The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches
Abstract
1. Introduction
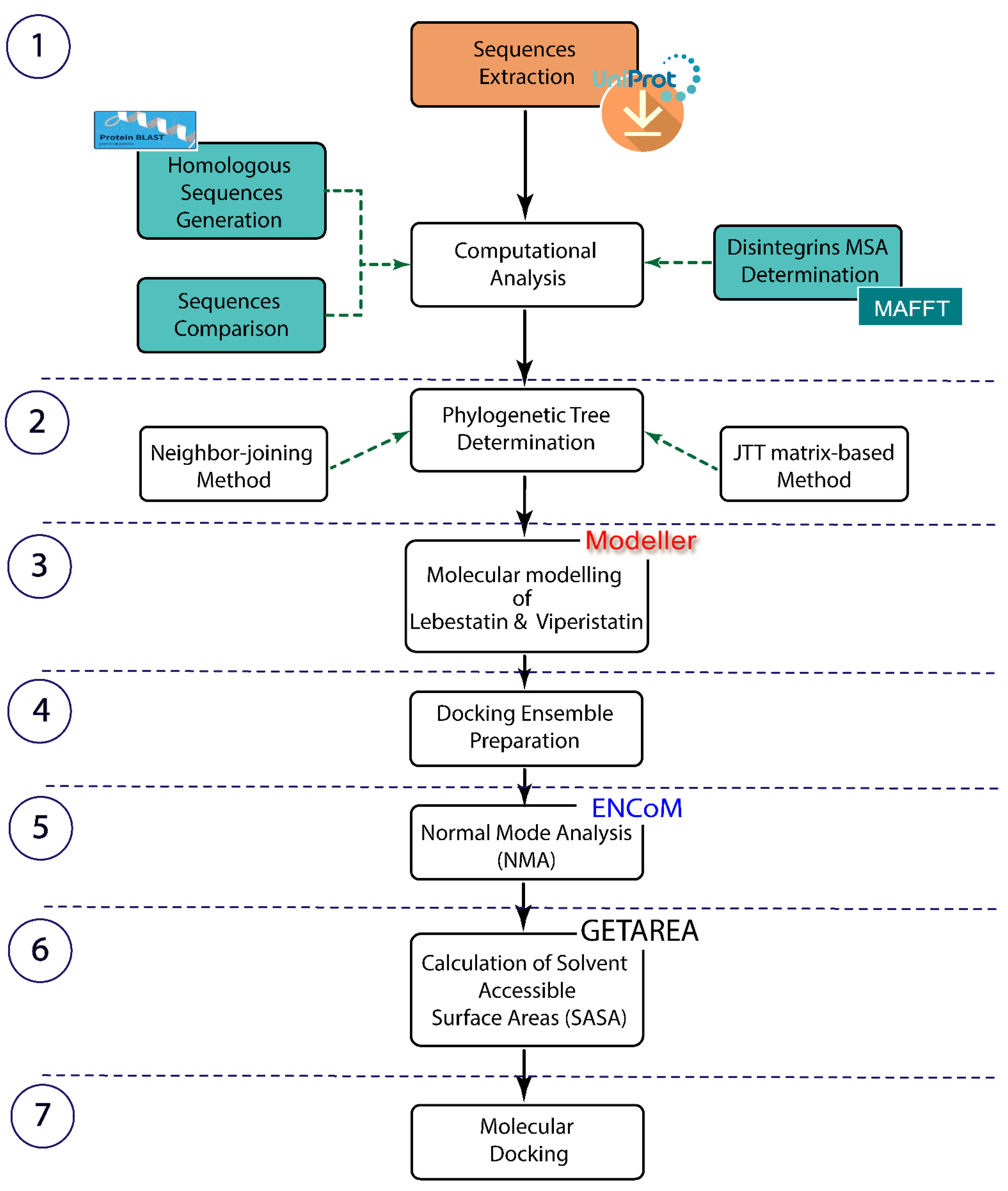
2. Results
2.1. Bioinformatics Approaches
2.2. Disintegrins Phylogenetic Tree Analysis
2.3. Molecular Modelling
2.4. Normal Modes Analysis
2.5. Calculation of Solvent Accessible Surface Areas
2.6. Molecular Docking (Protein-Protein Docking)
3. Discussion
4. Materials and Methods
4.1. Sequences Extraction and Computational Analysis
4.2. Phylogenetic Tree Determination
4.3. Lebestatin and Viperistatin Molecular Modelling
4.4. Docking Ensemble Preparation
4.5. Normal Modes Analysis (NMA)
4.6. Calculation of Solvent Accessible Surface Areas
4.7. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion venom components as potential candidates for drug development. Toxicon Off. J. Int. Soc. Toxinol. 2015, 93, 125–135. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Homology modeling in drug discovery: Overview, current applications, and future perspectives. Chem. Biol. Drug Des. 2018, 93, 12–20. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, F.; Wu, F.-X.; Li, Y.; Wang, J.; Li, M. Protein-protein interaction site prediction through combining local and global features with deep neural networks. Bioinformatics 2020, 36, 1114–1120. [Google Scholar] [CrossRef]
- Chen, C.; Huang, H.; Wu, C.H. Protein Bioinformatics Databases and Resources. Protein Bioinform. 2017, 1558, 3–39. [Google Scholar] [CrossRef]
- Ejaz, S.; Hashmi, F.B.; Malik, W.N.; Ashraf, M.; Nasim, F.U.-H.; Iqbal, M. Applications of Venom Proteins as Potential Anticancer Agents. Protein Pept. Lett. 2018, 25, 688–701. [Google Scholar] [CrossRef]
- Harvey, A.L. Toxins and drug discovery. Toxicon Off. J. Int. Soc. Toxinol. 2014, 92, 193–200. [Google Scholar] [CrossRef]
- Macêdo, J.; Fox, J.; Castro, M.S. Disintegrins from Snake Venoms and their Applications in Cancer Research and Therapy. Curr. Protein Pept. Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef]
- Kisiel, D.G.; Calvete, J.; Katzhendler, J.; Fertala, A.; Lazarovici, P.; Marcinkiewicz, C. Structural determinants of the selectivity of KTS-disintegrins for the α1β1 integrin. FEBS Lett. 2004, 577, 478–482. [Google Scholar] [CrossRef]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; A Mousa, S.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar]
- Daidone, I.; Aschi, M.; Patamia, M.; Bozzi, A.; Petruzzelli, R. Structural and dynamical properties of KTS-disintegrins: A comparison between Obtustatin and Lebestatin. Biopolymers 2012, 99, 47–54. [Google Scholar] [CrossRef]
- Arnaout, M.; Mahalingam, B.; Xiong, J.-P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005, 21, 381–410. [Google Scholar] [CrossRef]
- Lee, J.-O.; Bankston, L.A.; Liddington, M.A.A.R.C. Two conformations of the integrin A-domain (I-domain): A pathway for activation? Structure 1995, 3, 1333–1340. [Google Scholar] [CrossRef]
- Brown, M.C.; Eble, J.A.; Calvete, J.J.; Marcinkiewicz, C. Structural requirements of KTS-disintegrins for inhibition of α1β1 integrin. Biochem. J. 2008, 417, 95–101. [Google Scholar] [CrossRef]
- Shi, M.; Pedchenko, V.; Greer, B.H.; Van Horn, W.D.; Santoro, S.A.; Sanders, C.R.; Hudson, B.G.; Eichman, B.F.; Zent, R.; Pozzi, A. Enhancing Integrin α1 Inserted (I) Domain Affinity to Ligand Potentiates Integrin α1β1-mediated Down-regulation of Collagen Synthesis. J. Biol. Chem. 2012, 287, 35139–35152. [Google Scholar] [CrossRef]
- Anderson, L.R.; Owens, T.W.; Naylor, M.J. Integrins in development and cancer. Biophys. Rev. 2013, 6, 191–202. [Google Scholar] [CrossRef][Green Version]
- Calvanese, L.; Falcigno, L.; D’Auria, G. Essential dynamics analysis captures the concerted motion of the integrin-binding site in jerdostatin, an RTS disintegrin. Biopolymers 2014, 103, 158–166. [Google Scholar] [CrossRef]
- Calvete, J.J.; Moreno-Murciano, M.P.; Theakston, R.D.G.; Kisiel, D.G.; Marcinkiewicz, C. Snake venom disintegrins: Novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem. J. 2003, 372, 725–734. [Google Scholar] [CrossRef]
- Calvete, J.J.; Marcinkiewicz, C.; Monleón, D.; Esteve, V.; Celda, B.; Juárez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon Off. J. Int. Soc. Toxinol. 2005, 45, 1063–1074. [Google Scholar] [CrossRef]
- Bazaa, A.; Marrakchi, N.; El Ayeb, M.; Sanz, L.; Calvete, J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakesCerastes cerastes, Cerastes vipera andMacrovipera lebetina. Proteomics 2005, 5, 4223–4235. [Google Scholar] [CrossRef]
- Ba, S.M.A.; Ma, K.Y.W.; Quattrocchi, E.; Alonso-Quinones, H.; Sominidi-Damodaran, S.; Meves, A. The role of integrins in melanoma: A review. Int. J. Dermatol. 2020, 59, 525–534. [Google Scholar] [CrossRef]
- Marcinkiewicz, C.; Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell–matrix interactions. Int. J. Biochem. Cell Biol. 2013, 45, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon Off. J. Int. Soc. Toxinol. 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Marcinkiewicz, C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon 2011, 58, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Olfa, K.-Z.; José, L.; Salma, D.; Amine, B.; Najet, S.A.; Nicolas, A.; Maxime, L.; Raoudha, Z.; Kamel, M.; Jacques, M.; et al. Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Lab. Investig. 2005, 85, 1507–1516. [Google Scholar] [CrossRef]
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen-receptor-dependent melanoma metastasis. Cancer Biol. Ther. 2009, 8, 1507–1516. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Estrada, J.C.; David, V.; Wermelinger, L.S.; Almeida, F.C.L.; Zingali, R.B. Structure-Function Relationship of the Disintegrin Family: Sequence Signature and Integrin Interaction. Front. Mol. Biosci. 2021, 8, 783301. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2018, 47, D464–D474. [Google Scholar] [CrossRef]
- Bhagwat, M.; Aravind, L. PSI-BLAST Tutorial. In Comparative genomics; Humana Press: Totowa, NJ, USA, 2007; Volume 395, pp. 177–186. [Google Scholar] [CrossRef]
- Kazutaka, K.; Misakwa, K.; Kei-ichi, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. [20] VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [CrossRef]
- Chin, Y.; Headey, S.; Mohanty, B.; Patil, R.; McEwan, P.A.; Swarbrick, J.D.; Mulhern, T.; Emsley, J.; Simpson, J.S.; Scanlon, M.J. The Structure of Integrin α1I Domain in Complex with a Collagen-mimetic Peptide. J. Biol. Chem. 2013, 288, 36796–36809. [Google Scholar] [CrossRef]
- Luo, B.-H.; Carman, C.V.; Springer, T.A. Structural Basis of Integrin Regulation and Signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef]
- Frappier, V.; Chartier, M.; Najmanovich, R.J. ENCoM server: Exploring protein conformational space and the effect of mutations on protein function and stability. Nucleic Acids Res. 2015, 43, W395–W400. [Google Scholar] [CrossRef]
- Bonneau, R.; Strauss, C.E.; A Rohl, C.; Chivian, D.; Bradley, P.; Malmström, L.; Robertson, T.; Baker, D. De Novo Prediction of Three-dimensional Structures for Major Protein Families. J. Mol. Biol. 2002, 322, 65–78. [Google Scholar] [CrossRef]
- Suhre, K.; Sanejouand, Y.-H. ElNemo: A normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res. 2004, 32, W610–W614. [Google Scholar] [CrossRef]
- Tama, F.; Gadea, F.X.; Marques, O.; Sanejouand, Y.-H. Building-block approach for determining low-frequency normal modes of macromolecules. Proteins Struct. Funct. Bioinform. 2000, 41, 1–7. [Google Scholar] [CrossRef]
- Tsodikov, O.V.; Record, M.T., Jr.; Sergeev, Y.V. Novel computer program for fast exact calculation of accessible and molecular surface areas and average surface curvature. J. Comput. Chem. 2002, 23, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; De Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [PubMed]




| K21 | T22 | S23 | L/R24 | V/S/L38 | G41 | |
|---|---|---|---|---|---|---|
| Viperistatin | 39.7% | 22% | 100% | 98.4% | 0% | 100% |
| Lebestatin | 17.1% | 27.2% | 84.3% | 96.2% | 0% | 100% |
| Obtustatin | 35% | 34.3% | 84% | 91.4% | 0% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamessi, O.; Ben Mabrouk, H.; Kamoun, S.; Hkimi, C.; Ghedira, K.; Kharrat, R. The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches. Molecules 2023, 28, 325. https://doi.org/10.3390/molecules28010325
Khamessi O, Ben Mabrouk H, Kamoun S, Hkimi C, Ghedira K, Kharrat R. The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches. Molecules. 2023; 28(1):325. https://doi.org/10.3390/molecules28010325
Chicago/Turabian StyleKhamessi, Oussema, Hazem Ben Mabrouk, Selim Kamoun, Chaima Hkimi, Kais Ghedira, and Riadh Kharrat. 2023. "The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches" Molecules 28, no. 1: 325. https://doi.org/10.3390/molecules28010325
APA StyleKhamessi, O., Ben Mabrouk, H., Kamoun, S., Hkimi, C., Ghedira, K., & Kharrat, R. (2023). The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches. Molecules, 28(1), 325. https://doi.org/10.3390/molecules28010325






