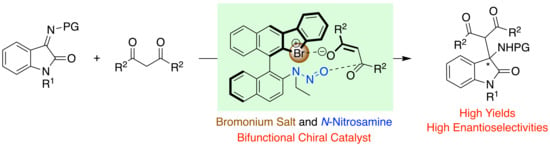
Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Catalysts
2.2. Application of Chiral Catalysts
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Chiral Bromonium Salts
3.2.1. Synthesis of 2
(R)-N-(2’-amino-3’-iodo-[1,1’-binaphthalen]-2-yl)acetamide (2)
3.2.2. Synthesis of 4a–c
(R)-6-(2-(ethyl(nitroso)amino)naphthalen-1-yl)benzo[b]naphtho [2,3-d]bromol-5-ium Tetrafluoroborate (4a)
(R)-6-(2-(ethyl(nitroso)amino)naphthalen-1-yl)benzo[b]naphtho [2,3-d]iodol-5-ium Tetrafluoroborate (4b)
(R)-6-(2-(ethyl(nitroso)amino)naphthalen-1-yl)benzo[b]naphtho [2,3-d]chlorol-5-ium Tetrafluoroborate (4c)
3.2.3. Synthesis of 4d
(R)-6-(2-(ethyl(nitroso)amino)naphthalen-1-yl)benzo[b]naphtho [2,3-d]bromol-5-ium chloride (4d)
3.3. General Procedure for Mannich Reaction of Imines with Active Methylenes
3.3.1. dimethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-2-oxoindolin-3-yl)malonate (7a)
3.3.2. dimethyl (R)-2-(3-((tert-butoxycarbonyl)amino)-1-methyl-2-oxoindolin-3-yl)malonate (7b) [48]
3.3.3. dimethyl (R)-2-(3-((tert-butoxycarbonyl)amino)-2-oxo-1-phenylindolin-3-yl)malonate (7c)
3.3.4. dimethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-4-chloro-2-oxoindolin-3-yl)malonate (7d)
3.3.5. dimethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-5-methyl-2-oxoindolin-3-yl)malonate (7e)
3.3.6. dimethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-5-chloro-2-oxoindolin-3-yl)malonate (7f)
3.3.7. dimethyl (R)-2-(1-benzyl-6-bromo-3-((tert-butoxycarbonyl)amino)-2-oxoindolin-3-yl)malonate (7g)
3.3.8. dimethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-7-chloro-2-oxoindolin-3-yl)malonate (7h)
3.3.9. dimethyl (R)-2-(1-benzyl-3-(((benzyloxy)carbonyl)amino)-2-oxoindolin-3-yl)malonate (7i)
3.3.10. dibenzyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-2-oxoindolin-3-yl)malonate (7j)
3.3.11. diethyl (R)-2-(1-benzyl-3-((tert-butoxycarbonyl)amino)-2-oxoindolin-3-yl)malonate (7k) [47]
3.3.12. tert-butyl (S)-(1-benzyl-3-(2,4-dioxopentan-3-yl)-2-oxoindolin-3-yl)carbamate (7m) [48]
3.3.13. tert-butyl (S)-(1-benzyl-3-(1,3-dioxo-1,3-diphenylpropan-2-yl)-2-oxoindolin-3-yl)carbamate (7n) [58]
3.4. Synthesis of 8
dibenzo[b,d]bromol-5-ium Tetrafluoroborate (8) [28]
3.5. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, S.; Mehmeti, K.; Veiga, A.X.; Nekoueishahraki, B.; Gräfenstein, J.; Erdélyi, M. Halogen bond asymmetry in solution. J. Am. Chem. Soc. 2018, 140, 13503–13513. [Google Scholar] [CrossRef] [PubMed]
- Robidas, R.; Legault, C.Y.; Huber, S.M. A low cost, high accuracy method for halogen bonding complexes. Phys. Chem. Chem. Phys. 2021, 23, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J. The halogen bond: Nature and applications. Phys. Sci. Rev. 2017, 2, 20170136. [Google Scholar] [CrossRef]
- Nandy, A.; Kazi, I.; Guha, S.; Sekar, G. Visible-light-driven halogen-bond-assisted direct synthesis of heteroaryl thioethers using transition-metal-free one-pot C–I bond formation/C–S cross-coupling reaction. J. Org. Chem. 2021, 86, 2570–2581. [Google Scholar] [CrossRef]
- Decato, D.A.; Sun, J.; Bollera, M.R.; Berryman, O.B. Pushing the limits of the hydrogen bond enhanced halogen bond—The case of the C–H hydrogen bond. Chem. Sci. 2022, 13, 11156–11162. [Google Scholar] [CrossRef]
- Yang, H.; Wong, M.W. Application of halogen bonding to organocatalysis: A theoretical perspective. Molecules 2020, 25, 1045. [Google Scholar] [CrossRef]
- Jónsson, H.F.; Sethio, D.; Wolf, J.; Huber, S.M.; Fiksdahl, A.; Erdelyi, M. Halogen bond activation in gold catalysis. ACS Catal. 2022, 12, 7210–7220. [Google Scholar] [CrossRef]
- Minakata, S.; Miwa, H.; Yamamoto, K.; Hirayama, A.; Okumura, S. Diastereodivergent intermolecular 1,2-diamination of unactivated alkenes enabled by iodine catalysis. J. Am. Chem. Soc. 2021, 143, 4112–4118. [Google Scholar] [CrossRef]
- Riel, A.M.S.; Decato, D.A.; Sun, J.; Berryman, O.B. Halogen bonding organocatalysis enhanced through intramolecular hydrogen bonds. Chem. Commun. 2022, 58, 1378–1381. [Google Scholar] [CrossRef]
- Kniep, F.; Jungbauer, S.H.; Zhang, Q.; Walter, S.M.; Schindler, S.; Schnapperelle, I.; Herdtweck, E.; Huber, S.M. Organocatalysis by neutral multidentate halogen-bond donors. Angew. Chem. Int. Ed. 2013, 52, 7028–7032. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nakatsuji, Y.; Li, S.; Tsuzuki, S.; Takemoto, Y. Direct N-glycofunctionalization of amides with glycosyl trichloroacetimidate by thiourea/halogen bond donor co-catalysis. Angew. Chem. Int. Ed. 2018, 57, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Loh, C.C.J. A multistage halogen bond catalyzed strain-release glycosylation unravels new hedgehog signaling inhibitors. J. Am. Chem. Soc. 2019, 141, 5381–5391. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, S.; Suzuki, T.; Yamanaka, M.; Tsutsumi, R.; Arai, T. Catalysis based on C−I⋅⋅⋅π halogen bonds: Electrophilic activation of 2-alkenylindoles by cationic halogen-bond donors for [4+2] cycloadditions. Angew. Chem. Int. Ed. 2019, 58, 10220–10224. [Google Scholar] [CrossRef]
- Sutar, R.L.; Huber, S.M. Catalysis of organic reactions through halogen bonding. ACS Catal. 2019, 9, 9622–9639. [Google Scholar] [CrossRef]
- Robidas, R.; Reinhard, D.L.; Huber, S.M.; Legault, C.Y. A quantum-chemical analysis on the lewis acidity of diarylhalonium ions. ChemPhysChem 2022. [Google Scholar] [CrossRef]
- Oishi, S.; Fujinami, T.; Masui, Y.; Suzuki, T.; Kato, M.; Ohtsuka, N.; Momiyama, N. Three-center-four-electron halogen bond enables non-metallic complex catalysis for Mukaiyama-Mannich-type reaction. iScience 2022, 25, 105220. [Google Scholar] [CrossRef]
- Zong, L.; Ban, X.; Kee, C.W.; Tan, C.-H. Catalytic Enantioselective alkylation of sulfenate anions to chiral heterocyclic sulfoxides using halogenated pentanidium salts. Angew. Chem. Int. Ed. 2014, 53, 11849–11853. [Google Scholar] [CrossRef]
- Lu, Y.H.; Nakatsuji, H.; Okumura, Y.; Yao, L.; Ishihara, K. Enantioselective halo-oxy- and halo-azacyclizations induced by chiral amidophosphate catalysts and halo-lewis acids. J. Am. Chem. Soc. 2018, 140, 6039–6043. [Google Scholar] [CrossRef]
- Kuwano, S.; Suzuki, T.; Hosaka, Y.; Arai, T. A chiral organic base catalyst with halogen-bonding-donor functionality: Asymmetric Mannich reactions of malononitrile with N-Boc aldimines and ketimines. Chem. Commun. 2018, 54, 3847–3850. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, J.; Tan, S.M.; Tan, D.; Lee, R.; Tan, C.-H. An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction. Science 2019, 363, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Yeung, Y.-Y. Halogen-bond-catalyzed addition of carbon-based nucleophiles to N-acylimminium ions. Org. Lett. 2019, 21, 5665–5669. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Engelage, E.; Stoll, R.; Huber, S.M. Bidentate chiral Bis(imidazolium)-based halogen-bond donors: Synthesis and applications in enantioselective recognition and catalysis. Angew. Chem. Int. Ed. 2020, 59, 6806–6810. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, S.; Nishida, Y.; Suzuki, T.; Arai, T. Catalytic asymmetric mannich-type reaction of malononitrile with N-Boc α-Ketiminoesters using chiral organic base catalyst with halogen bond donor functionality. Adv. Synth. Catal. 2020, 362, 1674–1678. [Google Scholar] [CrossRef]
- Ochiai, M.; Miyamoto, K.; Kaneaki, T.; Hayashi, S.; Nakanishi, W. Highly regioselective amination of unactivated alkanes by hypervalent sulfonylimino-λ3-bromane. Science 2011, 332, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Riedmüller, S.; Nachtsheim, B.J. Palladium-catalyzed synthesis of N-arylated carbazoles using anilines and cyclic diaryliodonium salts. Beilstein J. Org. Chem. 2013, 9, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Murray, J.S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. Halogen bonding in hypervalent iodine and bromine derivatives: Halonium salts. IUCrJ 2017, 4, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, M.; Dherbassy, Q.; Wencel-Delord, J. Cyclic diaryl λ3-bromanes as original aryne precursors. Angew. Chem. Int. Ed. 2021, 60, 14852–14857. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Saito, M.; Tsuji, S.; Takagi, T.; Shiro, M.; Uchiyama, M.; Ochiai, M. Benchtop-stable hypervalent Bromine(III) compounds: Versatile strategy and platform for air- and moisture-stable λ3-bromanes. J. Am. Chem. Soc. 2021, 143, 9327–9331. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef]
- Parra, A. Chiral hypervalent iodines: Active players in asymmetric synthesis. Chem. Rev. 2019, 119, 12033–12088. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S.M. Iodine(III) derivatives as halogen bonding organocatalysts. Angew. Chem. Int. Ed. 2018, 57, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Engelage, E.; Cramer, C.J.; Huber, S.M. Hypervalent Iodine(III) compounds as biaxial halogen bond donors. J. Am. Chem. Soc. 2020, 142, 8633–8640. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Ofial, A.R.; Mayr, H.; Legault, C.Y. Lewis acidity scale of diaryliodonium ions toward oxygen, nitrogen, and halogen lewis bases. J. Am. Chem. Soc. 2020, 142, 5221–5233. [Google Scholar] [CrossRef]
- Heinen, F.; Reinhard, D.L.; Engelage, E.; Huber, S.M. A bidentate Iodine(III)-based halogen-bond donor as a powerful organocatalyst. Angew. Chem. Int. Ed. 2021, 60, 5069–5073. [Google Scholar] [CrossRef]
- Robidas, R.; Reinhard, D.L.; Legault, C.Y.; Huber, S.M. Iodine(III)-based halogen bond donors: Properties and applications. Chem. Rec. 2021, 21, 1912–1927. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ishikawa, S.; Mino, T.; Sakamoto, M. Bromonium salts: Diaryl-λ3-bromanes as halogen-bonding organocatalysts. Chem. Commun. 2021, 57, 2519–2522. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Liu, Z.-J. Diaryliodonium salts as efficient Lewis acid catalysts for direct three component Mannich reactions. RSC Adv. 2015, 5, 25485–25488. [Google Scholar] [CrossRef]
- Yoshida, Y.; Mino, T.; Sakamoto, M. Chiral hypervalent Bromine(III) (bromonium salt): Hydrogen- and halogen-bonding bifunctional asymmetric catalysis by diaryl-λ3-bromanes. ACS Catal. 2021, 11, 13028–13033. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujimura, T.; Mino, T.; Sakamoto, M. Chiral binaphthyl-based iodonium salt (hypervalent Iodine(III)) as hydrogen- and halogen-bonding bifunctional catalyst: Insight into abnormal counteranion effect and asymmetric synthesis of N,S-acetals. Adv. Synth. Catal. 2022, 364, 1091–1098. [Google Scholar] [CrossRef]
- Beard, J.C.; Swager, T.M. An organic chemist’s guide to N-nitrosamines: Their structure, reactivity, and role as contaminants. J. Org. Chem. 2021, 86, 2037–2057. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pi, C.; Cui, X.; Wu, Y. Rh(III)-catalyzed tandem acylmethylation/nitroso migration/cyclization of N-nitrosoanilines with sulfoxonium ylides in one pot: Approach to 3-nitrosoindoles. Org. Lett. 2020, 22, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, N. Cationic Cobalt(III) catalyzed indole synthesis: The regioselective intermolecular cyclization of N-nitrosoanilines and alkynes. Angew. Chem. Int. Ed. 2016, 55, 4035–4039. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, Y.; Gao, Y.; Sun, C.; Xu, C.; Zhu, J. Rhodium(III)-catalyzed N-nitroso-directed C-H olefination of arenes. High-yield, versatile coupling under mild conditions. J. Am. Chem. Soc. 2013, 135, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, X.; Shao, Y.; Xie, H.; Deng, Y.; Ke, Z.; Jiang, H.; Zeng, W. Co(III)-catalyzed coupling-cyclization of aryl C–H Bonds with α-diazoketones involving wolff rearrangement. ACS Catal. 2018, 8, 1308–1312. [Google Scholar] [CrossRef]
- Yoshimura, M.; Muraoka, T.; Nakatsuka, H.; Huang, H.; Kitamura, M. Synthesis of 3,3′-diaryl-substituted 2,2′-diamino- 1,1′-binaphthyl and its derivatives. J. Org. Chem. 2010, 75, 4315–4318. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, D.; Feng, J.; Li, P.; Zhao, D.; Wang, R. Synthesis of N-alkoxycarbonyl ketimines derived from isatins and their application in enantioselective synthesis of 3-aminooxindoles. Org. Lett. 2012, 14, 2512–2515. [Google Scholar] [CrossRef]
- Rao, K.S.; Ramesh, P.; Chowhan, L.R.; Trivedi, R. Asymmetric Mannich reaction: Highly enantioselective synthesis of 3-amino-oxindoles via chiral squaramide based H-bond donor catalysis. RSC Adv. 2016, 6, 84242–84247. [Google Scholar] [CrossRef]
- Il’in, M.V.; Sysoeva, A.A.; Novikov, A.S.; Bolotin, D.S. Diaryliodoniums as hybrid hydrogen- and halogen-bond-donating organocatalysts for the Groebke-Blackburn-Bienaymé reaction. J. Org. Chem. 2022, 87, 4569–4579. [Google Scholar] [CrossRef]
- Available online: http://supramolecular.org (accessed on 26 December 2022).
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, 16th ed.; revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, 16th ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Hu, F.-L.; Wei, Y.; Shi, M.; Pindi, S.; Li, G. Asymmetric catalytic aza-Morita–Baylis–Hillman reaction for the synthesis of 3-substituted-3-aminooxindoles with chiral quaternary carbon centers. Org. Biomol. Chem. 2013, 11, 1921–1924. [Google Scholar] [CrossRef] [PubMed]
- Seayad, A.M.; Ramalingam, B.; Yoshinaga, K.; Nagata, T.; Chai, C.L.L. Highly enantioselective titanium-catalyzed cyanation of imines at room temperature. Org. Lett. 2010, 12, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Jing, C.; Zhai, C.; Hu, W. A novel method for synthesizing N-alkoxycarbonyl aryl α-imino esters and their applications in enantioselective transformations. Adv. Synth. Catal. 2012, 354, 301–307. [Google Scholar] [CrossRef]
- Da Silva, C.D.G.; Katla, R.; dos Santos, B.F.; Tavares, J.M.C., Jr.; Albuquerque, T.B.; Kupfer, V.L.; Rinaldi, A.W.; Domingues, N.L.C. Cobalt used as a novel and reusable catalyst: A new and one-pot synthesis of isatin-derived N,S-acetals using substituted isatins and thiols. Synthesis 2019, 51, 4014–4022. [Google Scholar] [CrossRef]
- Rodríguez-Ferrer, P.; Sanz-Novo, M.; Maestro, A.; Andrés, J.M.; Pedrosa, R. Synthesis of enantioenriched 3-amino-3-substituted oxindoles by stereoselective mannich reaction catalyzed by supported bifunctional thioureas. Adv. Synth. Catal. 2019, 361, 3645–3655. [Google Scholar] [CrossRef]









 | |||||
|---|---|---|---|---|---|
| Entry | Solvent | Temp. (°C) | X (mol%) | Yield (%) a | Ee (%) |
| 1 | toluene | −20 | 2.5 | >99 | 68 |
| 2 | THF | −20 | 2.5 | 90 | 62 |
| 3 | CH2Cl2 | −20 | 2.5 | 97 | 43 |
| 4 | Et2O | −20 | 2.5 | 92 | 2 |
| 5 | CH3CN | −20 | 2.5 | 96 | Rac. |
| 6 | toluene | −10 | 2.5 | >99 | 36 |
| 7 | toluene | −30 | 2.5 | 91 | 68 |
| 8 | toluene | −20 | 1.0 | 92 | Rac. |
| 9 | toluene | −20 | 5.0 | 91 | 59 |
| 10 | toluene | −20 | - | 88 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, Y.; Ao, T.; Mino, T.; Sakamoto, M. Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst. Molecules 2023, 28, 384. https://doi.org/10.3390/molecules28010384
Yoshida Y, Ao T, Mino T, Sakamoto M. Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst. Molecules. 2023; 28(1):384. https://doi.org/10.3390/molecules28010384
Chicago/Turabian StyleYoshida, Yasushi, Tatsuya Ao, Takashi Mino, and Masami Sakamoto. 2023. "Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst" Molecules 28, no. 1: 384. https://doi.org/10.3390/molecules28010384
APA StyleYoshida, Y., Ao, T., Mino, T., & Sakamoto, M. (2023). Chiral Bromonium Salt (Hypervalent Bromine(III)) with N-Nitrosamine as a Halogen-Bonding Bifunctional Catalyst. Molecules, 28(1), 384. https://doi.org/10.3390/molecules28010384