Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Physicochemical Properties of NADES
2.2. Effect of NADES Type on Olive Leaf Extraction by Different Assisted Extraction Methods
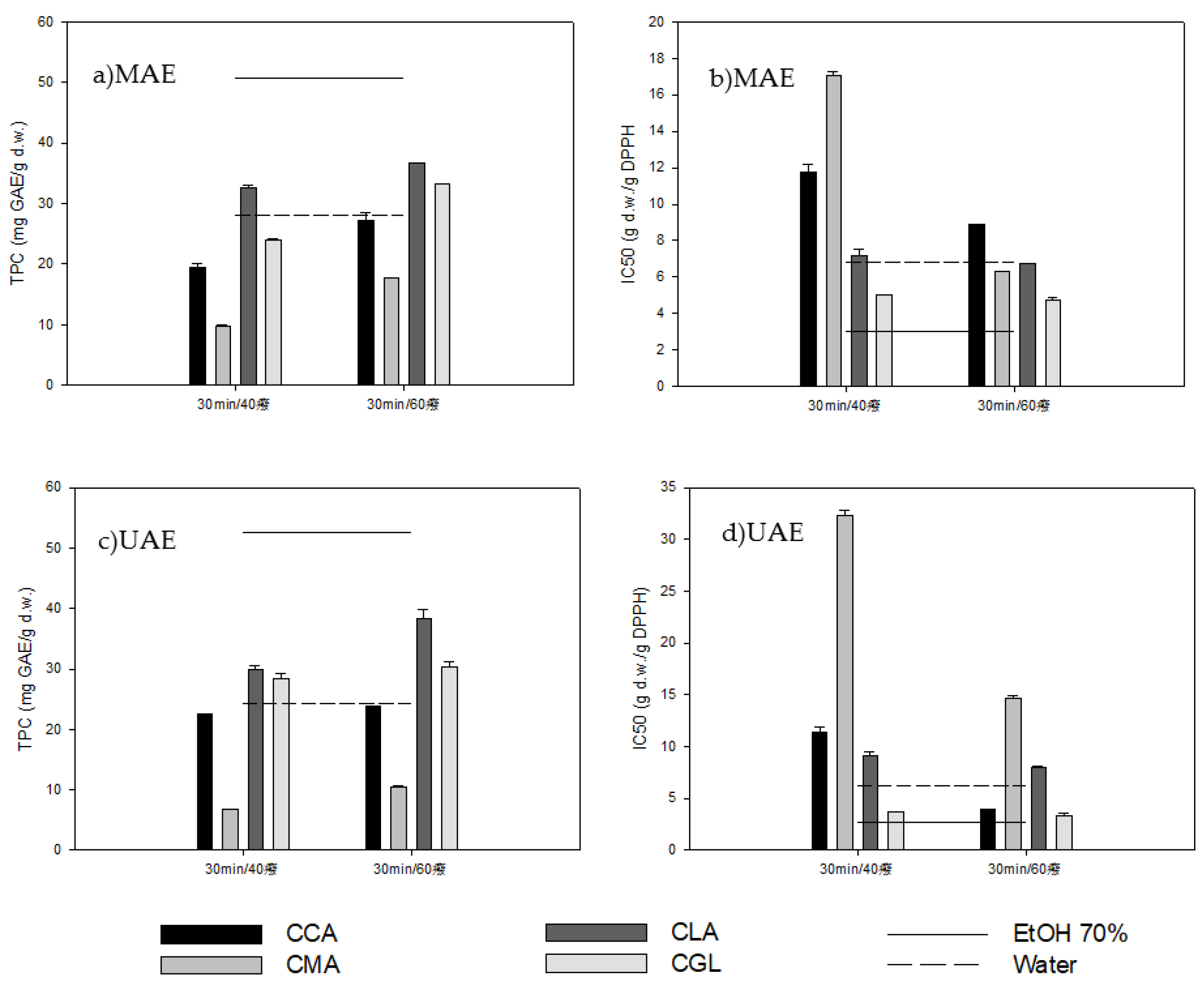
2.2.1. Microwave-Assisted Extraction (MAE)
2.2.2. Ultrasound-Assisted Extraction (UAE)
2.2.3. Homogenate-Assisted Extraction (HAE)
2.2.4. High Hydrostatic Pressure-Assisted Extraction (HHPAE)
2.3. Phenolic Profiles of the NADES Extracts
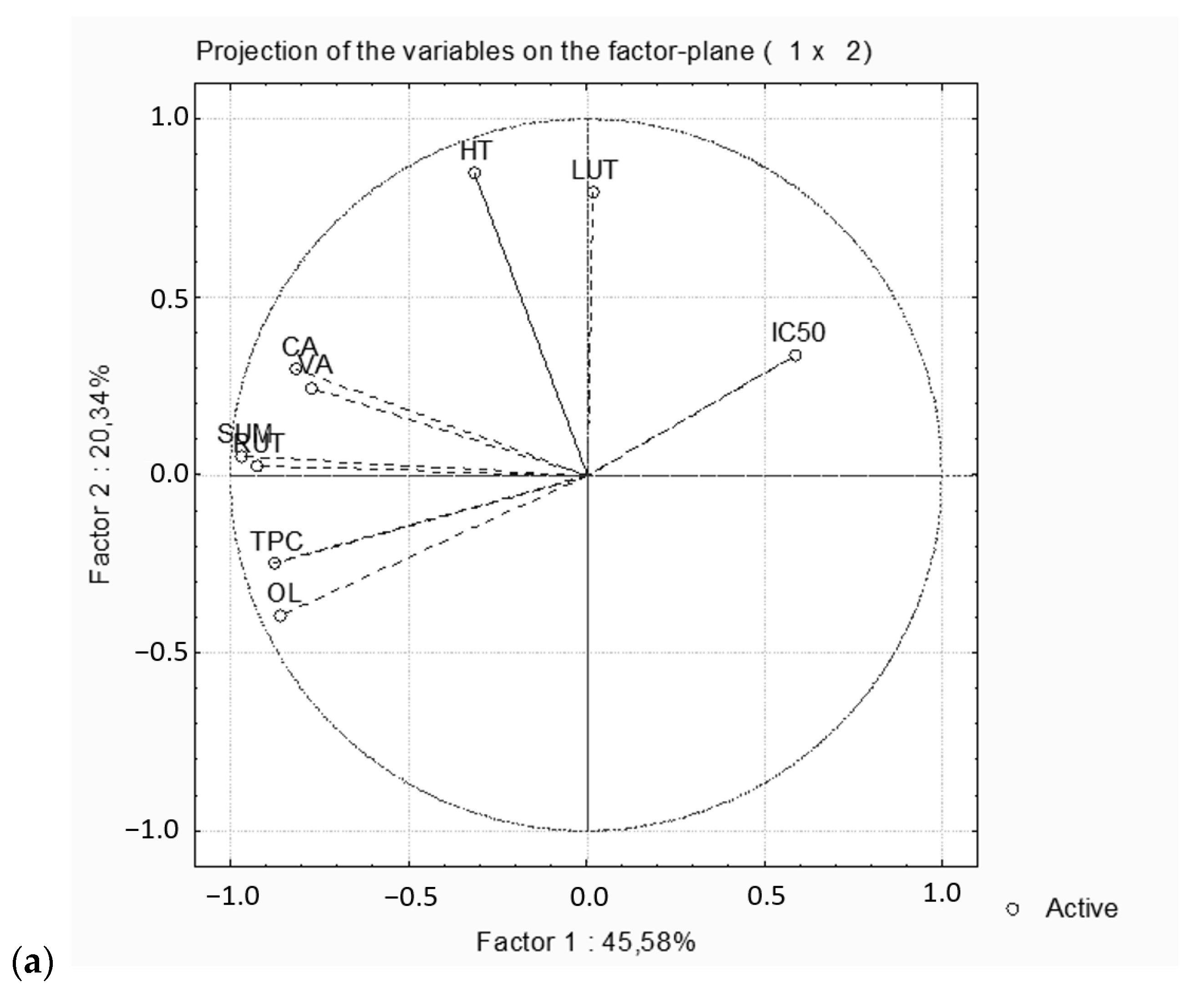
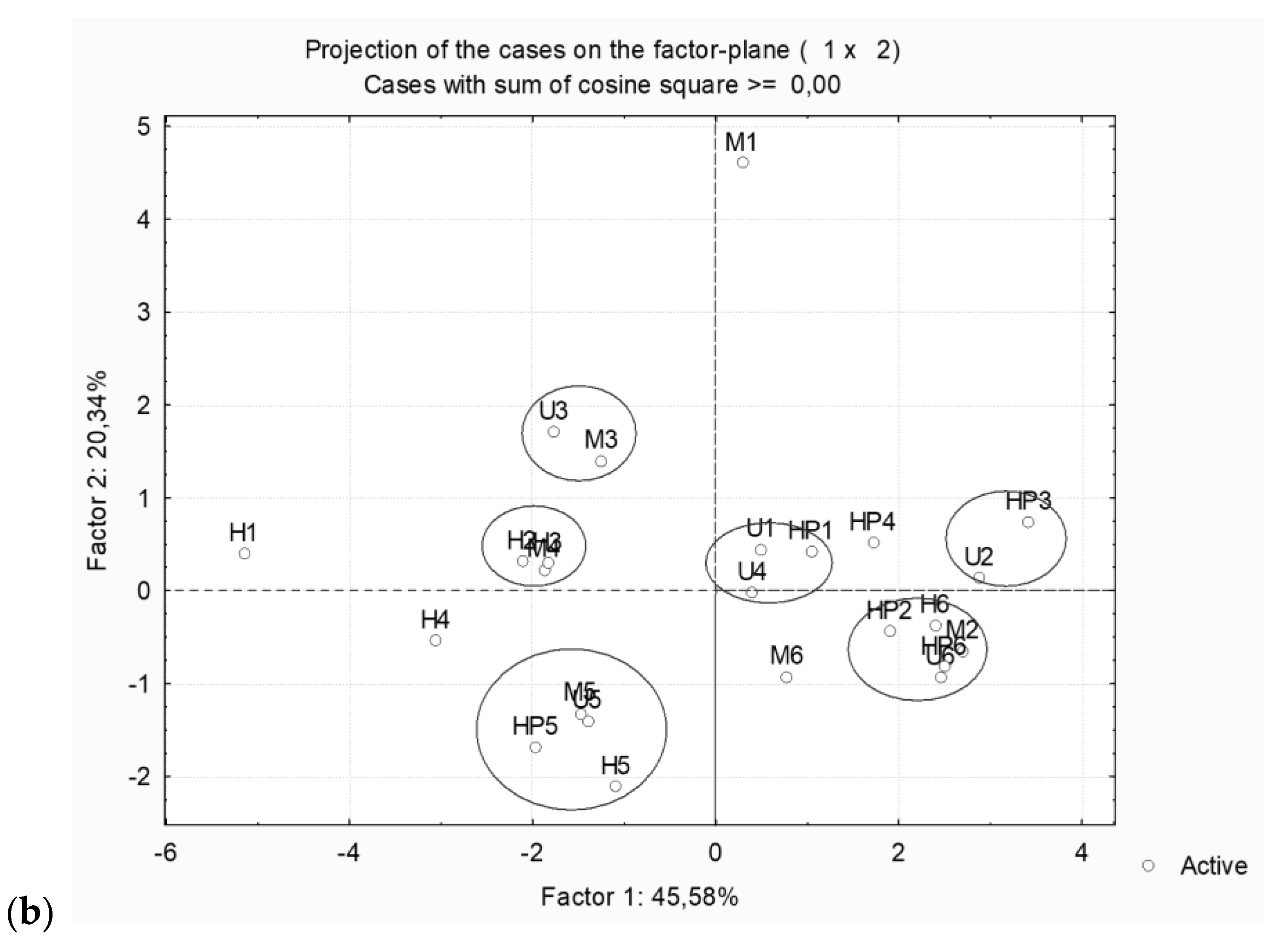
2.4. Statistical Analysis
2.5. Comparison of Assisted Extraction Methods
3. Materials and Methods
3.1. Raw Materials
3.2. Chemicals and Reagents
3.3. NADES Preparation
3.4. Olive Leaf Phenolic Compound Extraction
3.4.1. Microwave-Assisted Extraction (MAE)
3.4.2. Ultrasound-Assisted Extraction (UAE)
3.4.3. Homogenate-Assisted Extraction (HAE)
3.4.4. High Hydrostatic Pressure-Assisted Extraction (HHPAE)
3.5. Total Phenolic Content (TPC)
3.6. Antioxidant Activity
3.7. HPLC–DAD Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boskou, D.; Tsimidou, M.; Blekas, G. 5—Polar Phenolic Compounds. In Olive Oil; AOCS Press: Urbana, IL, USA, 2006; pp. 73–92. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Leitgeb, M.; Knez, Z.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef] [PubMed]
- Altıok, E.; Bayçın, D.; Bayraktar, O.; Ülkü, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Souilem, S.; Fki, I.; Kobayashi, I.; Khalid, N.; Neves, M.A.; Isoda, H.; Sayadi, S.; Nakajima, M. Emerging Technologies for Recovery of Value-Added Components from Olive Leaves and Their Applications in Food/Feed Industries. Food Bioprocess Technol. 2016, 10, 229–248. [Google Scholar] [CrossRef]
- Benavente-Garcı́a, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Gikas, E.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.-L. Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Čabarkapa, A.; Živković, L.; Žukovec, D.; Djelić, N.; Bajić, V.; Dekanski, D.; Spremo-Potparević, B. Protective effect of dry olive leaf extract in adrenaline induced DNA damage evaluated using in vitro comet assay with human peripheral leukocytes. Toxicol. In Vitro 2014, 28, 451–456. [Google Scholar] [CrossRef]
- Samet, I.; Han, J.; Jlaiel, L.; Sayadi, S.; Isoda, H. Olive (Olea europaea) Leaf Extract Induces Apoptosis and Monocyte/Macrophage Differentiation in Human Chronic Myelogenous Leukemia K562 Cells: Insight into the Underlying Mechanism. Oxidative Med. Cell. Longev. 2014, 2014, 927619. [Google Scholar] [CrossRef] [Green Version]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of Microwave-Assisted Extraction for the Characterization of Olive Leaf Phenolic Compounds by Using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Zhang, L.; Huang, P.L.; Chang, Y.-T.; Huang, P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Korukluoglu, M.; Sahan, Y.; Yigit, A.; Karakas, R. Antifungal activity of olive leaf (Olea europaea L.) extracts from the Trilye Region of Turkey. Ann. Microbiol. 2006, 56, 359–362. [Google Scholar] [CrossRef]
- Singh, I.; Mok, M.; Christensen, A.-M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perugini, P.; Vettor, M.; Rona, C.; Troisi, L.; Villanova, L.; Genta, I.; Conti, B.; Pavanetto, F. Efficacy of oleuropein against UVB irradiation: Preliminary evaluation. Int. J. Cosmet. Sci. 2008, 30, 113–120. [Google Scholar] [CrossRef]
- Thangavel, N.; Al Bratty, M.; Al Hazmi, H.A.; Najmi, A.; Ali Alaqi, R.O. Molecular Docking and Molecular Dynamics Aided Virtual Search of OliveNetTM Directory for Secoiridoids to Combat SARS-CoV-2 Infection and Associated Hyperinflammatory Responses. Front. Mol. Biosci. 2021, 7, 627767. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytotherapy Res. 2020, 35, 1298–1312. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-Lowering and Antioxidant Effects of Hydroxytyrosol and Its Triacetylated Derivative Recovered from Olive Tree Leaves in Cholesterol-Fed Rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Kotovicz, V.; Wypych, F.; Zanoelo, E.F. Pulsed hydrostatic pressure and ultrasound assisted extraction of soluble matter from mate leaves (Ilex paraguariensis): Experiments and modeling. Sep. Purif. Technol. 2014, 132, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Zu, Y.-G.; Fu, Y.-J.; Yang, Y.-C.; Li, S.-M.; Li, Z.-N.; Wink, M. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2010, 11, 637–643. [Google Scholar] [CrossRef]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Shouqin, Z.; Jun, X.; Changzheng, W. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Plaza-Morales, M.; Giovagnoli-Vicuña, C.; Jamett, F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT Food Sci. Technol. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Ünlü, A.E. Green and Non-conventional Extraction of Bioactive Compounds from Olive Leaves: Screening of Novel Natural Deep Eutectic Solvents and Investigation of Process Parameters. Waste Biomass Valorization 2021, 12, 5329–5346. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez-Fernández, M.Y.; Burrieza, J.E.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining green extracts rich in phenolic compounds from underexploited food by-products using natural deep eutectic solvents. Opportunities and challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-Ethanolic Mixtures for the Recovery of Phenols from Mediterranean Plant Materials. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Mohamad, M.; Ali, M.W.; Ripin, A.; Ahmad, A. Effect of Extraction Process Parameters on the Yield of Bioactive Compounds from the Roots of Eurycoma Longifolia. J. Teknol. 2013, 60, 51–57. [Google Scholar] [CrossRef]
- Akhmazillah, M.F.N.; Farid, M.M.; Silva, F.V.M. High pressure processing (HPP) of honey for the improvement of nutritional value. Innov. Food Sci. Emerg. Technol. 2013, 20, 59–63. [Google Scholar] [CrossRef]
- García, A.; Rodriguez-Juan, E.; Rodriguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esclapez, M.D.; Garcia-Perez, J.V.; Mulet, A.; Carcel, J.A. Ultrasound-Assisted Extraction of Natural Products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Brühl, L. Official Methods and Recommended Practices of the American Oil Chemist’s Society, Physical and Chemical Characteristics of Oils, Fats and Waxes, Section I. Ed. The AOCS Methods Editor and the AOCS Technical Department. 54 pages. AOCS Press, Champaign, 1996. Lipid/Fett 1997, 99, 197. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics; Wiley: Hoboken, NJ, USA, 2002; pp. 1–8. Available online: http://onlinelibrary.wiley.com/doi/10.1002/0471142913.fai0101s06/abstract (accessed on 30 September 2017).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Code | Components | Mole Ratio | Water Addition (% v/v) |
|---|---|---|---|
| CCA | Choline chloride/Citric Acid | 1:2 | 20 |
| CLA | Choline chloride/Lactic Acid | 1:2 | 20 |
| CMA | Choline chloride/Maltose | 1:2 | 20 |
| CGL | Choline chloride/Glycerol | 1:2 | 20 |
| NADES | Extraction Conditions | Phenolic Compounds * | SUM *** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Oleuropein (OL) ** | Hydroxytyrosol (HT) ** | Caffeic Acid (CA) ** | Vanillin (VA) ** | Rutin (RU) ** | Luteolin (LU) ** | ||||
| (a) MAE | t (min) | T(°C) | |||||||
| CCA | 40 | 3.32 ± 0.12 a | 6.41 ± 0.47 | 2.45 ± 0.34 | 0.59 ± 0.07 | 3.57 ± 0.38 | 0.05 ± 0.00 | 16.39 ± 0.71 a | |
| 60 | n.d. | 16.18 ± 0.35 | 0.06 ± 0.01 | 0.57 ± 0.05 | 4.06 ± 0.78 | 0.90 ± 0.05 | 21.77 ± 0.86 a | ||
| CMA | 30 | 40 | n.d. | 1.12 ± 0.10 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.01 | n.d. | 1.18 ± 0.10 b |
| 60 | n.d. | 1.12 ± 0.02 | n.d. | n.d. | n.d. | n.d. | 1.12 ± 0.02 b | ||
| CLA | 30 | 40 | 8.23 ± 1.19 b | 13.64 ± 0.07 | 0.07 ± 0.00 | 1.14 ± 0.10 | 5.89 ± 0.32 | 0.08 ± 0.01 | 29.05 ± 1.24 a |
| 60 | 7.93 ± 1.07 b | 13.44 ± 0.39 | 0.06 ± 0.01 | 1.05 ± 0.07 | 5.73 ± 0.30 | 0.08 ± 0.02 | 28.29 ± 1.18 a | ||
| CGL | 30 | 40 | 11.27 ± 3.50 b | 1.56 ± 0.05 | 0.05 ± 0.01 | 0.26 ± 0.05 | 2.17 ± 0.10 | 0.18 ± 0.04 | 15.49 ± 3.50 a |
| 60 | 14.61 ± 1.37 b | 2.64 ± 0.19 | 0.10 ± 0.02 | 1.58 ± 0.24 | 7.91 ± 0.59 | 0.14 ± 0.00 | 26.98 ± 1.52 a | ||
| EtOH 70% | 30 | 60 | 18.94 ± 0.50 | n.d. | 0.07 ± 0.01 | n.d. | 7.51 ± 0.03 | 0.08 ± 0.01 | 26.6 ± 0.50 |
| WATER | 30 | 60 | 10.60 ± 0.56 | 0.56 ± 0.10 | 0.02 ± 0.00 | 0.4 ± 0.03 | 3.77 ± 0.29 | n.d. | 15.35 ± 0.64 |
| (b) UAE | t (min) | T (°C) | |||||||
| CCA | 30 | 40 | 4.46 ± 0.85 ab | 9.14 ± 0.12 bc | 0.04 ± 0.00 | 0.71 ± 0.05 ab | 2.5 ± 0.15 a | 0.06 ± 0.01 | 16.91 ± 0.87 b |
| 60 | 5.02 ± 1.02 ab | 8.17 ± 0.20 bc | 0.04 ± 0.01 | 0.65 ± 0.00 ab | 3.11 ± 0.70 a | 0.06 ± 0.00 | 17.05 ± 1.25 b | ||
| CMA | 30 | 40 | 4.64 ± 1.23 a | 0.69 ± 0.05 a | 0.01 ± 0.00 | 0.15 ± 0.03 a | 0.65 ± 0.04 a | 0.01 ± 0.01 | 6.15 ± 1.23 a |
| 60 | 2.83 ± 0.92 a | 2.96 ± 0.10 a | 0.01 ± 0.00 | 0.09 ± 0.03 a | 0.96 ± 0.03 a | 0.01 ± 0.01 | 6.86 ± 0.93 a | ||
| CLA | 30 | 40 | 8.80 ± 3.55 c | 9.58 ± 0.47 c | 0.04 ± 0.01 | 0.91 ± 0.03 b | 4.70 ± 0.22 b | 0.09 ± 0.01 | 24.12 ± 3.58 c |
| 60 | 9.96 ± 3.21 c | 13.87 ± 0.50 c | 0.08 ± 0.01 | 1.38 ± 0.07 b | 7.02 ± 0.28 b | 0.13 ± 0.01 | 32.44 ± 3.26 c | ||
| CGL | 30 | 40 | 6.57 ± 0.55 bc | 2.96 ± 0.08 ab | 0.04 ± 0.00 | 0.67 ± 0.08 b | 0.49 ± 0.11 a | 0.12 ± 0.01 | 10.85 ± 0.57 ab |
| 60 | 8.53 ± 0.63 bc | 5.07 ± 0.12 ab | 0.06 ± 0.01 | 0.81 ± 0.09 b | 0.64 ± 0.08 a | 0.07 ± 0.00 | 15.18 ± 0.65 ab | ||
| EtOH 70% | 30 | 60 | 19.89 ± 1.08 | 2.67 ± 0.00 | 0.03 ± 0.00 | 0.39 ± 0.00 | 5.91 ± 0.00 | n.d. | 28.89 ± 1.08 |
| WATER | 30 | 60 | 1.77 ± 0.05 | n.d. | 0.01 ± 0.00 | n.d. | n.d. | n.d. | 1.78 ± 0.05 |
| NADES | Extraction Conditions | Phenolic Compounds * | SUM *** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Oleuropein (OL) ** | Hydroxytyrosol (HT) ** | Caffeic Acid (CA) ** | Vanillin (VA) ** | Rutin (RU) ** | Luteolin (LU) ** | ||||
| (a) HAE | T (°C) | Speed (rpm) | |||||||
| CCA | 40 | 4000 | 7.55 ± 0.10 | 12.63 ± 0.20 c | 0.08 ± 0.01 | 1.15 ± 0.08 a | 10.70 ± 0.35 | n.d. | 32.11 ± 0.42 b |
| 12,000 | 7.61 ± 0.32 | 13.60 ± 0.10 c | 0.07 ± 0.01 | 1.29 ± 0.05 a | 11.08 ± 0.20 | n.d. | 33.65 ± 0.39 b | ||
| 60 | 4000 | 19.07 ± 0.22 | 7.99 ± 0.18 c | 0.06 ± 0.02 | 0.53 ± 0.07 a | 5.01 ± 0.28 | n.d. | 32.66 ± 0.41 b | |
| 12,000 | 32.88 ± 0.08 | 10.07 ± 0.12 c | 0.20 ± 0.01 | 1.71 ± 0.06 a | 8.00 ± 0.75 | n.d. | 52.86 ± 0.77 b | ||
| CMA | 40 | 4000 | 13.22 ± 1.51 | 4.92 ± 1.33 b | 0.07 ± 0.00 | 1.49 ± 0.10 ab | 5.56 ± 0.36 | n.d. | 25.26 ± 2.05 a |
| 12,000 | 14.24 ± 1.52 | 5.25 ± 0.33 b | 0.08 ± 0.01 | 1.18 ± 0.18 ab | 5.17 ± 0.01 | n.d. | 25.92 ± 1.57 a | ||
| 60 | 4000 | 13.35 ± 1.01 | 5.26 ± 0.65 b | 0.09 ± 0.02 | 1.39 ± 0.11 ab | 5.57 ± 0.15 | n.d. | 25.66 ± 1.22 a | |
| 12,000 | 17.57 ± 0.19 | 6.40 ± 0.02 b | 0.10 ± 0.02 | 1.98 ± 0.27 ab | 5.91 ± 0.46 | n.d. | 31.96 ± 0.57 a | ||
| CLA | 40 | 4000 | 9.19 ± 1.30 | 3.24 ± 0.34 ab | 0.04 ± 0.01 | 0.92 ± 0.10 a | 5.76 ± 0.49 | n.d. | 19.15 ± 1.43 a |
| 12,000 | 14.71 ± 0.88 | n.d. | 0.07 ± 0.02 | 0.92 ± 0.04 a | 8.41 ± 0.95 | n.d. | 24.11 ± 1.30 a | ||
| 60 | 4000 | 14.52 ± 1.67 | 2.88 ± 0.00 ab | 0.10 ± 0.03 | 0.83 ± 0.33 a | 7.58 ± 1.48 | n.d. | 25.91 ± 2.26 a | |
| 12,000 | 12.47 ± 1.46 | 9.90 ± 0.93 ab | 0.08 ± 0.00 | 1.23 ± 0.04 a | 5.12 ± 0.60 | n.d. | 28.80 ± 1.83 a | ||
| CGL | 40 | 4000 | 14.64 ± 1.35 | n.d. | 0.08 ± 0.01 | 1.50 ± 0.06 b | 8.03 ± 0.46 | n.d. | 24.25 ± 1.43 a |
| 12,000 | 15.65 ± 1.06 | n.d. | 0.09 ± 0.01 | 1.48 ± 0.05 b | 8.98 ± 1.06 | n.d. | 26.20 ± 1.50 a | ||
| 60 | 4000 | 18.25 ± 0.46 | n.d. | 0.09 ± 0.03 | 2.16 ± 0.07 b | 8.19 ± 0.25 | n.d. | 28.69 ± 0.53 a | |
| 12,000 | 19.16 ± 1.15 | n.d. | 0.12 ± 0.02 | 2.34 ± 0.06 b | 8.55 ± 0.95 | n.d. | 30.17 ± 1.49 a | ||
| EtOH 70% | 60 | 12,000 | 26.67 ± 1.13 | n.d. | n.d. | 0.11 ± 0.02 | 4.97 ± 0.52 | n.d. | 31.75 ± 1.24 |
| WATER | 60 | 12,000 | 1.09 ± 0.06 | 1.23 ± 0.05 | 0.02 ± 0.00 | 0.29 ± 0.05 | 0.29 ± 0.03 | 0.02 ± 0.00 | 2.94 ± 0.10 |
| (b) HHPAE | HP (MPa) | t (min) | |||||||
| CCA | 300 | 5 | 1.26 ± 0.26 c | 3.32 ± 0.08 c | 0.01 ± 0.00 ab | 0.24 ± 0.02 | 1.24 ± 0.02 | 0.02 ± 0.00 a | 6.09 ± 0.27 c |
| 10 | 1.50 ± 0.19 c | 4.85 ± 0.10 c | 0.02 ± 0.00 ab | 0.35 ± 0.02 | 1.88 ± 0.02 | 0.03 ± 0.00 a | 8.63 ± 0.22 c | ||
| 600 | 5 | 2.20 ± 0.27 c | 6.08 ± 0.06 c | 0.02 ± 0.00 ab | 0.41 ± 0.02 | 2.59 ± 0.05 | 0.03 ± 0.00 a | 11.33 ± 0.28 c | |
| 10 | 2.39 ± 0.09 c | 7.35 ± 0.06 c | 0.03 ± 0.00 ab | 0.61 ± 0.04 | 3.18 ± 0.16 | 0.04 ± 0.01 a | 13.6 ± 0.20 c | ||
| CMA | 300 | 5 | 0.35 ± 0.07 ab | 0.50 ± 0.02 a | n.d. | 0.70 ± 0.03 | 0.04 ± 0.00 | 0.01 ± 0.00 a | 1.60 ± 0.08 a |
| 10 | 1.07 ± 0.35 ab | 0.33 ± 0.02 a | 0.01 ± 0.00 ab | 0.10 ± 0.03 | 0.85 ± 0.04 | 0.03 ± 0.00 a | 2.39 ± 0.35 a | ||
| 600 | 5 | 1.03 ± 0.50 ab | 0.74 ± 0.02 a | 0.01 ± 0.00 ab | 0.21 ± 0.00 | 1.41 ± 0.03 | 0.04 ± 0.01 a | 3.44 ± 0.50 a | |
| 10 | 1.53 ± 0.05 ab | 0.69 ± 0.07 a | 0.03 ± 0.00 ab | 0.24 ± 0.06 | 1.92 ± 0.01 | 0.06 ± 0.01 a | 4.47 ± 0.11 a | ||
| CLA | 300 | 5 | 0.54 ± 0.02 a | 1.76 ± 0.01 b | 0.01 ± 0.00 a | 0.13 ± 0.00 | 0.76 ± 0.00 | 0.01 ± 0.00 a | 3.21 ± 0.02 ab |
| 10 | 0.82 ± 0.08 a | 2.39 ± 0.00 b | 0.01 ± 0.00 a | 0.21 ± 0.01 | 0.82 ± 0.03 | 0.01 ± 0.00 a | 4.26 ± 0.09 ab | ||
| 600 | 5 | 0.98 ± 0.10 a | 2.87 ± 0.00 b | 0.01 ± 0.00 a | 0.24 ± 0.01 | 1.30 ± 0.01 | 0.02 ± 0.00 a | 5.42 ± 0.10 ab | |
| 10 | 1.05 ± 0.04 a | 2.85 ± 0.01 b | 0.01 ± 0.00 a | 0.24 ± 0.01 | 1.22 ± 0.06 | 0.02 ± 0.00 a | 5.39 ± 0.12 ab | ||
| CGL | 300 | 5 | 0.52 ± 0.04 bc | 0.97 ± 0.01 b | 0.04 ± 0.01 b | 0.08 ± 0.02 | 0.15 ± 0.02 | 0.02 ± 0.00 b | 1.78 ± 0.05 b |
| 10 | 2.60 ± 0.34 bc | 1.37 ± 0.03 b | 0.03 ± 0.00 b | 0.55 ± 0.01 | 2.43 ± 0.01 | 0.11 ± 0.01 b | 7.09 ± 0.34 b | ||
| 600 | 5 | 1.06 ± 0.12 bc | 2.47 ± 0.13 b | 0.02 ± 0.01 b | 0.13 ± 0.01 | 0.19 ± 0.01 | 0.26 ± 0.02 b | 4.13 ± 0.18 b | |
| 10 | 2.22 ± 0.29 bc | 4.19 ± 0.01 b | 0.03 ± 0.00 b | 0.46 ± 0.02 | 1.66 ± 0.01 | 0.15 ± 0.02 b | 8.71 ± 0.29 b | ||
| EtOH 70% | 600 | 10 | 29.18 ± 0.87 | n.d. | 0.02 ± 0.00 | 1.03 ± 0.03 | 6.73 ± 0.55 | n.d. | 36.96 ± 1.03 |
| WATER | 600 | 10 | 1.31 ± 0.05 | 0.53 ± 0.02 | n.d. | n.d. | 0.67 ± 0,01 | n.d. | 2.51 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siamandoura, P.; Tzia, C. Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules 2023, 28, 353. https://doi.org/10.3390/molecules28010353
Siamandoura P, Tzia C. Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules. 2023; 28(1):353. https://doi.org/10.3390/molecules28010353
Chicago/Turabian StyleSiamandoura, Paraskevi, and Constantina Tzia. 2023. "Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents" Molecules 28, no. 1: 353. https://doi.org/10.3390/molecules28010353
APA StyleSiamandoura, P., & Tzia, C. (2023). Comparative Study of Novel Methods for Olive Leaf Phenolic Compound Extraction Using NADES as Solvents. Molecules, 28(1), 353. https://doi.org/10.3390/molecules28010353




