The Effects of Propolis on Viral Respiratory Diseases
Abstract
:1. Introduction
2. Methodology
3. Progress in Studies of Propolis
4. Propolis Activity against Coronaviruses
4.1. Preclinical Studies
4.2. Clinical Trials
5. Propolis Activity against Influenza A Virus and Parainfluenza Virus
5.1. Preclinical Studies
5.2. Clinical Trials
6. Activity against Human Rhinoviruses
6.1. Preclinical Studies
6.2. Clinical Trials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojczyk, E.; Klama-Baryła, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and modern research on propolis and its application in wound healing and other fields of medicine and contributions by Polish studies. J. Ethnopharmacol. 2020, 262, 113159. [Google Scholar] [CrossRef] [PubMed]
- Seçilmiş, Y.; Silici, S. Bee product efficacy in children with upper respiratory tract infections. Turk J. Pediatr. 2020, 62, 634–640. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell'Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef]
- Yuksel, S.; Akyol, S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. J. Intercult. Ethnopharmacol. 2016, 5, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Perera, C.O.; Tandean, S.; Abdulah, R.; Herman, H.; Christoper, A.; Chandrasekaran, K.; Putra, A.; Lesmana, R. The Potential use of propolis as a primary or an adjunctive therapy in respiratory tract-related diseases and disorders: A systematic scoping review. Biomed. Pharmacother. 2022, 146, 112595. [Google Scholar] [CrossRef] [PubMed]
- Ferkol, T.; Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic burden of COVID-19: A Systematic Review. ClinicoEcon. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef]
- Ripari, N.; Sartori, A.A.; Honorio, M.S.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis antiviral and immunomodulatory activity: A review and perspectives for COVID-19 treatment. J. Pharm. Pharmacol. 2021, 73, 281–299. [Google Scholar] [CrossRef]
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Yeh, C.F.; Wang, K.C.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; San Chang, J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar]
- Zhou, W.; Yin, A.; Shan, J.; Wang, S.; Cai, B.; Di, L. Study on the rationality for antiviral activity of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple preparations improved by chito-oligosaccharide via integral pharmacokinetics. Molecules 2017, 22, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Saddique, M.A.B.; Tahir, H.; Amjad, M.D.; Ahmad, A.; Masood, U.; Khan, D. A Short Review on Key Role of Plants and their Extracts in Boosting up Immune Response to Combat COVID-19. Infect. Disord. Drug. Targets 2022, 22, e270521193625. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Rafieian-Kopaei, M. Mechanistic aspects of medicinal plants and secondary metabolites against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Curr. Pharm. Des. 2021, 27, 3996–4007. [Google Scholar] [CrossRef] [PubMed]
- Jamshidnia, M.; Sewell, R.D.E.; Rafieian-Kopaei, M. An Update on Promising Agents against COVID-19: Secondary metabolites and mechanistic aspects. Curr. Pharm. Des. 2022, 28, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Dilokthornsakul, W.; Kosiyaporn, R.; Wuttipongwaragon, R.; Dilokthornsakul, P. Potential effects of propolis and honey in COVID-19 prevention and treatment: A systematic review of in silico and clinical studies. J. Integr. Med. 2022, 20, 114–125. [Google Scholar] [CrossRef]
- Ghosh, S.; Al-Sharify, Z.T.; Maleka, M.F.; Onyeaka, H.; Maleke, M.; Maolloum, A.; Godoy, L.; Meskini, M.; Rami, M.R.; Ahmadi, S.; et al. Propolis efficacy on SARS-COV viruses: A review on antimicrobial activities and molecular simulations. Environ. Sci. Pollut. Res. Int. 2022, 29, 58628–58647. [Google Scholar] [CrossRef]
- Soleymani, S.; Naghizadeh, A.; Karimi, M.; Zarei, A.; Mardi, R.; Kordafshari, G.; Esmaealzadeh, N.; Zargaran, A. COVID-19: General strategies for herbal therapies. J. Evid. Based Integr. Med. 2022, 27, 1–18. [Google Scholar] [CrossRef]
- Sberna, G.; Biagi, M.; Marafini, G.; Nardacci, R.; Biava, M.; Colavita, F.; Piselli, P.; Miraldi, E.; D’Offizi, G.; Capobianchi, M.R.; et al. In vitro evaluation of antiviral efficacy of a standardized hydroalcoholic extract of poplar type propolis against SARS-CoV-2. Front Microbiol. 2022, 13, 799546. [Google Scholar] [CrossRef]
- Bijelic, K.; Hitl, M.; Kladar, N. Phytochemicals in the Prevention and Treatment of SARS-CoV-2—Clinical Evidence. Antibiotics 2022, 11, 1614. [Google Scholar] [CrossRef]
- Fiorini, A.C.; Scorza, C.A.; de Almeida AC, G.; Fonseca, M.; Finsterer, J.; Fonseca, F.L.; Scorza, F.A. Antiviral activity of Brazilian green propolis extract against SARS-CoV-2 (severe acute respiratory syndrome-coronavirus 2) infection: Case report and review. Clinics 2021, 76, e2357. [Google Scholar] [CrossRef] [PubMed]
- Güler, H.İ.; Ay Şal, F.; Can, Z.; Kara, Y.; Yildiz, O.; Beldüz, A.O.; Canakci, S.; Kolayli, S. Targeting CoV-2 spike RBD and ACE-2 interaction with flavonoids of Anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics. Turk J. Biol. 2021, 45, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Harisna, A.H.; Nurdiansyah, R.; Syaifie, P.H.; Nugroho, D.W.; Saputro, K.E.; Prakoso, F.C.D.; Rochman, N.T.; Maulana, N.N.; Noviyanto, A.; Mardliyati, E.; et al. In silico investigation of potential inhibitors to main protease and spike protein of SARS-CoV-2 in propolis. Biochem. Biophys. Rep. 2021, 26, 100969. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phyther. Res. 2020, 35, 743–750. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Bachevski, D.; Damevska, K.; Simeonovski, V.; Dimova, M. Back to the basics: Propolis and COVID-19. Dermatol. Ther. 2020, 33, e13780. [Google Scholar] [CrossRef]
- Maruta, H.; He, H. PAK1-blockers: Potential Therapeutics against COVID-19. Med. Drug Discov. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, antibacterial, antifungal, and antiparasitic properties of propolis: A review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horcinová-Sedlácková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Ozarowski, M.; Karpinski, T.M.; Alam, R.; Łochynska, M. Antifungal Properties of Chemically Defined Propolis from Various Geographical Regions. Microorganisms 2022, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Münstedt, K. Bee products and the treatment of blister-like lesions around the mouth, skin and genitalia caused by herpes viruses -A systematic review. Complement. Ther. Med. 2019, 43, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08). J. Funct. Foods 2014, 8, 214–223. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.M.; Perumalsamy, H.; Wang, X.; Ahn, Y.J. Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. J. Apic. Res. 2020, 59, 413–425. [Google Scholar] [CrossRef]
- Sokolonski, A.R.; Fonseca, M.S.; Machado, B.A.S.; Deegan, K.R.; Araújo, R.P.C.; Umsza-Guez, M.A.; Meyer, R.; Portela, R.W. Activity of antifungal drugs and Brazilian red and green propolis extracted with different methodologies against oral isolates of Candida spp. BMC Complement. Med. Ther. 2021, 21, 286. [Google Scholar] [CrossRef]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In vitro activity of propolis on oral microorganisms and biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical diversity and challenges in quality control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Galvéz-Ruíz, J.C.; Ikner, L.A.; Umsza-Guez, M.A.; de Paula Castro, T.L.; Gerba, C.P. In vitro antiviral effect of Mexican and Brazilian propolis and phenolic compounds against human coronavirus 229E. Int. J. Environ. Health Res. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (COVID-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Sauter, D.; Wang, K.; Zhang, R.; Sun, B.; Karioti, A.; Bilia, A.R.; Efferth, T.; Schwarz, W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014, 80, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.J.; Michaelis, M.; Hsu, H.K.; Tsai, C.C.; Yang, K.D.; Wu, Y.C.; Cinatl, J., Jr.; Doerr, H.W. Toona sinensis Roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P.; et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004, 78, 11334. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ting, Z.; Jiang, D.; Sheng, C.; Fan, Y.; Qi, J. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS ONE 2014, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Sarkar, A.; Maulik, U. Molecular docking study of potential phytochemicals and their efects on the complex of SARS-CoV2 spike protein and human ACE2. Sci. Rep. 2020, 10, 17699. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.D.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceicao, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef]
- Miryan, M.; Soleimani, D.; Dehghani, L.; Sohrabi, K.; Khorvash, F.; Bagherniya, M.; Sayedi, S.M.; Askari, G. The effect of propolis supplementation on clinical symptoms in patients with coronavirus (COVID-19): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 996. [Google Scholar] [CrossRef] [PubMed]
- Kosari, M.; Noureddini, M.; Khamechi, S.P.; Najafi, A.; Ghaderi, A.; Sehat, M.; Banafshe, H.R. The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial. Phytother Res. 2021, 35, 4000–4006. [Google Scholar] [CrossRef]
- Investigation of Clinical Effectiveness of Propolis Extracts as Food Supplements in Patients with SARS-CoV-2(COVID-19). Identifier: NCT04916821. Available online: https://beta.clinicaltrials.gov/study/NCT04916821?distance=50&cond=SARS-CoV-2&term=propolis&rank=1 (accessed on 1 September 2022).
- Zorlu, D. COVID-19 and anatolian propolis: A case report. Acta Med. Mediterr. 2021, 37, 1229–1233. [Google Scholar]
- Yusuf, A.P.; Zhang, J.Y.; Li, J.Q.; Muhammad, A.; Abubakar, M.B. Herbal medications and natural products for patients with covid-19 and diabetes mellitus: Potentials and challenges. Phytomed Plus 2022, 2, 100280. [Google Scholar] [CrossRef] [PubMed]
- Balica, G.; Vostinaru, O.; Stefanescu, C.; Mogosan, C.; Iaru, I.; Cristina, A.; Pop, C.E. Potential Role of Propolis in the Prevention and Treatment of Metabolic Diseases. Plants 2021, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Ghazaly, M.A.; El-Khatib, A.S.; Hatem, A.M.; de Vries, P.J.; El-Shafei, S.; Khattab, M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam. Clin. Pharmacol. 2003, 17, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The cardiovascular therapeutic potential of propolis—A comprehensive review. Biology 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.M.; Zhao, C.Y.; Tarquinio, K.M.; Brown, S.P. Causes and consequences of COVID-19-associated bacterial infections. Front. Microbiol. 2021, 12, 682571. [Google Scholar] [CrossRef]
- Pourajam, S.; Kalantari, E.; Talebzadeh, H.; Mellali, H.; Sami, R.; Soltaninejad, F.; Amra, B.; Sajadi, M.; Alenaseri, M.; Kalantari, F.; et al. Secondary bacterial infection and clinical characteristics in patients with COVID-19 admitted to two intensive care units of an academic hospital in Iran during the first wave of the pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 784130. [Google Scholar] [CrossRef]
- Hoque, M.N.; Akter, S.; Mishu, I.D.; Islam, M.R.; Rahman, M.S.; Akhter, M.; Islam, I.; Hasan, M.M.; Rahaman, M.M.; Sultana, M.; et al. Microbial co-infections in COVID-19: Associated microbiota and underlying mechanisms of pathogenesis. Microb. Pathog. 2021, 156, 104941. [Google Scholar] [CrossRef]
- Taufik, F.F.; Natzir, R.; Patellongi, I.; Santoso, A.; Hatta, M.; Junita, A.R.; Syukri, A.; Primaguna, M.R.; Dwiyanti, R.; Febrianti, A. In vivo and in vitro inhibition effect of propolis on Klebsiella pneumoniae: A review. Ann. Med. Surg. 2022, 81, 104388. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 14 October 2022).
- Governa, P.; Cusi, M.G.; Borgonetti, V.; Sforcin, J.M.; Terrosi, C.; Baini, G.; Miraldi, E.; Biagi, M. Beyond the biological effect of a chemically characterized poplar propolis: Antibacterial and antiviral activity and comparison with flurbiprofen in cytokines release by LPS stimulated human mononuclear cells. Biomedicines 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takemura, T.; Urushisaki, T.; Fukuoka, M.; Hosokawa-Muto, J.; Hata, T.; Okuda, Y.; Hori, S.; Tazawa, S.; Araki, Y.; Kuwata, K. 3,4-dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the Influenza A Virus. Evid. Based Complement. Alternat. Med. 2012, 2012, 946867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urushisaki, T.; Takemura, T.; Tazawa, S.; Fukuoka, M.; Hosokawa-Muto, J.; Araki, Y.; Kuwata, K. Caffeoylquinic acids are major constituents with potent anti-influenza effects in brazilian green propolis water extract. Evid. Based Complement. Alternat. Med. 2011, 2011, 254914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Shimizu, T.; Hino, A.; Tsutsumi, A.; Park, Y.K.; Watanabe, W.; Kurokawa, M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir. Chem. Chemother. 2008, 19, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Serkedjieva, J.; Manolova, N.; Bankova, V. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids). J. Nat. Prod. 1992, 55, 294–297. [Google Scholar] [CrossRef]
- Drago, L.; De Vecchi, E.; Nicola, L.; Gismondo, M.R. In vitro antimicrobial activity of a novel propolis formulation (Actichelated propolis). J. Appl. Microbiol. 2007, 103, 1914–1921. [Google Scholar] [CrossRef]
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicie 2021, 37, 100986. [Google Scholar] [CrossRef]
- Esposito, C.; Garzarella, E.U.; Bocchino, B.; D'Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; Galeotti, F.; Tenore, G.C.; Zaccaria, V.; et al. A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): A monocentric, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2021, 80, 153368. [Google Scholar] [CrossRef]
- Di Pierro, F.; Zanvit, A.; Colombo, M. Role of a proprietary propolis-based product on the wait-and-see approach in acute otitis media and in preventing evolution to tracheitis, bronchitis, or rhinosinusitis from nonstreptococcal pharyngitis. Int. J. Gen. Med. 2016, 9, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, H.A.; Varsano, I.; Kahan, E.; Sarrell, E.M.; Uziel, Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: A randomized, double-blind, placebo-controlled, multicenter study. Arch. Pediatr. Adolesc. Med. 2004, 158, 217–221. [Google Scholar] [CrossRef] [PubMed]
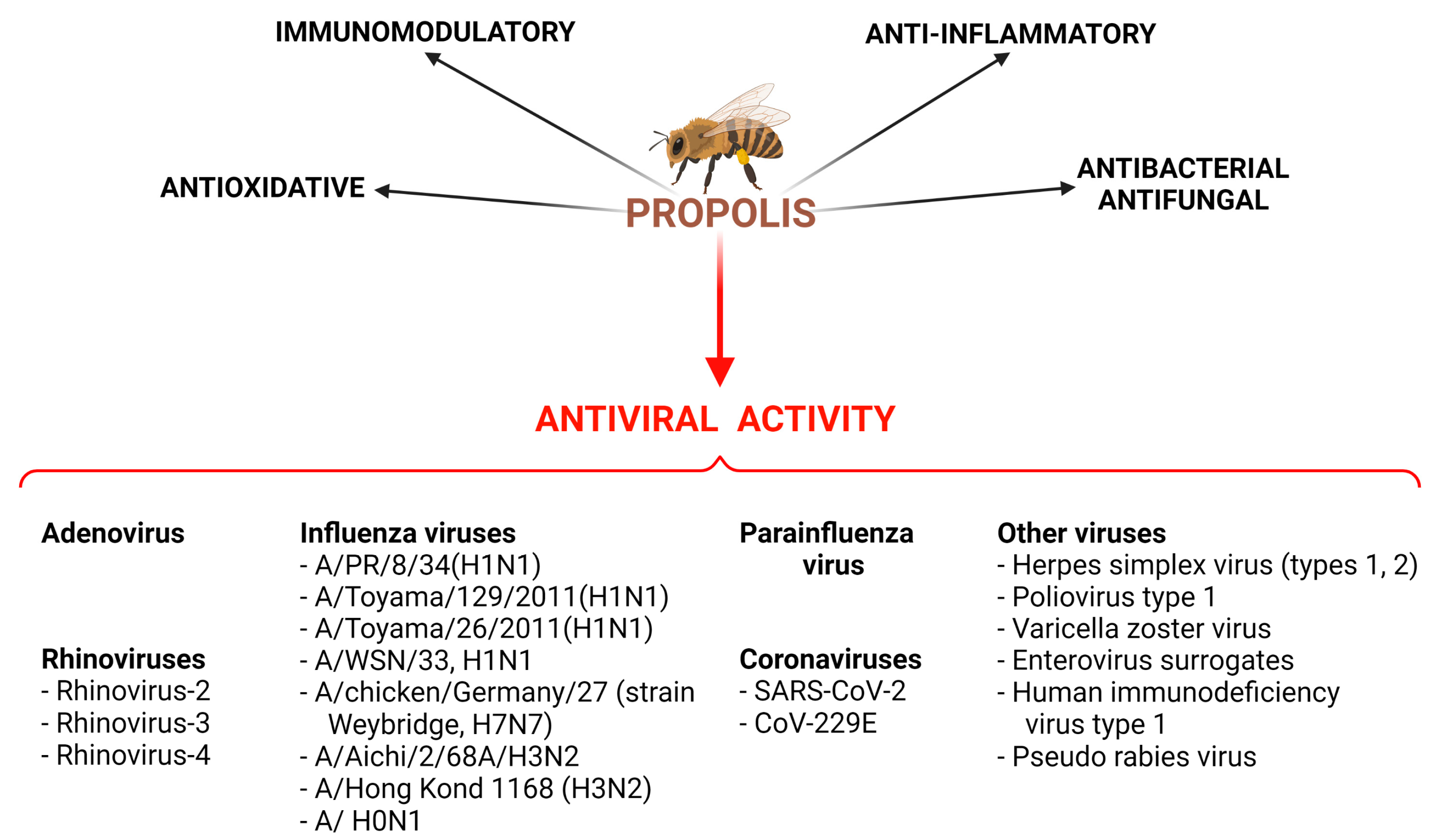

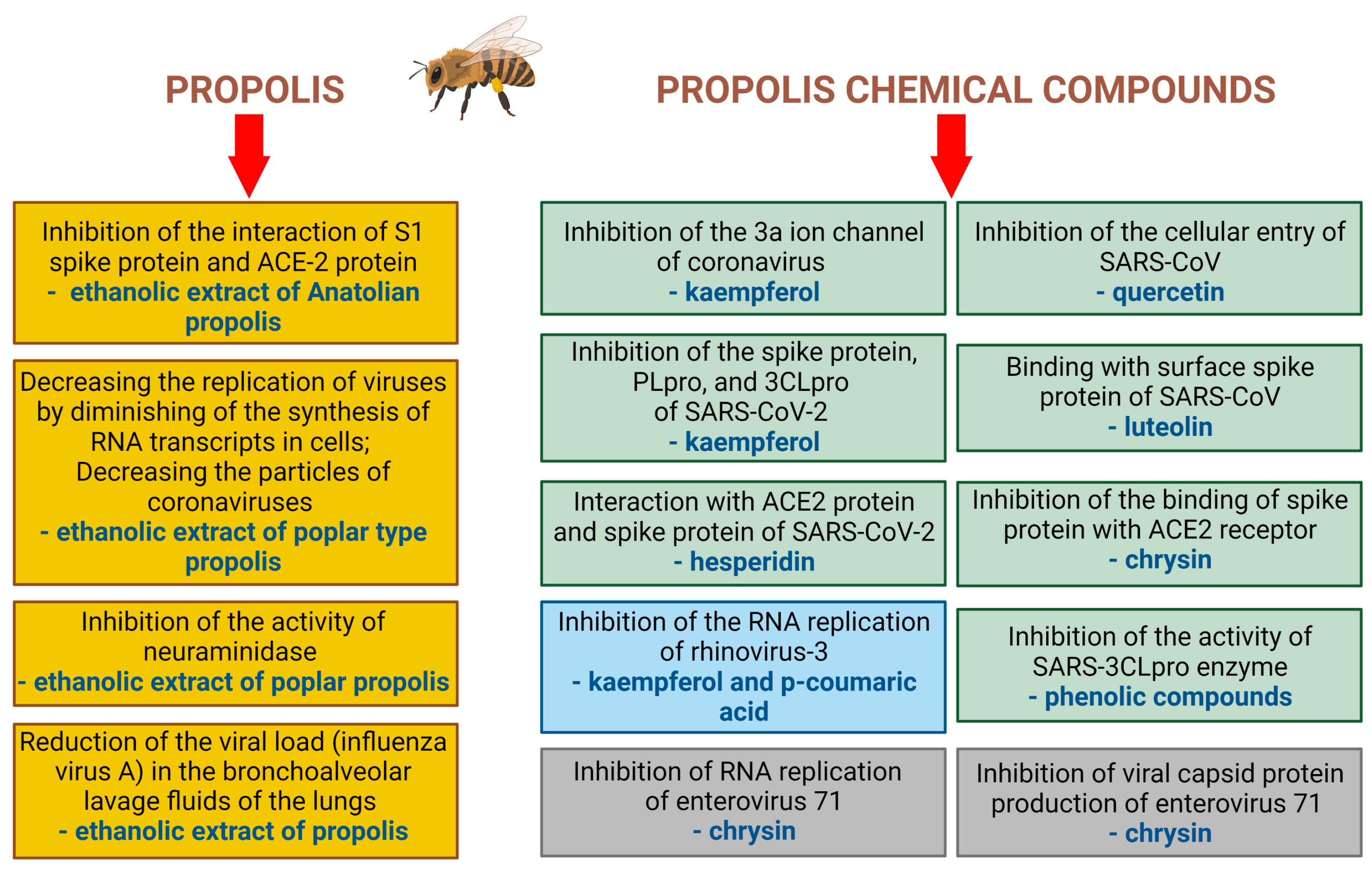
| Type of Virus | Type of Preparation of Propolis | Effects | Types of Studies | Ref. |
|---|---|---|---|---|
| CoV-229E | Extracts from green and brown propolis | Anti-viral activity against human CoV-229E after application of green propolis extract (EC50 = 19.08 µg/mL) and brown propolis extract (EC50 = 11.24 µg/mL) | In vitro: line of MRC-5 cells | Silva-Beltrán et al. [41] |
| SARS-CoV-2 | 70% ethanolic extract of Anatolian propolis | Inhibition of the interaction between the SARS-CoV-2 S1 spike protein and ACE-2 receptors in a concentration- dependent manner | In vitro: screening colorimetric assay kit | Güler et al. [22] |
| SARS-CoV-2 | 80% extract of poplar-type propolis | Decrease in the replication of viruses by diminishing of the synthesis of RNA transcripts in cells after application of extract at a concentration of 25 µg/mL Decrease in the number of virions and a reduction in the number of infected cells | In vitro: VERO E6 (African green monkey, kidney epithelial cell line), CALU3 (human lung epithelial cell lines) | Sberna et al. [19] |
| Avian influenza virus A/chicken/Germany/27 (strain Weybridge, H7N7) | 70% ethanolic extract from samples of propolis from Albania, Brazil, Bulgaria, Mongolia | Antiviral activity in eight samples of propolis Significant selectivity index (SI) for samples of Bulgarian propolis (SI = 8) and Brazilian propolis (IS = 35) | In vitro: primary chick embryo fibroblast (CEF) cells | Kujumgiev, 1999 [67] |
| A/H1N1 and A/H3N2 | Et2O fraction of ethanolic extract of propolis | Decreasing the infectious activity of A/H1N1 and A/H3N2 in vitro at concentrations of 50 µg/mL and 100 µg/mL, respectively | In vitro: embryonated hen’s eggs | Serkedjieva, 1992 [69] |
| Influenza A/PR/8/34 virus | Ethanolic extract of Brazilian propolis | Increased survival time of infected mice and improved the symptoms of influenza in animals after 10 mg/kg administered three times daily Values of EC50 were from <10 to 116.6 µg/mL for thirteen samples | In vivo: mice (DBA/2) infected by influenza virus In vitro: Madin–Darby canine kidney (MDCK) cells | Shimizu, 2008 [68] |
| Influenza virus, parainfluenza virus, adenovirus | Actichelated® propolis; hydroalcoholic extract from propolis | Antiviral activity of Actichelated® propolis at concentrations from 0.032 g/l to 0.128 g/l is higher than that of hydroalcoholic extract against influenza virus, parainfluenza virus, adenovirus; no cytotoxic effects | In vitro: Hep-2 cell monolayer with isolated viruses | Drago, 2007 [70] |
| Influenza virus A/WSN/33 (H1N1) | Water extract of Brazilian green propolis | Increased cell viability at concentrations of 100 to 300 μg/mL of propolis extract Cell survival EC50 = 183 μg/mL | In vitro: Madin–Darby canine kidney (MDCK) cells | Urushisaki, 2011 [66] |
| Influenza A virus (H1N1) | 80% ethanolic extract of Italian propolis | Inhibition of viral replication Inhibiting the neuraminidase activity IC50 = 35.29 µg/mL | In vitro: Madin-Darby canine kidney (MDCK) cells | Governa, 2019 [64] |
| Human rhinovirus: HRV-2, HRV-3, HRV-4 | 80% ethanolic extract of Brazilian propolis and its fractions obtained using hexane, chloroform and ethyl acetate, butanol, water | antiviral activity against HVR-4 with the following IC50 values: 5.00 µg/mL-chloroform-soluble fraction 5.2 µg/mL-ethyl acetate-soluble fraction 8.9 µg/mL-hexane-soluble fraction 15.4 µg/mL-ethanolic extract 26.7 µg/mL-water-soluble fraction 78.4 µg/mL-butanol-soluble fraction | In vitro: human epithelial adenocarcinoma cervix cell line HeLa (ATCC CCL-2) | Kwon, 2019 [36] |
| Type of Virus/Disease | Type of Preparation of Propolis; Dosage and Method of Administration | Population | Effects | Type of Study | Ref. |
|---|---|---|---|---|---|
| SARS-CoV-2 | Standardized extract of green propolis (Propomax® capsules) Extract of propolis at doses of 400 mg (40 patients) and 800 mg (42 patients) per day for 7 days | Patients with COVID-19 from 18 to 80 years of age, n = 82 | Reduction in their hospitalization time; improvement in clinical symptoms of COVID-19 | Pilot randomized clinical study | Silveira et al. [48] |
| SARS-CoV-2 | Extract of Iranian green propolis 300 mg of propolis extract in tablets administered three times a day for 14 days | Patients with COVID-19 from 18 to 75 years of age, n = 40 | Improvement in clinical symptoms of COVID-19 | Randomized, double-blind, placebo-controlled clinical trial | Miryan et al. [49] |
| SARS-CoV-2 | Propolis with extract of Hyoscyamus niger Syrup at dose of 10 mL three times a day for 6 days | Patients with COVID-19 from 18 to 65 years of age, n = 50 | Decrease in dry cough, breath score, sore throat and chest pain | Randomized clinical study | Kosari et al. [50] |
| SARS-CoV-2 | Aqueous propolis extract at a dose of 2 mL (50 mg/mL) orally given 3 times a day for 7 days, or at a dose of 1 mL (64 mg/mL) with 1 mL oily perga extract (120 mg/mL) given orally 3 times daily for 7 days | Patients with COVID-19 | In progress | Randomized clinical study | NCT04916821 [51] |
| Mixed etiology: acute otitis media and/or nonstreptococcal pharyngitis | Mixture of propolis-phytosome (Propolisina®) containing 75 mg/sachet of pure propolis | Children over 2 years of age, n = 28 | Effective in patients with nonstreptococcal and viral pharyngitis caused by paramyxoviruses, rhinoviruses, adenoviruses. Propolis decreased symptoms such as sore throat, fever and pharyngeal erythema | Open-label, retrospective, controlled clinical study | Di Pierro et al. [73] |
| Mixed etiology of upper respiratory tract diseases | Herbal preparation of propolis (50 mg/mL), Echinacea (50 mg/mL) and vitamin C (10 mg/mL) at doses of 5.0 mL and 7.5 mL twice daily for 12 weeks | Children from 1 to 5 years of age | 55% reduction in the number of illness episodes, 62% reduction in the number of days with fever and decrease in the total number of days with symptoms of respiratory illness | Randomized, double-blind, placebo-controlled study | Cohen et al. [74] |
| Mixed etiology of upper respiratory tract diseases | Oral spray of propolis (M.E.D.® propolis) at a dose of 2–4 sprays (0.8–1.6 mL of propolis) three times per day (5 days) | Patients from 18 to 77 years of age, n = 58 | Remission of symptoms after three days of medication (in 83% of patients) | Randomized, double-blind, placebo-controlled clinical trial | Esposito et al. [72] |
| Mixed etiology: viral and bacterial tonsillopharyngitis | Complex product containing honey, royal jelly and propolis at a dose of 20–40 mg/kg for 10 days | Patients from 5 to 12 years of age | Effective in the treatment of infections of the upper respiratory tract | Double-blind clinical trial | Seçilmiş, 2020 [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359. https://doi.org/10.3390/molecules28010359
Ożarowski M, Karpiński TM. The Effects of Propolis on Viral Respiratory Diseases. Molecules. 2023; 28(1):359. https://doi.org/10.3390/molecules28010359
Chicago/Turabian StyleOżarowski, Marcin, and Tomasz M. Karpiński. 2023. "The Effects of Propolis on Viral Respiratory Diseases" Molecules 28, no. 1: 359. https://doi.org/10.3390/molecules28010359
APA StyleOżarowski, M., & Karpiński, T. M. (2023). The Effects of Propolis on Viral Respiratory Diseases. Molecules, 28(1), 359. https://doi.org/10.3390/molecules28010359







