Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Experimental Studies of the Antioxidant Activities of LC
2.1.1. Evaluation of LC on Neuron Viability
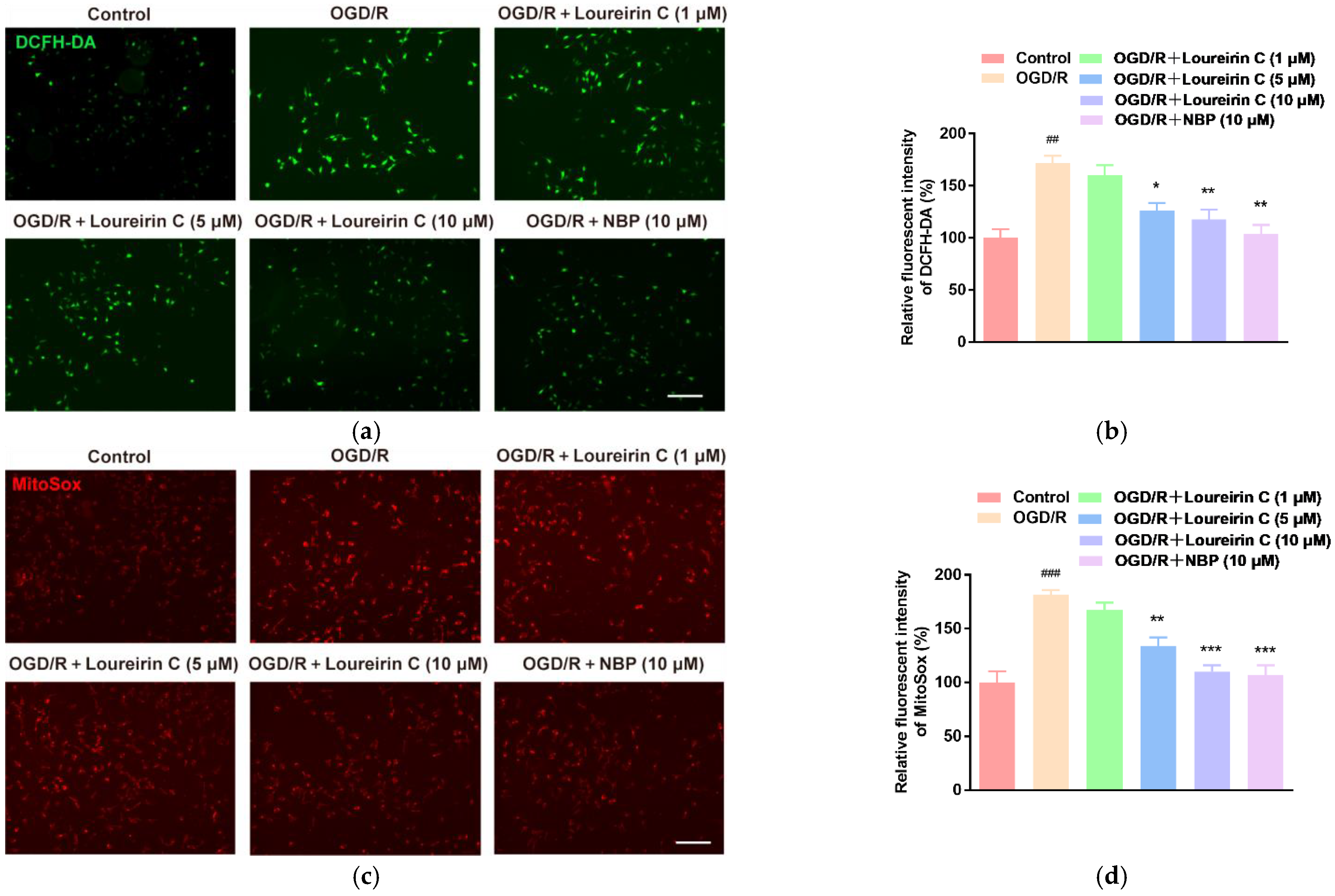
2.1.2. In Vitro Antioxidant Activities of LC
2.2. The Theoretical Studies of Free Radical Scavenging Mechanisms of LC
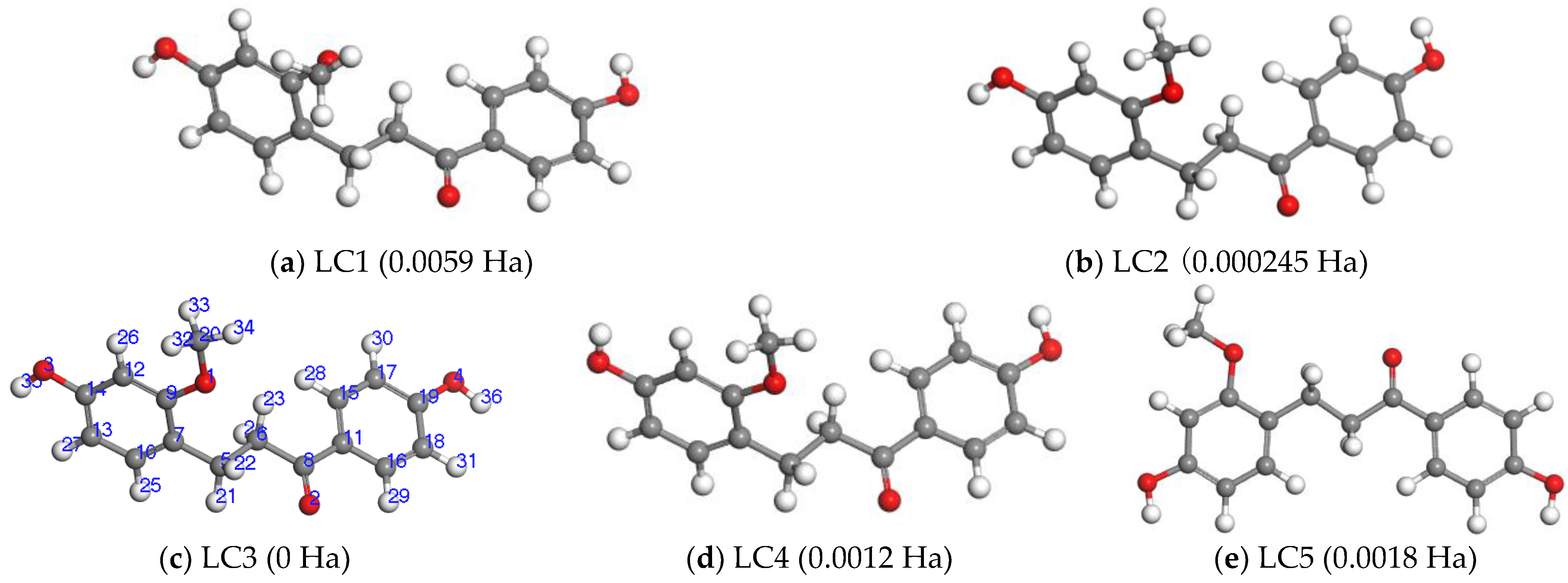
2.2.1. The Determination of the Preferred Conformation of LC
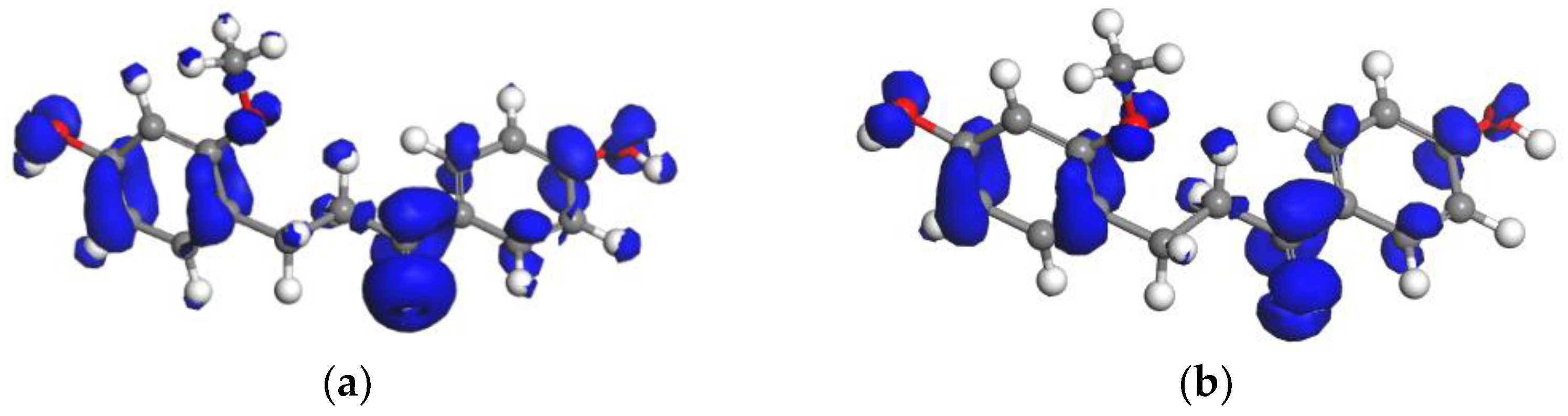
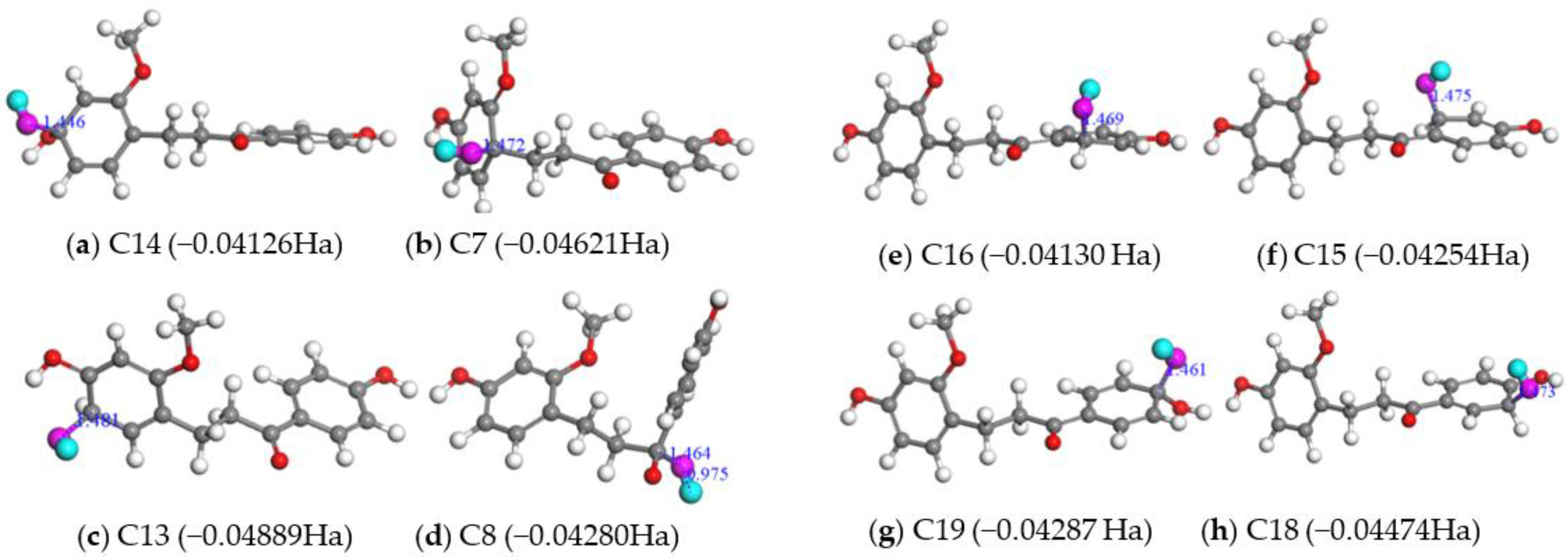
2.2.2. Fukui Function Analyses
2.2.3. The Reactivity Analyses
2.3. The Free Radical Scavenging Mechanisms of LC via Hydrogen Atom Abstraction Mechanism
2.3.1. Free Radical Scavenging Capacity via HAT
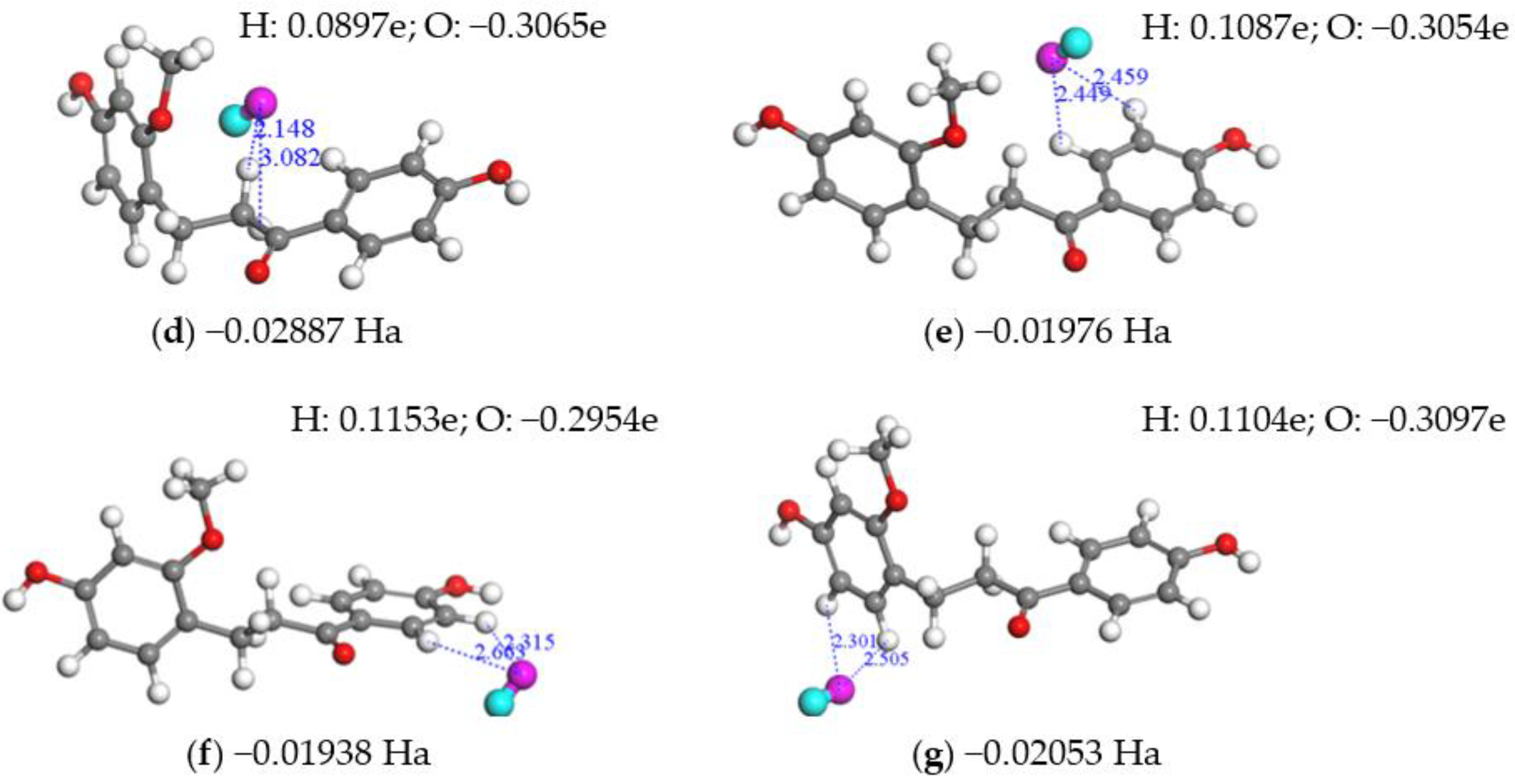
2.3.2. Free Radical Scavenging Capacity via SEPT
2.3.3. Free radical Scavenging Capacity via SPLET
2.3.4. The Antioxidant Mechanism for LC
3. Methods of Experiment and Calculation
3.1. LC Preparation
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. DCFH-DA Staining
3.5. MitoSox Staining
3.6. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.K.; Hu, S.B.; Sang, S.P.; Luo, L.S.; Yu, C.H. Age-period-cohort analysis of stroke mortality in China: Data from the global burden of disease study 2013. Stroke 2017, 48, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Fang, Y.J.; Zhang, Z.Y.; Luo, Y.J.; Zhang, A.K. Ferroptosis: An emerging therapeutic target in stroke. J. Neurochem. 2022, 160, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Dirnag, U.; Urra, X.; Planas, A.M. Neuroprotec-tion in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferropto-sis: An irondependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Y.; Wan, W.P.; Zhao, S.; Ma, Z.G. L-type calcium channels are involved in iron-induced neurotoxicity in primary cultured ventral mesencephalon neurons of rats. Neurosci. Bull. 2020, 36, 165–173. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Q.; Anrather, J.; Shi, F.D. Immune interventions in stroke. Nat. Rev. Neurol. 2015, 11, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Flemming, A. Calming inflammation to prevent stroke damage. Nat. Rev. Immunol. 2019, 19, 473. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.X.; Wu, X.M.; Ye, Y.Z.; Jian, Z.H.; Zeng, Z.L.; Gu, J. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front. Mol. Neurosci. 2020, 13, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia—Reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef]
- Matsumoto, S.; Murozono, M.; Kanazawa, M.; Nara, T.; Ozawa, T.; Watanabe, Y. Edaravone and cyclosporine a as neuroprotective agents for acute ischemic stroke. Acute Med. Surg. 2018, 5, 213–221. [Google Scholar] [CrossRef]
- Lee, X.R.; Xiang, G.L. Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin. Neurol. Neurosurg. 2018, 167, 157–161. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jun, B.; Belayev, L.; Heap, J.; Kautzmann, M.A.; Obenaus, A.; Menghani, H.; Marcell, S.J.; Khoutorova, L.; Yang, R.; et al. Elovanoids are a novel class of homeostatic lipid mediators that protect neural cell integrity upon injury. Sci. Adv. 2017, 3, e1700735. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agriindustrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Yi, T.; Chen, H.B.; Zhao, Z.Z.; Yu, Z.L.; Jiang, Z.H. Comparison of the chemical profiles and anti-platelet aggregation effects of two “Dragon’s Blood” drugs used in traditional Chinese medicine. J. Ethnopharmacol. 2011, 133, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Masaoud, M.; Ripperger, H.; Porzel, A.; Adam, G. Flavonoids of dragon’s blood from Dracaena cinnabari. Phytochemistry 1995, 38, 745–749. [Google Scholar] [CrossRef]
- Xin, N.; Li, Y.J.; Li, Y.; Dai, R.J.; Meng, W.W.; Chen, Y.; Schlappi, M.Y.; Deng, L. Dragon’s Blood extract has antithrom-botic properties, affecting platelet aggregation functions and anticoagulation activities. J. Ethnopharmacol. 2011, 135, 510–514. [Google Scholar] [CrossRef]
- Xin, N.; Yang, F.J.; Li, Y.; Li, Y.J.; Dai, R.J.; Meng, W.W.; Chen, Y.Y.; Deng, L. Dragon’s blood dropping pills have protective effects on focal cerebral ischemia rats model. Phytomedicine 2013, 21, 68–74. [Google Scholar] [CrossRef]
- Liu, F.; Dai, R.J.; Deng, Y.L.; Liu, X.J.; Lv, F. Virtual screening and activities in vitro of active components from total phenols part of Drgaon’s blood on promoting blood circulation. Trans. Beijing Inst. Technol. 2015, 35, 218–220. [Google Scholar]
- He, Q.; Li, Z.Y.; Wang, Y.T.; Hou, Y.H.; Li, L.Y.; Zhao, J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int. Immunopharmacol. 2017, 50, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.L.; Le, D.S.; Wang, C.Y.; Mao, G.H. Pterostilbene attenuates ischemic stroke by modulating miR-21-5p/PDCD4 axis in vivo and in vitro. J. Funct. Foods 2020, 75, 14275. [Google Scholar] [CrossRef]
- Mahmood, A.; Saqib, M.; Ali, M.; Abdullah, M.I.; Khalid, B. Theoretical investigation for the designing of novel antioxidants. Can. J. Chem. 2013, 91, 126–130. [Google Scholar] [CrossRef]
- Saqib, M.; Iqbal, S.; Naeem, S.; Mahmood, A. DFT for exploring the antioxidant potential of homogentisic and orsellinic acids. Pak. J. Pharm. Sci. 2013, 26, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, A.; Asemani, S.S. Theoretical investigation on antioxidant activity of vitamins and phenolic acids for designing a novel antioxidant. J. Mol. Struct. 2009, 930, 15–20. [Google Scholar] [CrossRef]
- Bian, C.; Wang, S.J.; Liu, Y.H.; Jing, X.L. Thermal stability of phenolic resin: New insights based on bond dissociation energy and reactivity of functional groups. RSC Adv. 2016, 6, 55007–55016. [Google Scholar] [CrossRef]
- Djeradi, H.; Rahmouni, A.; Cheriti, A. Antioxidant activity of flavonoids: A QSAR modeling using Fukui indices descriptors. J. Mol. Model. 2014, 20, 2476–2484. [Google Scholar] [CrossRef]
- Cao, J.S.; Ren, Q.; Chen, F.W.; Lu, T. Comparative study on the methods for predicting the reactive site of nucleophilic reaction. Sci. China Chem. 2015, 58, 1845–1852. [Google Scholar] [CrossRef]
- Guardia, J.J.; Moral, M.; Granadino-Roldan, J.M.; Garzon, A. DFT Study on the Mechanism and Kinetics of Reactions of Pterostilbene with Hydroxyl and Hydroperoxyl Radicals. Comput. Theor. Chem. 2016, 1077, 113–118. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Antonioletti, R.; Viglianti, A.; Traversi, G.; Leone, S.; Basso, E.; Cozzi, R. Scavenging of hydroxyl radical by resveratrol and related natural stilbenes after hydrogen peroxide attack on DNA. Chem. Biol. Interact. 2013, 206, 175–185. [Google Scholar] [CrossRef]
- Amic, D.; Lucic, B. Reliability of bond dissociation enthalpy calculated by the PM6 method and experimental TEAC values in antiradical QSAR of flavonoids. Bioorg. Med. Chem. 2010, 18, 28–35. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Su, G.Y.; Li, N.; Li, W.J.; Chen, G.; Chen, R.; Zhou, D.; Hou, Y. Preventive agents for neurodegenerative diseases from resin of Dracaena cochinchinensis attenuate LPS-induced microglia over-activation. J. Nat. Med. 2019, 73, 318–330. [Google Scholar] [CrossRef]
- Xu, J.K.; Liu, J.Y.; Li, Q.; Mi, Y.; Zhou, D.; Meng, Q.Q.; Chen, G.; Li, N.; Hou, Y. Pterostilbene alleviates Aβ1-42-induced cognitive dysfunction via inhibition of oxidative stress by activating Nrf2 signaling pathway. Mol. Nutr. Food Res. 2021, 65, 2000711. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Saqib, M.; Iqbal, S.; Mahmood, A.; Akram, R. Theoretical investigation for exploring the antioxidant potential of chlorogenic acid: A density functional theory study. Int. J. Food Prop. 2016, 19, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.; Lukes, V.; Ilcin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Bartmess, J.E. Thermodynamics of the electron and the proton. J. Phys. Chem. 1994, 98, 6420–6424. [Google Scholar] [CrossRef]
- Contreras, R.R.; Fuentealba, P.; Galván, M.; Pérez, P. A direct evaluation of regional Fukui functions in molecules. Chem. Phys. Lett. 1999, 304, 405–413. [Google Scholar] [CrossRef]
- Ayers, P.W.; Parr, R.G. Variational principles for describing chemical reactions: The Fukui function and chemical hardness revisited. J. Am. Chem. Soc. 2000, 122, 2010–2018. [Google Scholar] [CrossRef]


| Parameter | LC1 | LC2 | LC3 | LC4 | LC5 |
|---|---|---|---|---|---|
| O3-H35 | 0.973 | 0.973 | 0.973 (0.976) | 0.973 | 0.973 |
| O3-C14 | 1.379 | 1.381 | 1.380 (1.380) | 1.381 | 1.381 |
| O4-H36 | 0.974 | 0.974 | 0.974 (0.977) | 0.974 | 0.974 |
| O4-C19 | 1.373 | 1.373 | 1.372 (1.367) | 1.373 | 1.372 |
| ∠C14-O3-H35 | 107.898 | 107.662 | 107.696 (108.149) | 108.504 | 107.586 |
| ∠C19-O4-H36 | 108.248 | 108.233 | 108.16 (108.838) | 108.297 | 108.194 |
| ∠C9-C7-C11-C15 | −58.971 | −54.396 | −53.880 (−61.099) | −59.786 | 178.021 |
| ΔE0/(Ha) | 0.006 | 0.001 | −919.487 (−0.026) | 0.006 | 0.002 |
| Atom | O1 | O2 | O3 | O4 | C5 | C6 | C7 | C8 | C9 | C10 |
| in gas | 0.021 | 0.102 | 0.052 | 0.045 | 0.008 | 0.016 | 0.035 | 0.068 | 0.021 | 0.023 |
| in water | 0.034 | 0.094 | 0.049 | 0.032 | 0.011 | 0.018 | 0.050 | 0.076 | 0.030 | 0.029 |
| Atom | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C19 | C20 |
| in gas | 0.021 | 0.021 | 0.048 | 0.037 | 0.031 | 0.035 | 0.031 | 0.031 | 0.047 | 0.011 |
| in water | 0.028 | 0.023 | 0.057 | 0.040 | 0.035 | 0.038 | 0.026 | 0.025 | 0.041 | 0.011 |
| HAT | SEPT | SPLET | |||
|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | |
| 95.9667 | |||||
| O3-H35 | 77.3364 | 295.7091 | 342.5314 | 49.1445 | |
| O4-H36 | 79.8917 | 298.2644 | 336.9088 | 57.3223 | |
| C16-H29 | 110.5625 | 328.9352 | 397.7635 | 27.1384 | |
| C18-H31 | 107.6919 | 326.0646 | 384.6286 | 37.4027 | |
| C17-H30 | 110.2604 | 328.6331 | 391.9849 | 32.6149 | |
| C15-H28 | 106.7397 | 325.1124 | 392.4227 | 28.6564 | |
| C6-H23 | 85.4831 | 303.8558 | 362.1344 | 37.6881 | |
| C5-H21 | 84.1793 | 302.5520 | 374.1161 | 24.4026 | |
| C10-H25 | 108.5907 | 326.9634 | 391.8397 | 31.0904 | |
| C13-H27 | 110.1209 | 328.4936 | 385.6423 | 38.8180 | |
| C12-H26 | 111.1434 | 329.5161 | 384.1790 | 41.3038 | |
| HAT | SEPT | SPLET | |||
|---|---|---|---|---|---|
| BDE | IP | PDE | PA | ETE | |
| 32.6024 | |||||
| O3-H35 | 74.5010 | 40.2800 | −17.8594 | 90.7418 | |
| O4-H36 | 75.3943 | 41.17328 | −19.1905 | 92.9662 | |
| C16-H29 | 106.8953 | 72.6743 | 26.0949 | 79.1818 | |
| C18-H31 | 110.8465 | 76.6255 | 27.7661 | 81.4618 | |
| C17-H30 | 110.3096 | 76.0886 | 27.6415 | 81.0495 | |
| C15-H28 | 107.9299 | 73.7089 | 30.0925 | 76.2188 | |
| C6-H23 | 82.5412 | 48.3202 | −0.6891 | 81.6117 | |
| C5-H21 | 79.3396 | 45.1186 | 22.7078 | 55.0132 | |
| C10-H25 | 108.4034 | 74.1824 | 34.6772 | 72.1076 | |
| C13-H27 | 110.4871 | 76.2661 | 32.4688 | 76.3997 | |
| C12-H26 | 110.7761 | 76.5551 | 27.6081 | 81.5494 | |
| H• | H+ | e− | |
|---|---|---|---|
| ΔsolvH [31] | −4 | −1090 | −236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-S.; Zhang, G.-Y.; Hou, Y. Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke. Molecules 2023, 28, 380. https://doi.org/10.3390/molecules28010380
Liu Y-S, Zhang G-Y, Hou Y. Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke. Molecules. 2023; 28(1):380. https://doi.org/10.3390/molecules28010380
Chicago/Turabian StyleLiu, Ye-Shu, Guo-Ying Zhang, and Yue Hou. 2023. "Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke" Molecules 28, no. 1: 380. https://doi.org/10.3390/molecules28010380
APA StyleLiu, Y. -S., Zhang, G. -Y., & Hou, Y. (2023). Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke. Molecules, 28(1), 380. https://doi.org/10.3390/molecules28010380








