Diffuse Reflectance Spectroscopy with Dilution: A Powerful Method for Halide Perovskites Study
Abstract
:1. Introduction
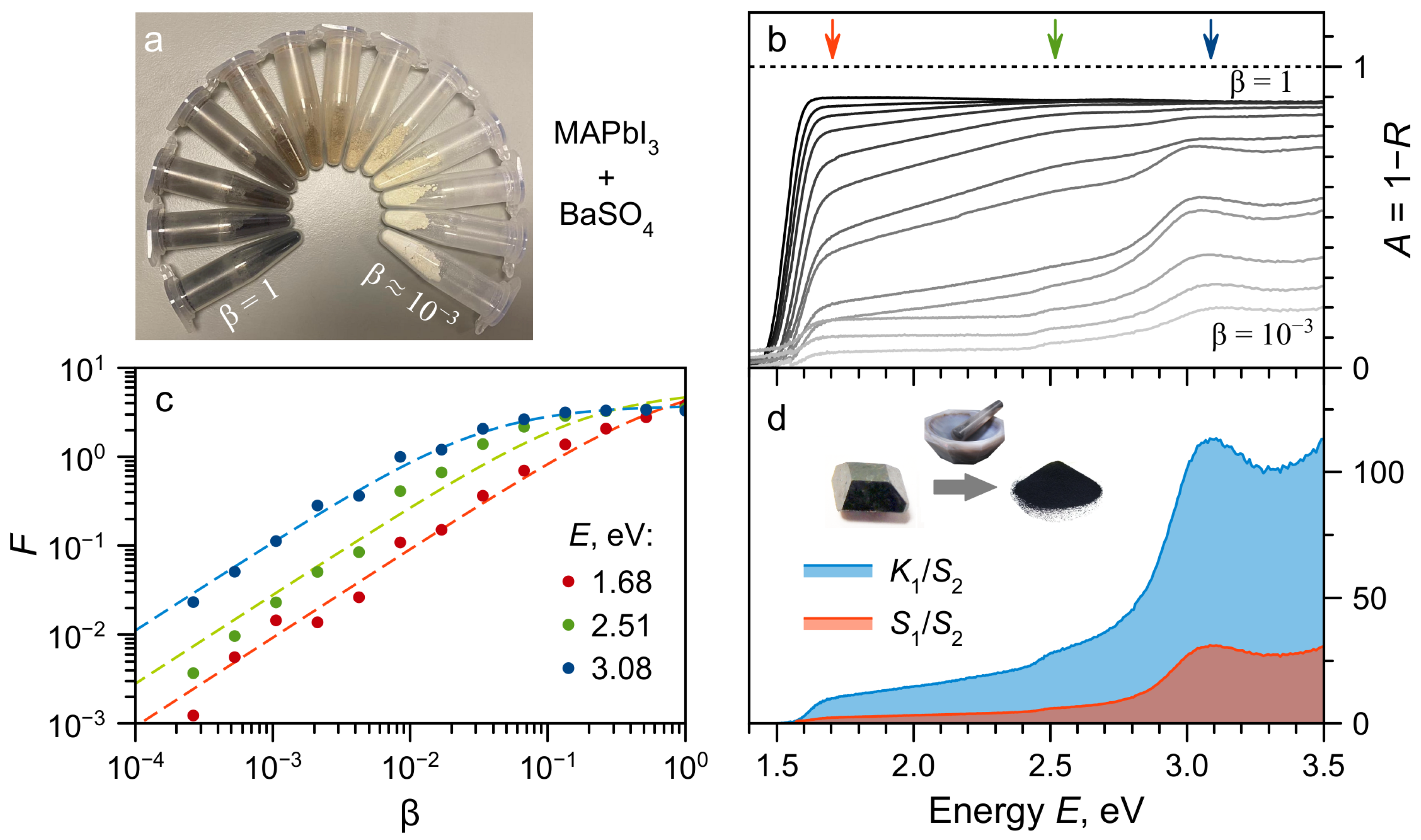
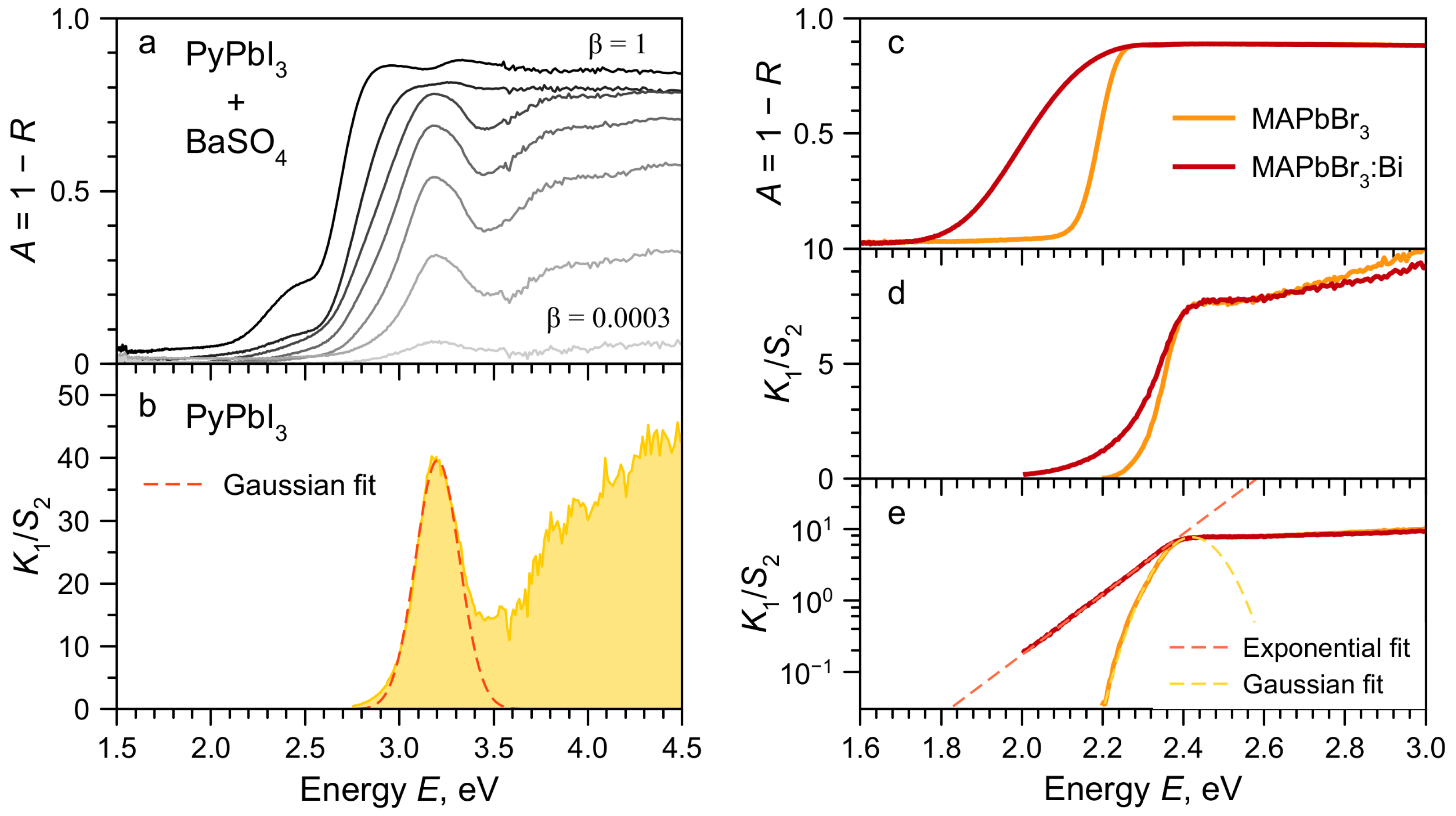
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Crystal Growth
3.2. Basic Characterization
3.3. DRS Measurements
3.4. DRS Spectra Fitting for Two Dilutions
3.5. Conversion between Mass and Volume Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DRS | Diffuse reflectance spectroscopy |
| FWHM | Full width at half maximum |
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, C.; Liu, X.; Yao, J.; Zhao, Y.S. Materials chemistry and engineering in metal halide perovskite lasers. Chem. Soc. Rev. 2020, 49, 951–982. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Wei, H.; Huang, J. Halide lead perovskites for ionizing radiation detection. Nat. Commun. 2019, 10, 1066. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Wu, T.; Hu, B. A Review on Organic–Inorganic Halide Perovskite Photodetectors: Device Engineering and Fundamental Physics. Adv. Mater. 2017, 29, 1605242. [Google Scholar] [CrossRef]
- Murzin, A.O.; Selivanov, N.I.; Kozlov, V.O.; Ryzhov, I.I.; Miyasaka, T.; Emeline, A.V.; Kapitonov, Y.V. Photoluminescence Excitation Spectroscopy of Defect-Related States in MAPbI3 Perovskite Single Crystals. Adv. Opt. Mater. 2021, 9, 2001327. [Google Scholar] [CrossRef]
- Smith, M.D.; Jaffe, A.; Dohner, E.R.; Lindenberg, A.M.; Karunadasa, H.I. Structural origins of broadband emission from layered Pb–Br hybrid perovskites. Chem. Sci. 2017, 8, 4497–4504. [Google Scholar] [CrossRef] [Green Version]
- Rakita, Y.; Cohen, S.R.; Kedem, N.K.; Hodes, G.; Cahen, D. Mechanical properties of APbX3 (A = Cs or CH3NH3; X= I or Br) perovskite single crystals. MRS Commun. 2015, 5, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, O.; Chernikov, A.; Norman, Z.M.; Zhong, Y.; Velauthapillai, A.; van der Zande, A.; Owen, J.S.; Heinz, T.F. Excitons in ultrathin organic-inorganic perovskite crystals. Phys. Rev. B 2015, 92, 045414. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, J.; Zhang, Y.; Wang, H.; Lin, G.; Xiong, X.; Zhou, W.; Luo, H.; Li, D. Fabrication of single phase 2D homologous perovskite microplates by mechanical exfoliation. 2D Mater. 2018, 5, 021001. [Google Scholar] [CrossRef]
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Techn. Physik 1931, 12, 593–609. See also English Translation by S. Westin (An Article on Optics of Paint Layers). Available online: http://www.graphics.cornell.edu/~westin/pubs/kubelka.pdf (accessed on 23 November 2022).
- Kodaira, T.; Nozue, Y.; Ohwashi, S.; Goto, T.; Terasaki, O. Optical properties of potassium clusters incorporated into zeolite LTA. Phys. Rev. B 1993, 48, 12245–12252. [Google Scholar] [CrossRef]
- Gustav, K. Reflectance Spectroscopy Principles, Methods, Applications; Springer: Berlin, Germany, 1969. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Davis, E.; Mott, N. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Lösche, A. N. F. MOTT, E. A. DAVIS. Electronic Processes in Non-Crystalline Materials Clarendon-Press, Oxford 1971 437 Seiten. £ 7,50. Kristall Technik 1972, 7, K55–K56. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, P.; Christol, P.; Mathieu, H. General formalism for excitonic absorption edges in confined systems with arbitrary dimensionality. J. Phys. IV 1993, 03, C5-377–C5-380. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, C. Optical Dispersion by Wannier Excitons. Phys. Rev. Lett. 1995, 75, 4090–4093. [Google Scholar] [CrossRef]
- Patterson, E.M.; Shelden, C.E.; Stockton, B.H. Kubelka-Munk optical properties of a barium sulfate white reflectance standard. Appl. Opt. 1977, 16, 729–732. [Google Scholar] [CrossRef]
- Selivanov, N.I.; Murzin, A.O.; Yudin, V.I.; Kapitonov, Y.V.; Emeline, A.V. Counterdiffusion-in-gel growth of high optical and crystal quality MAPbX3 (MA = CH3NH3+, X = I-, Br-) lead-halide perovskite single crystals. CrystEngComm 2022, 24, 2976–2981. [Google Scholar] [CrossRef]
- Panzer, F.; Li, C.; Meier, T.; Köhler, A.; Huettner, S. Impact of Structural Dynamics on the Optical Properties of Methylammonium Lead Iodide Perovskites. Adv. Energy Mater. 2017, 7, 1700286. [Google Scholar] [CrossRef] [Green Version]
- Bohn, B.J.; Simon, T.; Gramlich, M.; Richter, A.F.; Polavarapu, L.; Urban, A.S.; Feldmann, J. Dephasing and Quantum Beating of Excitons in Methylammonium Lead Iodide Perovskite Nanoplatelets. ACS Photonics 2018, 5, 648–654. [Google Scholar] [CrossRef]
- Blancon, J.C.; Stier, A.V.; Tsai, H.; Nie, W.; Stoumpos, C.C.; Traoré, B.; Pedesseau, L.; Kepenekian, M.; Katsutani, F.; Noe, G.T.; et al. Scaling law for excitons in 2D perovskite quantum wells. Nat. Commun. 2018, 9, 2254. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, M.; Ishihara, T.; Goto, T. Exciton Features in 0-, 2-, and 3-Dimensional Networks of [PbI6]4-Octahedra. J. Phys. Soc. Jpn. 1994, 63, 3870–3879. [Google Scholar] [CrossRef]
- Selivanov, N.I.; Murashkina, A.A.; Kevorkyants, R.; Emeline, A.V.; Bahnemann, D.W. Pyridinium lead tribromide and pyridinium lead triiodide: Quasi-one-dimensional perovskites with an optically active aromatic π-system. Dalton Trans. 2018, 47, 16313–16319. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Murashkina, A.A.; Shilovskikh, V.V.; Kapitonov, Y.V.; Ryabchuk, V.K.; Emeline, A.V.; Miyasaka, T. Invalidity of Band-Gap Engineering Concept for Bi3+ Heterovalent Doping in CsPbBr3 Halide Perovskite. J. Phys. Chem. Lett. 2018, 9, 5408–5411. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Weber, D. CH3NH3PbX3, ein Pb(II)-System mit kubischer Perowskitstruktur/ CH3NH3PbX3, a Pb(II)-System with Cubic Perovskite Structure. Z. Naturforschung B 1978, 33, 1443–1445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzin, A.O.; Samsonova, A.Y.; Stoumpos, C.C.; Selivanov, N.I.; Emeline, A.V.; Kapitonov, Y.V. Diffuse Reflectance Spectroscopy with Dilution: A Powerful Method for Halide Perovskites Study. Molecules 2023, 28, 350. https://doi.org/10.3390/molecules28010350
Murzin AO, Samsonova AY, Stoumpos CC, Selivanov NI, Emeline AV, Kapitonov YV. Diffuse Reflectance Spectroscopy with Dilution: A Powerful Method for Halide Perovskites Study. Molecules. 2023; 28(1):350. https://doi.org/10.3390/molecules28010350
Chicago/Turabian StyleMurzin, Aleksei O., Anna Yu. Samsonova, Constantinos C. Stoumpos, Nikita I. Selivanov, Alexei V. Emeline, and Yury V. Kapitonov. 2023. "Diffuse Reflectance Spectroscopy with Dilution: A Powerful Method for Halide Perovskites Study" Molecules 28, no. 1: 350. https://doi.org/10.3390/molecules28010350
APA StyleMurzin, A. O., Samsonova, A. Y., Stoumpos, C. C., Selivanov, N. I., Emeline, A. V., & Kapitonov, Y. V. (2023). Diffuse Reflectance Spectroscopy with Dilution: A Powerful Method for Halide Perovskites Study. Molecules, 28(1), 350. https://doi.org/10.3390/molecules28010350







