gonzalez Lopez, J.; Pesciaroli, C.; Barghini, P.; Fenice, M. Immobilization of Delftia Tsuruhatensis in Macro-Porous Cellulose and Biodegradation of Phenolic Compounds in Repeated Batch Process. J. Biotechnol. 2012, 157, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Satish, K.; Neeraj; Viraj, K.M.; Santosh, K.K. Biodegradation of Phenol by Free and Immobilized Candida Tropicalis NPD1401. Afr. J. Biotechnol. 2018, 17, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.K.; Srivastava, A.; Singh, V.P. Bacterial Degradation of Nitrophenols and Their Derivatives. J. Hazard. Mater. 2014, 266, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent Advances in Degradation of Chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909. [Google Scholar] [CrossRef]
- Arora, P.K.; Sasikala, C.; Ramana, C.V. Degradation of Chlorinated Nitroaromatic Compounds. Appl. Microbiol. Biotechnol. 2012, 93, 2265–2277. [Google Scholar] [CrossRef]
- Singh, T.; Bhatiya, A.K.; Mishra, P.K.; Srivastava, N. An effective approach for the degradation of phenolic waste. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthaku, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–243. [Google Scholar] [CrossRef]
- Arora, P.K.; Jain, R.K. Arthrobacter Nitrophenolicus Sp. Nov. a New 2-Chloro-4-Nitrophenol Degrading Bacterium Isolated from Contaminated Soil. 3 Biotech 2013, 3, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Arora, P.K.; Jain, R.K. Metabolism of 2-Chloro-4-Nitrophenol in a Gram Negative Bacterium, Burkholderia Sp. RKJ 800. PLoS ONE 2012, 7, e38676. [Google Scholar] [CrossRef] [Green Version]
- Dulak, K.; Sordon, S.; Matera, A.; Kozak, B.; Huszcza, E.; Popłoński, J. Novel Flavonoid C-8 Hydroxylase from Rhodotorula Glutinis: Identification, Characterization and Substrate Scope. Microb. Cell Fact. 2022, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.C.; Cyle, K.T.; Martinez, C.E.; Karasz, D.C.; Newman, J.D.; Buckley, D.H. Paraburkholderia Solitsugae Sp. Nov. and Paraburkholderia Elongata Sp. Nov., Phenolic Acid-Degrading Bacteria Isolated from Forest Soil and Emended Description of Paraburkholderia Madseniana. Int. J. Syst. Evol. Microbiol. 2020, 70, 5093–5105. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of Phenolic Compounds by Basidiomycota and Its Phenol Oxidases: A Review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal Laccases-Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Ikehata, K.; Buchanan, I.D.; Smith, D.W. Recent Developments in the Production of Extracellular Fungal Peroxidases and Laccases for Waste Treatment. J. Environ. Eng. Sci. 2004, 3, 1–19. [Google Scholar] [CrossRef]
- Shukla, A.C. Applied Mycology Entrepreneurship with Fungi; Springer: Cham, Switzerland, 2022; ISBN 9783030906481. [Google Scholar]
- Otto, B.; Schlosser, D. First Laccase in Green Algae: Purification and Characterization of an Extracellular Phenol Oxidase from Tetracystis Aeria. Planta 2014, 240, 1225–1236. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Utilization of Phenolic Compounds by Microalgae. Algal. Res. 2019, 42, 101602. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Strain Improvement of Chlorella Sp. for Phenol Biodegradation by Adaptive Laboratory Evolution. Bioresour. Technol. 2016, 205, 264–268. [Google Scholar] [CrossRef]
- Di Caprio, F.; Scarponi, P.; Altimari, P.; Iaquaniello, G.; Pagnanelli, F. The Influence of Phenols Extracted from Olive Mill Wastewater on the Heterotrophic and Mixotrophic Growth of Scenedesmus sp. J. Chem. Technol. Biotechnol. 2018, 93, 3619–3626. [Google Scholar] [CrossRef]
- Pinto, G.; Pollio, A.; Previtera, L.; Stanzione, M.; Temussi, F. Removal of Low Molecular Weight Phenols from Olive Oil Mill Wastewater Using Microalgae. Biotechnol. Lett. 2003, 25, 1657–1659. [Google Scholar] [CrossRef]

| Type of Industry | Range of Total Phenol Concentration (mg·L−1) |
|---|---|
| Rubber | 3–10 |
| Leather | 4–6 |
| Ferrous | 5–9 |
| Pulp and paper | 22 |
| Fiberglass | 40–2564 |
| Petroleum-processing plant | 40–185 |
| Wood Preserving | 50–953 |
| Fabric | 100–150 |
| Petrochemical | 200–1200 |
| Coke ovens (without dephenolization) | 300–3900 |
| Olive washing | 400–1120 |
| Agri-food (winery, oil) | 400–10,700 |
| Phenolic resins | 1270–1345 |
| Coal conversion | 1700–7000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Palazon, B.; Gorrasi, S.; Rosa-Masegosa, A.; Pasqualetti, M.; Braconcini, M.; Fenice, M. Treatment of High-Polyphenol-Content Waters Using Biotechnological Approaches: The Latest Update. Molecules 2023, 28, 314. https://doi.org/10.3390/molecules28010314
Muñoz-Palazon B, Gorrasi S, Rosa-Masegosa A, Pasqualetti M, Braconcini M, Fenice M. Treatment of High-Polyphenol-Content Waters Using Biotechnological Approaches: The Latest Update. Molecules. 2023; 28(1):314. https://doi.org/10.3390/molecules28010314
Chicago/Turabian StyleMuñoz-Palazon, Barbara, Susanna Gorrasi, Aurora Rosa-Masegosa, Marcella Pasqualetti, Martina Braconcini, and Massimiliano Fenice. 2023. "Treatment of High-Polyphenol-Content Waters Using Biotechnological Approaches: The Latest Update" Molecules 28, no. 1: 314. https://doi.org/10.3390/molecules28010314
APA StyleMuñoz-Palazon, B., Gorrasi, S., Rosa-Masegosa, A., Pasqualetti, M., Braconcini, M., & Fenice, M. (2023). Treatment of High-Polyphenol-Content Waters Using Biotechnological Approaches: The Latest Update. Molecules, 28(1), 314. https://doi.org/10.3390/molecules28010314










