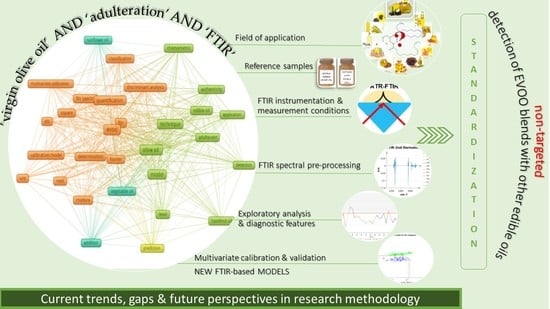
Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives
Abstract
:1. Introduction
1.1. Non-Targeted Authenticity Testing
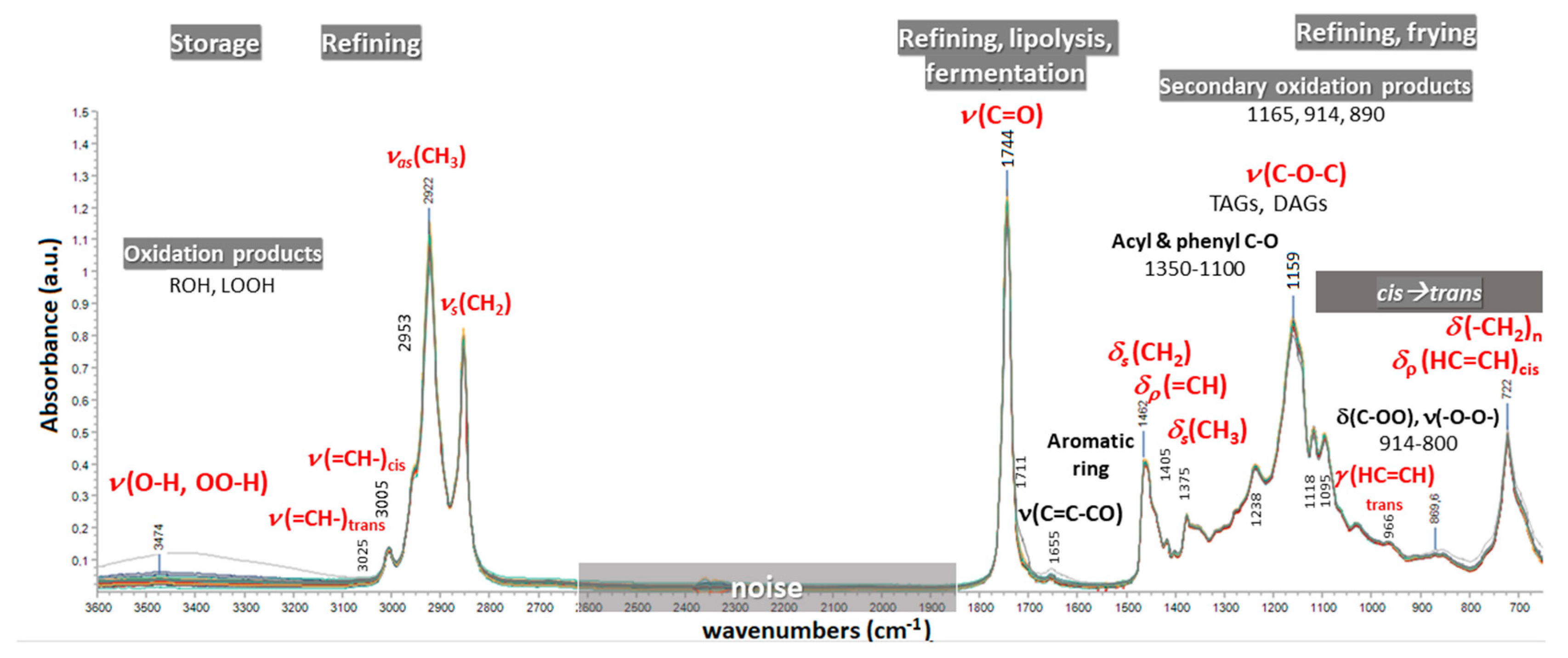
1.2. FTIR Spectroscopy in the Quality Control of EVOO/VOO
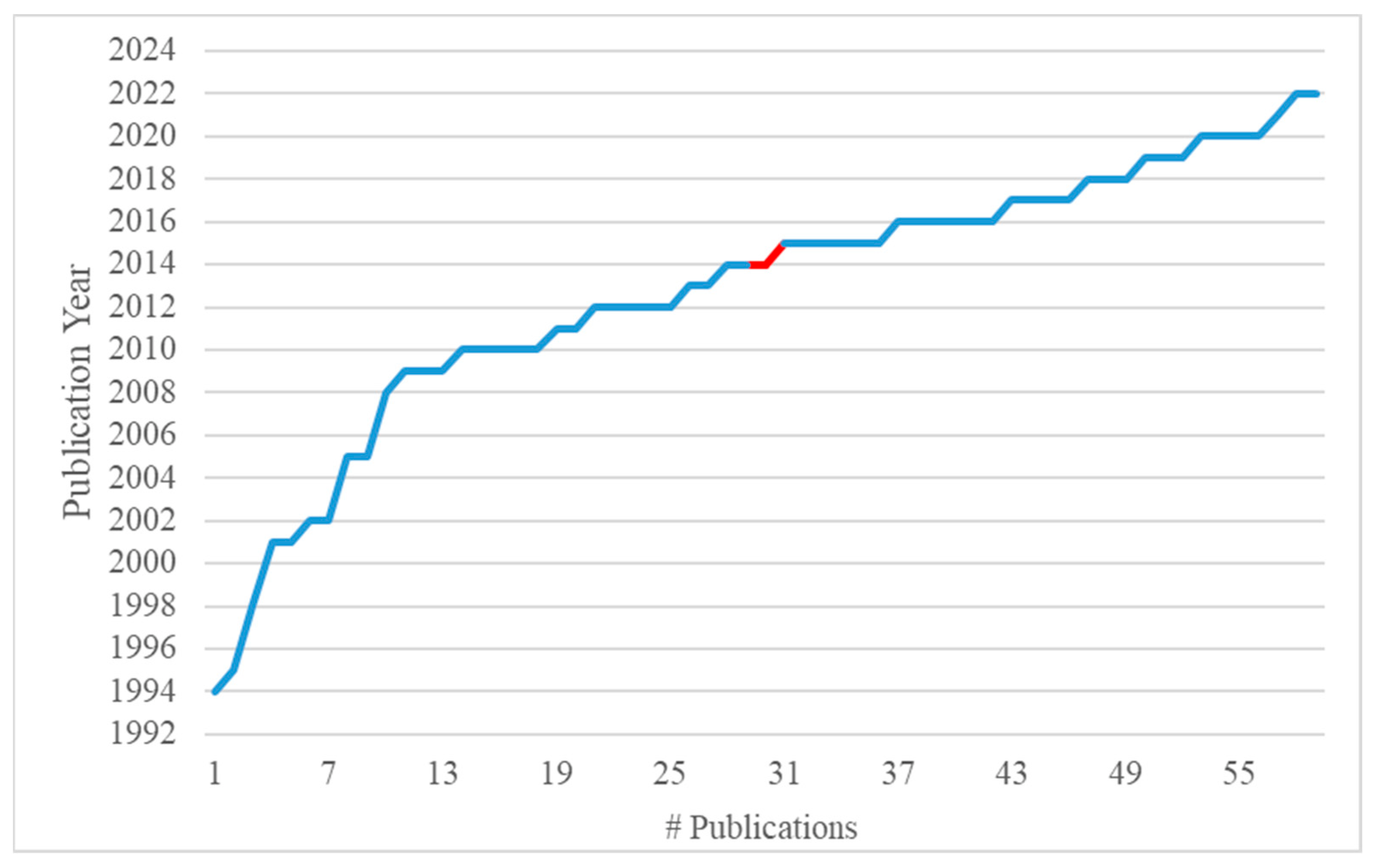
2. Concept and Methodology
3. Results and Discussion
3.1. Field of Application
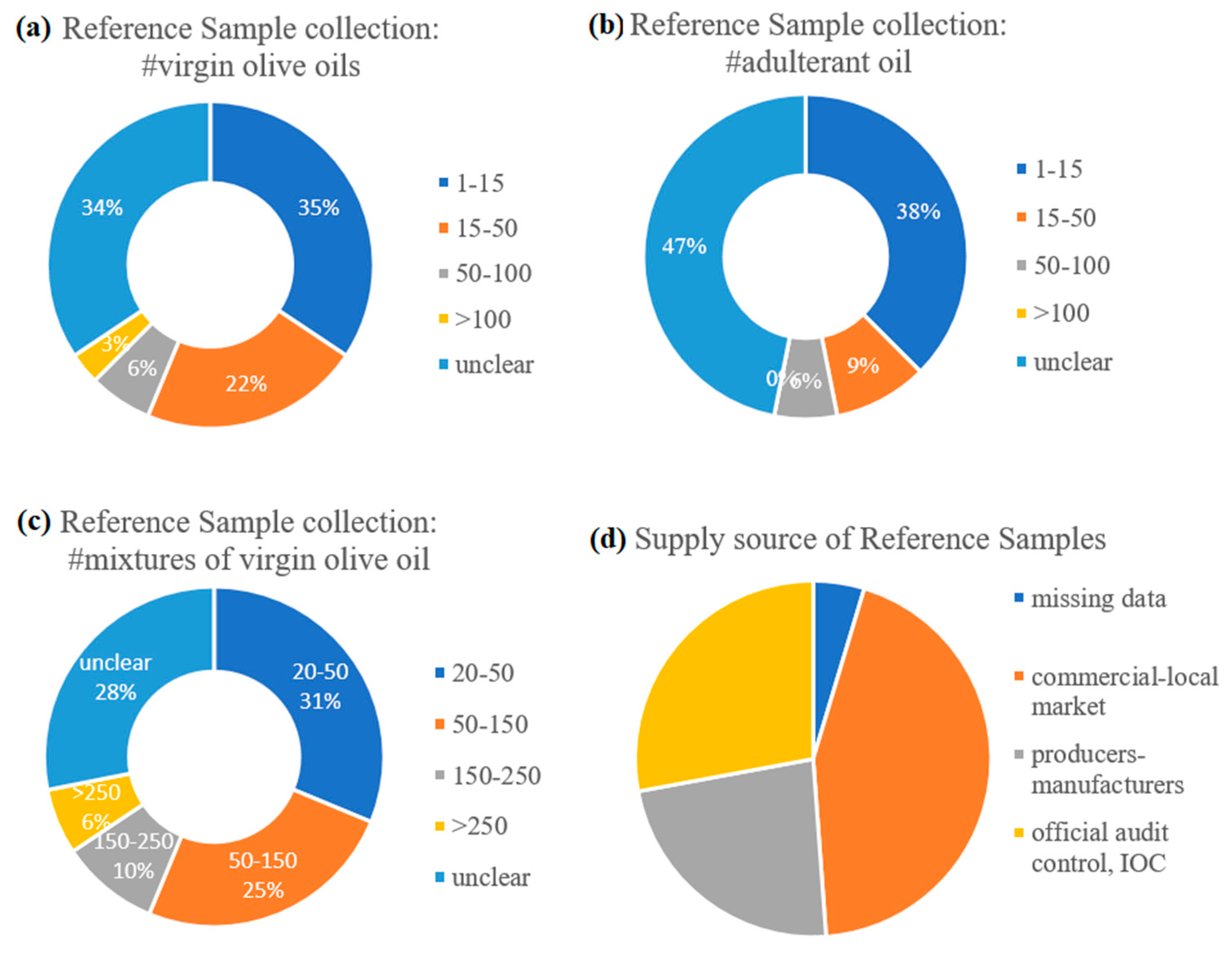
3.2. Sampling Methodology and Reference Samples
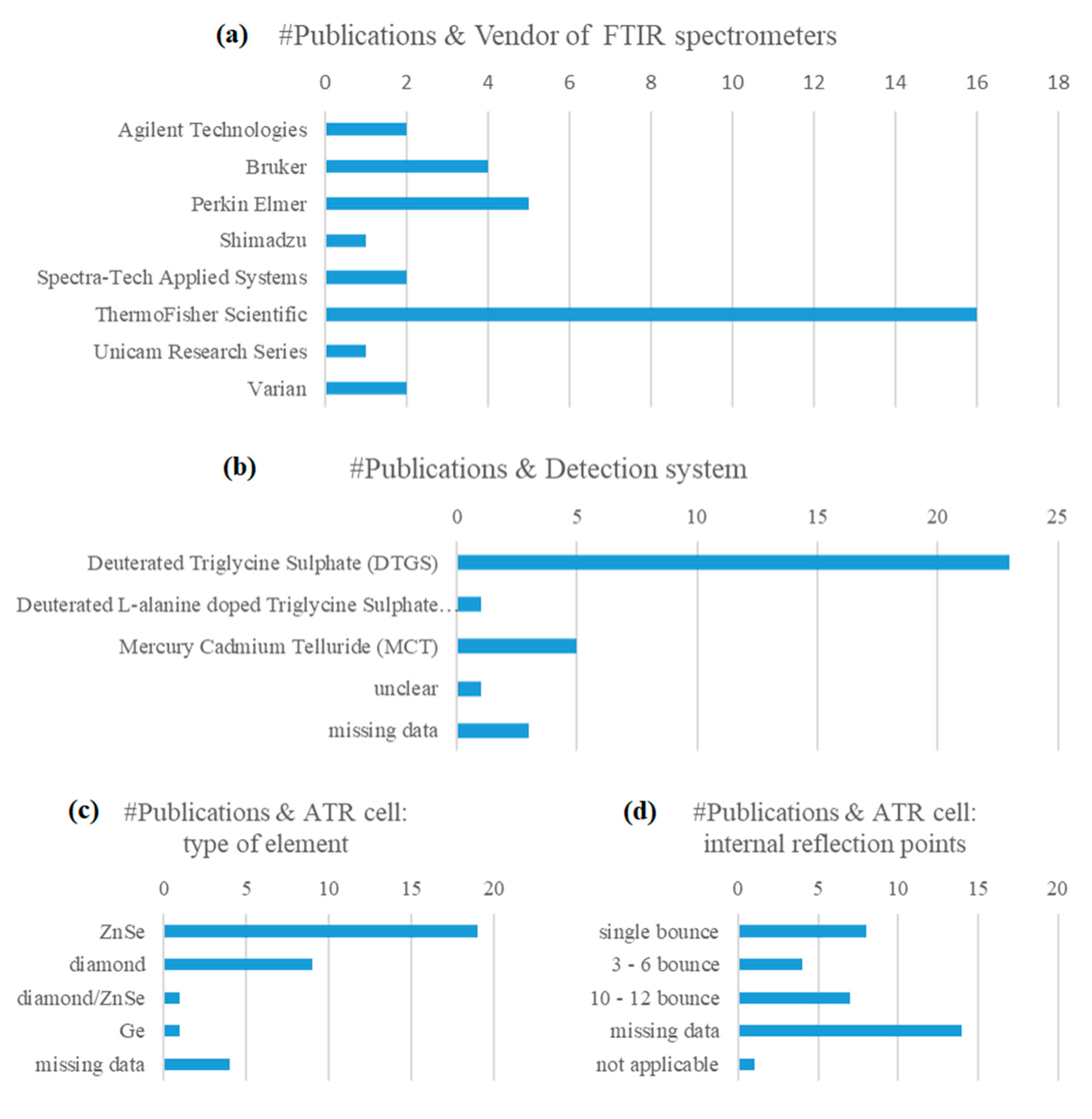
3.3. Spectra Acquisition Conditions
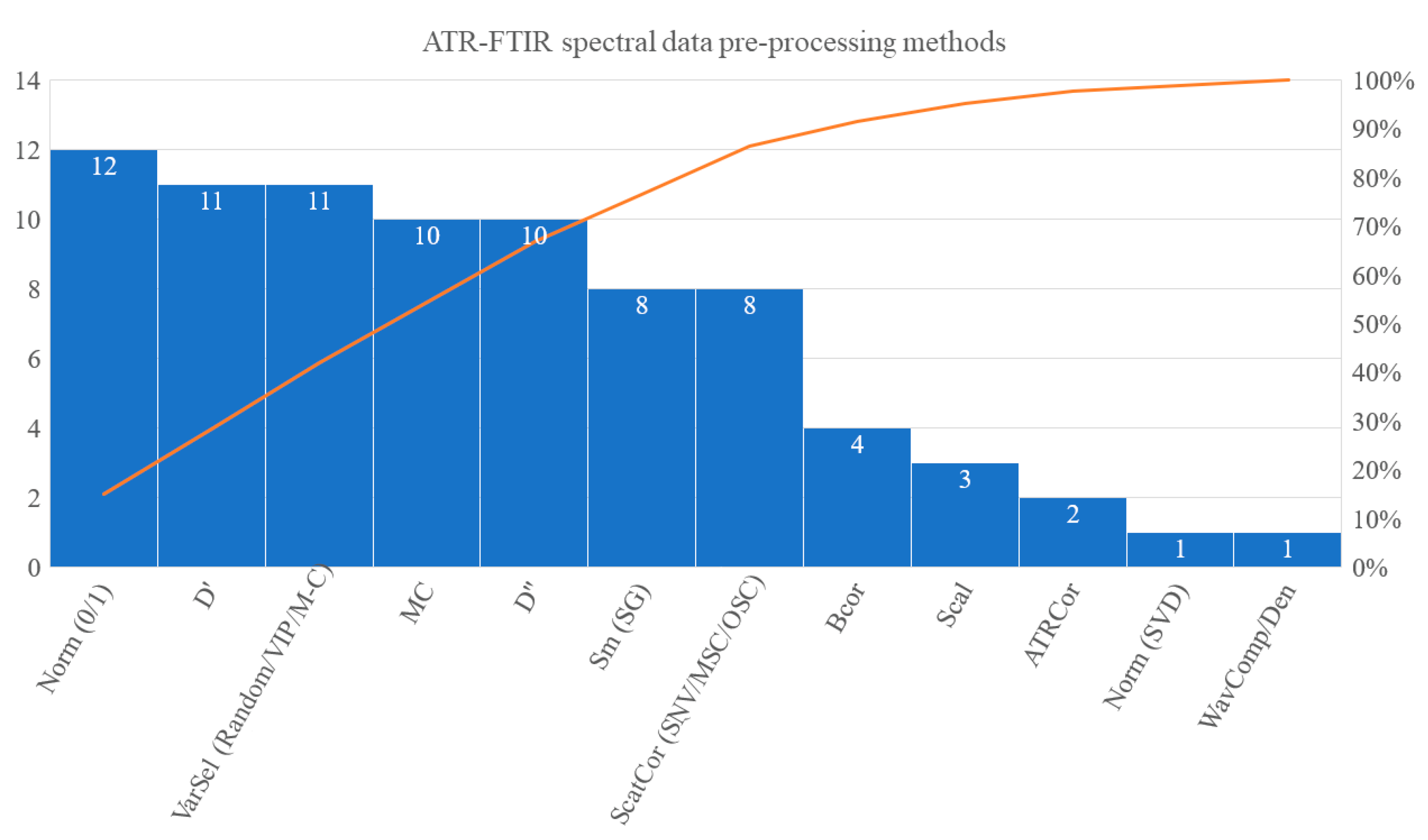
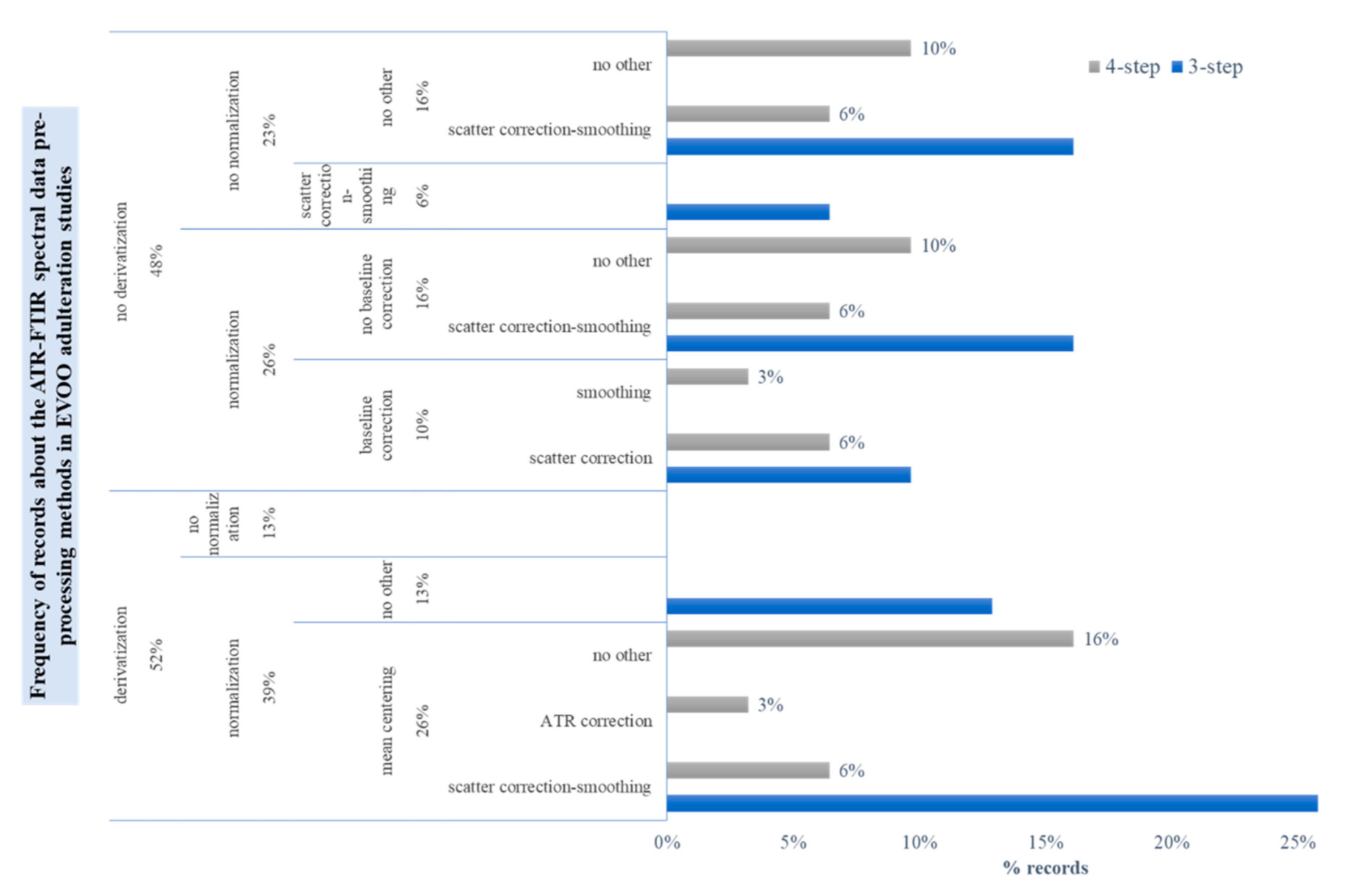
3.4. Spectral Pre-Processing Schemes
3.5. Exploratory Analyses
3.6. Modeling Strategies
3.7. Calibration
3.7.1. Case study I: Multivariate Regression of FTIR Spectral Data against Reference Fatty Acid Content Values
3.7.2. Case study II: Detection of EVOO Adulteration with Olive Pomace Oil
3.8. Discirminant Classification
3.9. Class Modelling Methods in Non-Targeted Approach
3.10. Method Performance Criteria
4. Concluding Remarks and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Commission. European Commission Regulation (EEC) No 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Commun. 1991, L248, 1–83. [Google Scholar]
- European Commission. European Commission Regulation (EU) No 61/2011 of 24 January 2011 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 2011, L23, 1–14. [Google Scholar]
- European Commission. Study on the Implementation of Conformity Checks in the Olive Oil Sector throughout the European Union; European Commission: Brussels, Belgium, 2020; ISBN 9789276092643. [Google Scholar]
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive oil authentication: A comparative analysis of regulatory frameworks with especial emphasis on quality and authenticity indices, and recent analytical techniques developed for their assessment. A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Holroyd, S.E.; Moore, J.C.; Laurvick, K.; Gendel, S.M.; Xie, Z. Opportunities and challenges using non-targeted methods for food fraud detection. J. Agric. Food Chem. 2019, 67, 8425–8430. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Tsimidou, M.Z. Perspective of vibrational spectroscopy analytical methods in on-field/official control of olives and virgin olive oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1600148. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Cozzolino, D. Advantages, Opportunities, and challenges of vibrational spectroscopy as tool to monitor sustainable food systems. Food Anal. Methods 2022, 15, 1390–1396. [Google Scholar] [CrossRef]
- Brewster, V.L.; Goodacre, R. Vibrational spectroscopy for “Food Forensics”. In Infrared and Raman Spectroscopy in Forensic Science; Chalmers, J.M., Edwards, H.G.M., Hargreaves, M.D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 583–592. [Google Scholar]
- Skibsted, E.; Engelsen, S.B. Spectroscopy for Process Analytical Technology (PAT), 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780128032244. [Google Scholar]
- Rodionova, O.Y.; Oliveri, P.; Pomerantsev, A.L. Rigorous and compliant approaches to one-class classification. Chemom. Intell. Lab. Syst. 2016, 159, 89–96. [Google Scholar] [CrossRef]
- Alladio, E.; Giacomelli, L.; Biosa, G.; Di Corcia, D.; Gerace, E.; Salomone, A.; Vincenti, M. Development and validation of a Partial Least Squares-Discriminant Analysis (PLS-DA) model based on the determination of ethyl glucuronide (EtG) and fatty acid ethyl esters (FAEEs) in hair for the diagnosis of chronic alcohol abuse. Forensic Sci. Int. 2018, 282, 221–230. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Maggio, R.M.; Cerretani, L. Chemometric applications to assess quality and critical parameters of virgin and extra-virgin olive oil. A review. Anal. Chim. Acta 2016, 913, 1–21. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. Food Chemicals Codex (FCC) 11. In Appendix XVIII: USP Guidance on Developing and Validating Non-Targeted Methods for Adulteration Detection; USP: Rockville, MD, USA, 2019. [Google Scholar]
- Esbensen, K.H.; Ramsey, C.A. QC of sampling processes—A first overview: From field to test portion. J. AOAC Int. 2015, 98, 282–287. [Google Scholar] [CrossRef]
- Esbensen, K.H. Materials properties: Heterogeneity and appropriate sampling modes. J. AOAC Int. 2015, 98, 269–274. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. Food Chemicals Codex (FCC) 10. In Appendix XVII: Food Fraud Mitigation Guidance; USP: Rockville, MD, USA, 2016; pp. 1586–1621. [Google Scholar]
- Nunes, C.A. Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Res. Int. 2014, 60, 255–261. [Google Scholar] [CrossRef]
- Callao, M.P.; Ruisánchez, I. An overview of multivariate qualitative methods for food fraud detection. Food Control 2018, 86, 283–293. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Mossoba, M.M.; Seiler, A.; Steinhart, H.; Kramer, J.K.G.; Rodrigues-Saona, L.; Griffith, A.P.; Pierceall, R.; Van De Voort, F.R.; Sedman, J.; Ismail, A.A.; et al. Regulatory infrared spectroscopic method for the rapid determination of total isolated trans fat: A collaborative study. J. Am. Oil Chem. Soc. 2011, 88, 39–46. [Google Scholar] [CrossRef]
- Tena, N.; Aparicio-Ruiz, R.; García-González, D.L. Time course analysis of fractionated thermoxidized virgin olive oil by FTIR spectroscopy. J. Agric. Food Chem. 2013, 61, 3212–3218. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Bendini, A.; Cerretani, L. Rapid evaluation of oxidised fatty acid concentration in virgin olive oil using Fourier-transform infrared spectroscopy and multiple linear regression. Food Chem. 2011, 124, 679–684. [Google Scholar] [CrossRef]
- Squeo, G.; Grassi, S.; Paradiso, V.M.; Alamprese, C.; Caponio, F. FT-IR extra virgin olive oil classification based on ethyl ester content. Food Control 2019, 102, 149–156. [Google Scholar] [CrossRef]
- Rohman, A.; Ghazali, M.A.I.B.; Windarsih, A.; Irnawati; Riyanto, S.; Yusof, F.M.; Mustafa, S. Comprehensive review on application of FTIR spectroscopy coupled with chemometrics for authentication analysis of fats and oils in the food products. Molecules 2020, 25, 5485. [Google Scholar] [CrossRef] [PubMed]
- De la Mata, P.; Dominguez-Vidal, A.; Bosque-Sendra, J.M.; Ruiz-Medina, A.; Cuadros-Rodríguez, L.; Ayora-Cañada, M.J. Olive oil assessment in edible oil blends by means of ATR-FTIR and chemometrics. Food Control 2012, 23, 449–455. [Google Scholar] [CrossRef]
- Cozzolino, D. The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods. Anal. Methods 2015, 7, 9390–9400. [Google Scholar] [CrossRef]
- Mark, H.; Workman, J. Chemometrics in Spectroscopy, 2nd ed.; Mark, H., Workman, J., Eds.; Elsevier Academic Press: London, UK, 2018; ISBN 9780128053096. [Google Scholar]
- Nichani, K.; Uhlig, S.; Stoyke, M.; Kemmlein, S.; Ulberth, F.; Haase, I.; Döring, M.; Walch, S.G.; Gowik, P. Essential terminology and considerations for validation of non-targeted methods. Preprints 2021, 2021120420. [Google Scholar] [CrossRef]
- Kemsley, E.K.; Defernez, M.; Marini, F. Multivariate statistics: Considerations and confidences in food authenticity problems. Food Control 2019, 105, 102–112. [Google Scholar] [CrossRef]
- Persson, O.; Danell, R.; Schneider, J.W. How to use Bibexcel for various types of bibliometric analysis. In Celebrating Scholarly Communication Studies: A Festschrift for Olle Persson at His 60th Birthday; Åström, F., Danell, R., Larsen, B., Schneider, J., Eds.; International Society for Scientometrics and Informetrics: Leuven, Belgium, 2009; pp. 9–24. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M. SIMCA: A Method for analyzing chemical data in terms of similarity and analogy. In Chemometrics Theory and Application; ACS Symposium Series Vol. 52; Kowalski, B., Ed.; American Chemical Society: Washington, DC, USA, 1977; pp. 243–282. [Google Scholar]
- Wold, S.; Martens, H.; Wold, H. The multivariate calibration problem in chemistry solved by the PLS method. In Matrix Pencils; Lecture Notes in Mathematics; Kågström, B., Ruhe, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 286–293. [Google Scholar]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Wold, S.; Johansson, E.; Cocchi, M. PLS: Partial least squares projections to latent structures. In 3D QSAR in Drug Design: Theory, Methods and Applications; Kubinyi, H., Ed.; ESCOM: Leiden, The Netherlands, 1993. [Google Scholar]
- Barclay, V.J.; Bonner, R.F.; Hamilton, I.P. Application of wavelet transforms to experimental spectra: Smoothing, denoising, and data set compression. Anal. Chem. 1997, 69, 78–90. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Gallagher, N.B.; O’Sullivan, D. Selection of representative learning and test sets using the Onion method. Available online: https://eigenvector.com/wp-content/uploads/2020/01/Onion_SampleSelection.pdf (accessed on 12 April 2022).
- Engel, J.; Gerretzen, J.; Szymańska, E.; Jansen, J.J.; Downey, G.; Blanchet, L.; Buydens, L.M.C. Breaking with trends in pre-processing? TrAC-Trends Anal. Chem. 2013, 50, 96–106. [Google Scholar] [CrossRef]
- Jollife, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.C.; Liong, C.Y.; Jemain, A.A. A contemporary review on Data Preprocessing (DP) practice strategy in ATR-FTIR spectrum. Chemom. Intell. Lab. Syst. 2017, 163, 64–75. [Google Scholar] [CrossRef]
- Cocchi, M.; Biancolillo, A.; Marini, F. Chemometric Methods for Classification and Feature Selection, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 82, ISBN 9780444640444. [Google Scholar]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Massart, D.L.; Vandeginste, B.G.M.; Deming, S.N.; Michotte, Y.; Kaufman, L. Chemometrics: A textbook. In Data Handling in Science and Technology; Vandeginste, B.G.M., Kaufman, L., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2003; pp. 1–476. ISBN 0444426604. [Google Scholar]
- Lai, Y.W.; Kemsley, E.K.; Wilson, R.H. Quantitative analysis of potential adulterants of extra virgin olive oil using infrared spectroscopy. Food Chem. 1995, 53, 95–98. [Google Scholar] [CrossRef]
- Lai, Y.W.; Kemsley, E.K.; Wilson, R.H. Potential of Fourier Transform Infrared Spectroscopy for the authentication of vegetable oils. J. Agric. Food Chem. 1994, 42, 1154–1159. [Google Scholar] [CrossRef]
- Marigheto, N.A.; Kemsley, E.K.; Defernez, M.; Wilson, R.H. A comparison of mid-infrared and raman spectroscopies for the authentication of edible oils. J. Am. Oil Chem. Soc. 1998, 75, 987–992. [Google Scholar] [CrossRef]
- Ozen, B.F.; Mauer, L.J. Detection of hazelnut oil adulteration using FT-IR spectroscopy. J. Agric. Food Chem. 2002, 50, 3898–3901. [Google Scholar] [CrossRef]
- Maggio, R.M.; Cerretani, L.; Chiavaro, E.; Kaufman, T.S.; Bendini, A. A novel chemometric strategy for the estimation of extra virgin olive oil adulteration with edible oils. Food Control 2010, 21, 890–895. [Google Scholar] [CrossRef]
- Yang, H.; Irudayaraj, J. Comparison of near-infrared, Fourier transform-infrared, and Fourier transform-Raman methods for determining olive pomace oil adulteration in extra virgin olive oil. J. Am. Oil Chem. Soc. 2001, 78, 889–895. [Google Scholar] [CrossRef]
- Tay, A.; Singh, R.K.; Krishnan, S.S.; Gore, J.P. Authentication of olive oil adulterated with vegetable oils using Fourier transform infrared spectroscopy. LWT 2002, 35, 99–103. [Google Scholar] [CrossRef]
- Didham, M.; Truong, V.K.; Chapman, J.; Cozzolino, D. Sensing the addition of vegetable oils to olive oil: The ability of UV–VIS and MIR spectroscopy coupled with chemometric analysis. Food Anal. Methods 2020, 13, 601–607. [Google Scholar] [CrossRef]
- Pan, M.; Sun, S.; Zhou, Q.; Chen, J. A simple and portable screening method for adulterated olive oils using the hand-held FTIR spectrometer and chemometrics tools. J. Food Sci. 2018, 83, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Filoda, P.F.; Fetter, L.F.; Fornasier, F.; de Souza Schneider, R.d.C.; Helfer, G.A.; Tischer, B.; Teichmann, A.; da Costa, A.B. Fast methodology for identification of olive oil adulterated with a mix of different vegetable oils. Food Anal. Methods 2019, 12, 293–304. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Coelho, L.; Barros, A.; de Almeida, J.M.M.M. Study of adulteration of extra virgin olive oil with peanut oil using FTIR spectroscopy and chemometrics. Cogent Food Agric. 2015, 1, 1018695. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Kutsanedzie, F.Y.H.; Li, H.H.; Chen, Q.S. Evaluation of extra-virgin olive oil adulteration using FTIR spectroscopy combined with multivariate algorithms. Qual. Assur. Saf. Crops Foods 2018, 10, 411–421. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Özdikicierler, O.; Tsimidou, M.Z. Detection of ternary mixtures of virgin olive oil with canola, hazelnut or safflower oils via non-targeted ATR-FTIR fingerprinting and chemometrics. Food Control 2022, 142, 109240. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Shi, W.; Shen, Q. Partial Least Squares Discriminant Analysis model based on Variable Selection applied to identify the adulterated olive oil. Food Anal. Methods 2016, 9, 1713–1718. [Google Scholar] [CrossRef]
- Georgouli, K.; Martinez Del Rincon, J.; Koidis, A. Continuous statistical modelling for rapid detection of adulteration of extra virgin olive oil using mid infrared and Raman spectroscopic data. Food Chem. 2017, 217, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Küpper, L.; Heise, H.M.; Lampen, P.; Davies, A.N.; McIntyre, P. Authentication and quantification of extra virgin olive oils by attenuated total reflectance infrared spectroscopy using silver halide fiber probes and partial least-square calibration. Appl. Spectrosc. 2001, 55, 563–570. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Ozen, B. Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chem. 2009, 116, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A.; Che Man, Y.B. Quantification and classification of corn and sunflower oils as adulterants in olive oil using chemometrics and FTIR spectra. Sci. World J. 2012, 2012, 250795. [Google Scholar] [CrossRef] [Green Version]
- Aykas, D.P.; Karaman, A.D.; Keser, B.; Rodriguez-Saona, L. Non-targeted authentication approach for extra virgin olive oil. Foods 2020, 9, 221. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Min, S. Data fusion strategy in quantitative analysis of spectroscopy relevant to olive oil adulteration. Vib. Spectrosc. 2019, 101, 20–27. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Authentication of extra virgin olive oil from sesame oil using FTIR spectroscopy and gas chromatography. Int. J. Food Prop. 2012, 15, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A.; Che Man, Y.B.; Yusof, F.M. The use of FTIR spectroscopy and chemometrics for rapid authentication of extra virgin olive oil. J. Am. Oil Chem. Soc. 2014, 91, 207–213. [Google Scholar] [CrossRef]
- Jović, O.; Smolić, T.; Primožič, I.; Hrenar, T. Spectroscopic and chemometric analysis of binary and ternary edible oil mixtures: Qualitative and quantitative study. Anal. Chem. 2016, 88, 4516–4524. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Potential use of FTIR-ATR Spectroscopic method for determination of Virgin Coconut Oil and Extra Virgin Olive Oil in ternary mixture systems. Food Anal. Methods 2011, 4, 155–162. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. FTIR spectroscopy coupled with chemometrics of multivariate calibration and discriminant analysis for authentication of extra virgin olive oil. Int. J. Food Prop. 2017, 20, S1173–S1181. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Determination of extra virgin olive oil in quaternary mixture using FTIR spectroscopy and multivariate calibration. Spectroscopy 2011, 26, 203–211. [Google Scholar] [CrossRef]
- Khoshayand, M.R.; Abdollahi, H.; Shariatpanahi, M.; Saadatfard, A.; Mohammadi, A. Simultaneous spectrophotometric determination of paracetamol, ibuprofen and caffeine in pharmaceuticals by chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 491–499. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res. Int. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- Oussama, A.; Elabadi, F.; Platikanov, S.; Kzaiber, F.; Tauler, R. Detection of olive oil adulteration using FT-IR spectroscopy nd PLS with variable importance of projection (VIP) scores. J. Am. Oil Chem. Soc. 2012, 89, 1807–1812. [Google Scholar] [CrossRef]
- Sun, X.; Lin, W.; Li, X.; Shen, Q.; Luo, H. Detection and quantification of extra virgin olive oil adulteration with edible oils by FT-IR spectroscopy and chemometrics. Anal. Methods 2015, 7, 3939–3945. [Google Scholar] [CrossRef]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Dragoş, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar]
- Kandhro, A.A.; Saleem, R.; Laghari, A.H.; Sultana, R.; Khan, M.A.; Memon, D.H. Quantification of pure refined olive oil adulterant in extra virgin olive oil using diamond cell ATR-FTIR spectroscopy. J. Chem. Soc. Pak. 2014, 36, 699–706. [Google Scholar]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef]
- Jamwal, R.; Amit; Kumari, S.; Sharma, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Recent trends in the use of FTIR spectroscopy integrated with chemometrics for the detection of edible oil adulteration. Vib. Spectrosc. 2021, 113, 103222. [Google Scholar] [CrossRef]
- Bridges, C.C. Hierarchical Cluster Analysis. Psychol. Rep. 1966, 18, 851–854. [Google Scholar] [CrossRef]
- Roweis, S.T.; Saul, L.K. Nonlinear dimensionality reduction by locally linear embedding. Science 2000, 290, 2323–2326. [Google Scholar] [CrossRef] [Green Version]
- Buratti, S.; Ballabio, D.; Giovanelli, G.; Dominguez, C.M.Z.; Moles, A.; Benedetti, S.; Sinelli, N. Monitoring of alcoholic fermentation using near infrared and mid infrared spectroscopies combined with electronic nose and electronic tongue. Anal. Chim. Acta 2011, 697, 67–74. [Google Scholar] [CrossRef]
- Lörchner, C.; Horn, M.; Berger, F.; Fauhl-Hassek, C.; Glomb, M.A.; Esslinger, S. Quality control of spectroscopic data in non-targeted analysis—Development of a multivariate control chart. Food Control 2022, 133, 108601. [Google Scholar] [CrossRef]
- Obeidat, S.M.; Khanfar, M.S.; Obeidat, W.M. Classification of edible oils and uncovering adulteration of virgin olive oil using FTIR with the aid of chemometrics. Aust. J. Basic Appl. Sci. 2009, 3, 2048–2053. [Google Scholar]
- COI/T.15/NC No. 3/Rev. 4; Trade Standard Applying to Olive Oils and Olive Pomace Oils. International Olive Council (IOC): Madrid, Spain, 2019.
- Rodionova, O.Y.; Pomerantsev, A.L. Chemometric tools for food fraud detection: The role of target class in non-targeted analysis. Food Chem. 2020, 317, 126448. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. A comparative study of mid-infrared, UV–Visible and fluorescence spectroscopy in combination with chemometrics for the detection of adulteration of fresh olive oils with old olive oils. Food Control 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Ruisánchez, I.; Jiménez-Carvelo, A.M.; Callao, M.P. ROC curves for the optimization of one-class model parameters. A case study: Authenticating extra virgin olive oil from a Catalan protected designation of origin. Talanta 2021, 222, 121564. [Google Scholar] [CrossRef]

| Combination of Oils | Mixture Composition (%) | No. of Study under Review (Cited Reference) | ||
|---|---|---|---|---|
| Preparation | A—Adulterant Oil (Oil 1:Oil 2) | B—EVOO (n of Samples) | ||
| EVOO–CO–SuO | Incremental addition of A to B—varying composition of A | 2–20 (0:1, 1:3, 1:2, 1:1, v/v) | 80 (n = 5) 85 (n = 3) 90 (n = 3) 95 (n = 3) 98 (n = 3) | [63] |
| EVOO–GSO–RBO–WNO | Random design | 0–100 (miscellaneous ratios) | 0 (n = 4) 2.5–50 (n = 19) 50–98 (n = 3) 100 (n = 1) | [72] |
| OO–VCNO–PO | Random design | 0–100 (miscellaneous ratios) | 0 (n = 2) 2.5–40 (n = 9) 40–65 (n = 12) 100 (n = 1) | [70] |
| EVOO–SuO–RSO and EVOO–HOSuO–RSO and EVOO–HOSuO–SuO | Incremental addition of A to B | 0–100 (miscellaneous ratios) | 0 (n = 9) 10–40 (n = 34) 50–90 (n = 20) 100 (n = 1) | [69] |
| EVOO–PNO–RSO | Incremental addition of A to B—standard composition of A | ~2–40 (1:1, v/v) | ~60–98 (n = 120) | [58] |
| EVOO–CO–HO–SafO | Fixed volume addition of A to B—varying composition of A & G—optimal simplex design | 20 (0:1, 1:7, 1:3, 1:1, 3:1, 7:1, 1:0, v/v) 13 (1:1, v/v) 10 (1:0, 0:1, v/v) | 80 (n = 137) 87 (n = 3) 90 (n = 8) 100 (n = 85) | [59] |
| No. | Type of Adulterant Oil/Country | Reference Samples 1 (n) | OPO-VOO 2 (%, v/v) | Cworking Levels | Working Spectral Region (cm−1) | Spectral Data Pre-Processing | Criteria for Selection of Optimal LVs 3 | LVs (n) | ncal /nval Spectra | PRESS 4 | Fitness to the Model (R2) | REP 5 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [52] | Olive Pomace Oil/USA | 4 | 5:95 to 95:5 | 21 | 4000–650 | MSC | RMSECV 6 | 11 | 21 × 3/21 × 1 7 | 0.122 | 0.991 | 3.3 |
| [51] | Olive Pomace Oil/Italy | - 8 | 5:95 to 30:70 | 5 | 1876–912 | MC, D’, MW (10p) | F ratio of PRESS Haaland & Thomas criterion, 1988 | 4 | 3 × 3/2 × 3 | 0.002 | 0.973 | 16.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoudi, S.A.; Strani, L.; Cocchi, M. Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives. Molecules 2023, 28, 337. https://doi.org/10.3390/molecules28010337
Ordoudi SA, Strani L, Cocchi M. Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives. Molecules. 2023; 28(1):337. https://doi.org/10.3390/molecules28010337
Chicago/Turabian StyleOrdoudi, Stella A., Lorenzo Strani, and Marina Cocchi. 2023. "Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives" Molecules 28, no. 1: 337. https://doi.org/10.3390/molecules28010337
APA StyleOrdoudi, S. A., Strani, L., & Cocchi, M. (2023). Toward the Non-Targeted Detection of Adulterated Virgin Olive Oil with Edible Oils via FTIR Spectroscopy & Chemometrics: Research Methodology Trends, Gaps and Future Perspectives. Molecules, 28(1), 337. https://doi.org/10.3390/molecules28010337