Hepatoprotective Effect of a New FFAR1 Agonist—N-Alkylated Isobornylamine
Abstract
:1. Introduction
2. Results
2.1. Biochemical Blood Assay
2.2. Histological Examination
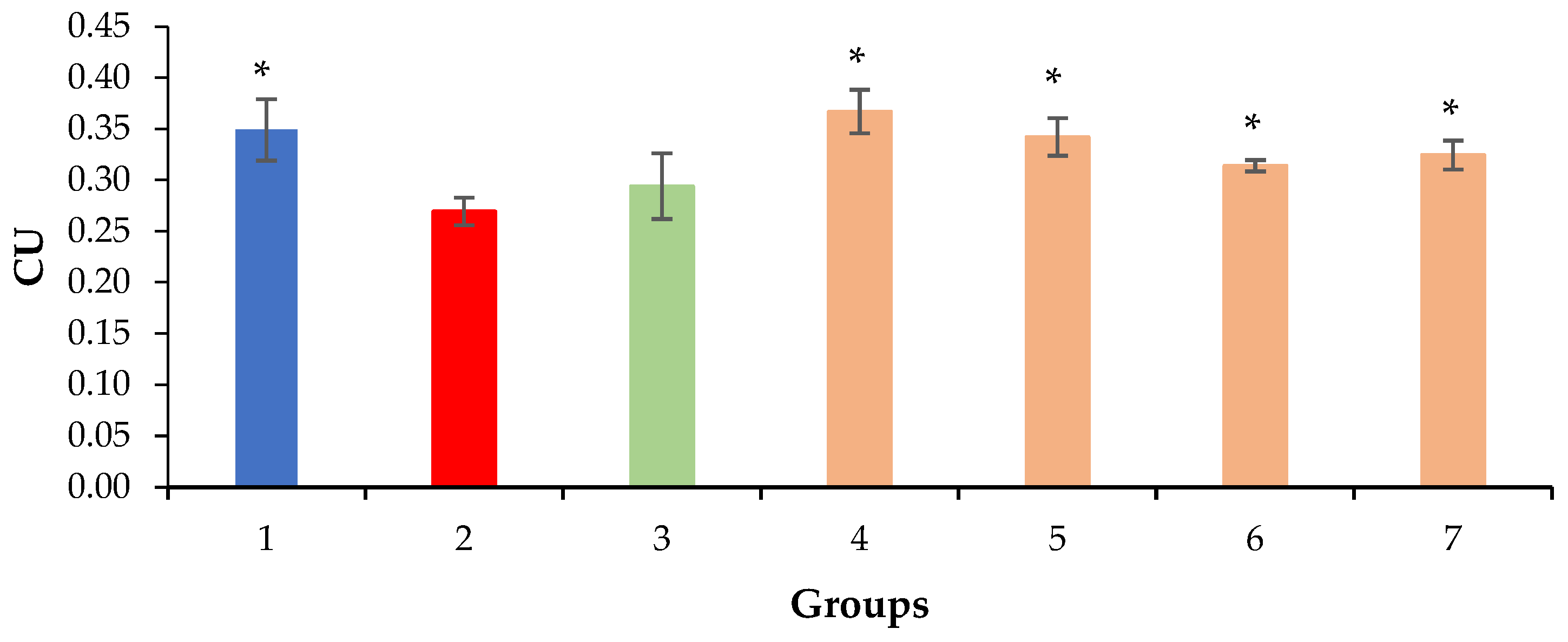
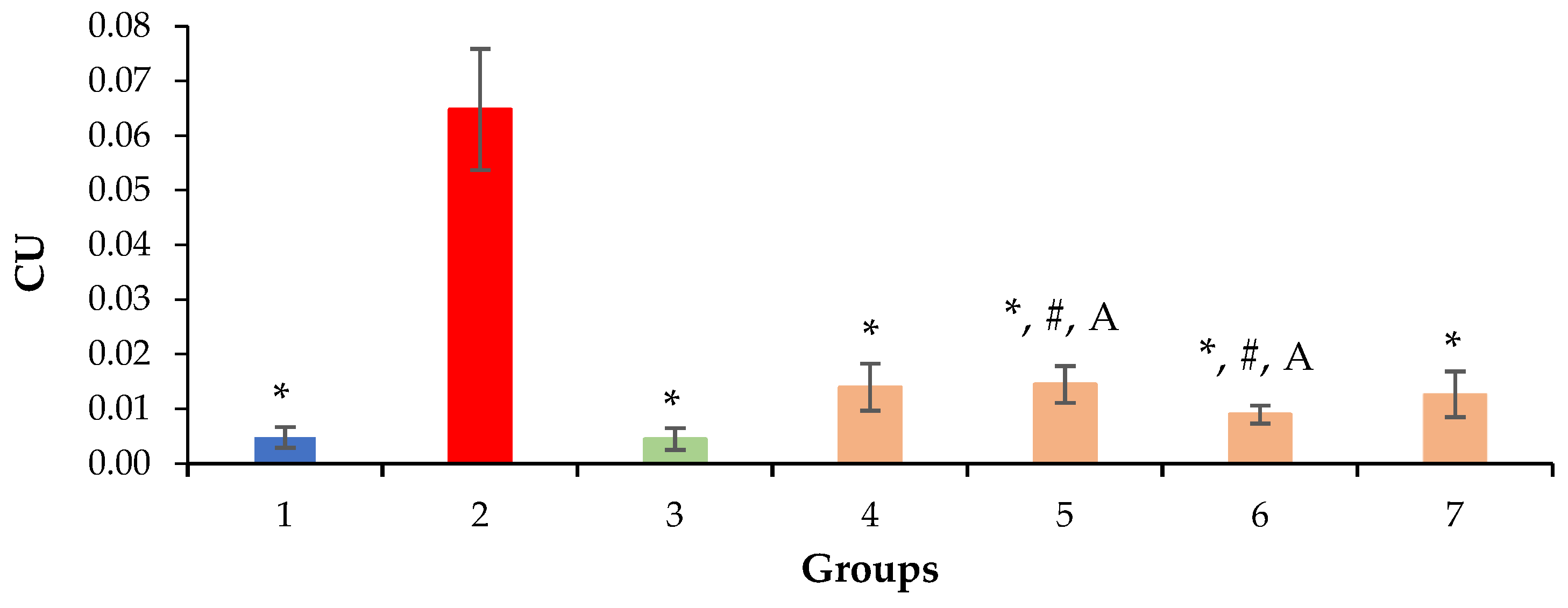
2.3. Relative Density of Nuclei and Cytoplasm of Hepatocytes, Sinusoids and Liver Necrosis
2.4. Acute Toxicity Study
2.5. In Vitro Assay
3. Discussion
4. Materials and Methods
4.1. Investigated Compound
4.2. Animals
4.3. Carbon Tetrachloride (CCl4)-Induced Liver Hepatotoxicity
4.4. Biochemical Assays
4.5. Histological Liver Examination
4.6. Morphometric Liver Analysis
4.7. Acute Toxicity Evaluation
4.8. In Vitro Experiments
4.8.1. Cell Culture
4.8.2. The Design of the Experiment on HepG2 Cells
4.8.3. Glucose Consumption and Lactate Release Assay
4.8.4. MTT Assay for Cell Viability
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed Saleem, T.S.; Madhusudhana Chetty, S.; Ramkanth, S.; Rajan, V.S.T.; Mahesh Kumar, K.; Gauthaman, K. Hepatoprotective Herbs—A Review. Int. J. Res. Pharm. Sci. 2010, 1, 1–5. [Google Scholar]
- Verma, V.K.; Sarwa, K.K.; Kumar, A.; Zaman, M.K. Comparison of Hepatoprotective Activity of Swertia Chirayita and Andrographis Paniculata Plant of North–East India against CCl4 Induced Hepatotoxic Rats. J. Pharm. Res. 2013, 7, 647–653. [Google Scholar] [CrossRef]
- Ray, G. Management of Liver Diseases: Current Perspectives. World J. Gastroenterol. 2022, 28, 5818–5826. [Google Scholar] [CrossRef]
- Maqbool, M.; Amin Dar, M.; Rasool, S.; Bashir, R. Hepatotoxicity and Hepatoprotective Agents: A Mini Review. PharmaTutor 2019, 7, 34–40. [Google Scholar] [CrossRef]
- Madrigal-Santillan, E.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Sumaya-Martinez, M.T.; Gutierrez-Salinas, J.; Bautista, M.; Morales-Gonzalez, A.; Garcia-Luna, Y.; Gonzalez-Rubio, M.; Aguilar-Faisal, J.L.; et al. Review of Natural Products with Hepatoprotective Effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.Y. G Protein-Coupled Receptors as Potential Targets for Nonalcoholic Fatty Liver Disease Treatment. World J. Gastroenterol. 2021, 27, 677–691. [Google Scholar] [CrossRef]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq Signaling Invitro Is Associated with Robust Incretin Secretagogue Action Ex Vivo and in Vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Kurtz, R.; Anderman, M.F.; Shepard, B.D. GPCRs Get Fatty: The Role of G Protein-Coupled Receptor Signaling in the Development and Progression of Nonalcoholic Fatty Liver Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G304–G318. [Google Scholar] [CrossRef]
- Kimura, T.; Pydi, S.P.; Pham, J.; Tanaka, N. Metabolic Functions of g Protein-Coupled Receptors in Hepatocytes—Potential Applications for Diabetes and Nafld. Biomolecules 2020, 10, 1445. [Google Scholar] [CrossRef]
- Ookawara, M.; Matsuda, K.; Watanabe, M.; Moritoh, Y. The GPR40 Full Agonist SCO-267 Improves Liver Parameters in a Mouse Model of Nonalcoholic Fatty Liver Disease without Affecting Glucose or Body Weight. J. Pharmacol. Exp. Ther. 2020, 375, 21–27. [Google Scholar] [CrossRef]
- Gagnon, L.; Leduc, M.; Thibodeau, J.F.; Zhang, M.Z.; Grouix, B.; Sarra-Bournet, F.; Gagnon, W.; Hince, K.; Tremblay, M.; Geerts, L.; et al. A Newly Discovered Antifibrotic Pathway Regulated by Two Fatty Acid Receptors: GPR40 and GPR84. Am. J. Pathol. 2018, 188, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- On, S.; Kim, H.Y.; Kim, H.S.; Park, J.; Kang, K.W. Involvement of G-Protein-Coupled Receptor 40 in the Inhibitory Effects of Docosahexaenoic Acid on SREBP1-Mediated Lipogenic Enzyme Expression in Primary Hepatocytes. Int. J. Mol. Sci. 2019, 20, 2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuranov, S.O.; Luzina, O.A.; Onopchenko, O.; Pishel, I.; Zozulya, S.; Gureev, M.; Salakhutdinov, N.F.; Krasavin, M. Exploring Bulky Natural and Natural-like Periphery in the Design of p-(Benzyloxy) Phenylpropionic Acid Agonists of Free Fatty Acid Receptor 1 (GPR40). Bioorg. Chem. 2020, 99, 103830. [Google Scholar] [CrossRef] [PubMed]
- Kuranov, S.; Luzina, O.; Khvostov, M.; Baev, D.; Kuznetsova, D.; Zhukova, N.; Vassiliev, P.; Kochetkov, A.; Tolstikova, T.; Salakhutdinov, N. Bornyl Derivatives of P-(Benzyloxy)Phenylpropionic Acid: In Vivo Evaluation of Antidiabetic Activity. Pharmaceuticals 2020, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hao, H.; Kang, A.; Liang, Y.; Xie, T.; Sun, S.; Dai, C.; Zheng, X.; Xie, L.; Li, J.; et al. Integral Pharmacokinetics of Multiple Lignan Components in Normal, CCl4-Induced Hepatic Injury and Hepatoprotective Agents Pretreated Rats and Correlations with Hepatic Injury Biomarkers. J. Ethnopharmacol. 2010, 131, 290–299. [Google Scholar] [CrossRef]
- Delgado-Montemayor, C.; Cordero-Perez, P.; Salazar-Aranda, R.; Waksman-Minsky, N. Models of Hepatoprotective Activity Assessment. Med. Univ. 2015, 17, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Mohi-ud-din, R.; Mir, R.H.; Sawhney, G.; Dar, M.A.; Bhat, Z.A. Possible Pathways of Hepatotoxicity Caused by Chemical Agents. Curr. Drug Metab. 2019, 20, 867–879. [Google Scholar] [CrossRef]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and Mechanism of Action of Haloalkanes: Carbon Tetrachloride as a Toxicological Model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef]
- Abdelaziz, D.H.A.; Ali, S.A. The Protective Effect of Phoenix Dactylifera L. Seeds against CCl4-Induced Hepatotoxicity in Rats. J. Ethnopharmacol. 2014, 155, 736–743. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, D.M.; Ma, S.B.; Zhao, X.; Wang, S.; Wei, G.; Wang, X.F.; Wen, A.D.; Wang, J.W. Protective Effect of Wedelolactone against CCl4-Induced Acute Liver Injury in Mice. Int. Immunopharmacol. 2016, 34, 44–52. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Matsuoka, M.; French, S.W. Experimental Models of Hepatic Fibrosis: A Review. Semin Liver Dis. 1990, 10, 56–65. [Google Scholar] [CrossRef]
- Yannaki, E.; Athanasiou, E.; Xagorari, A.; Constantinou, V.; Batsis, I.; Kaloyannidis, P.; Proya, E.; Anagnostopoulos, A.; Fassas, A. G-CSF-Primed Hematopoietic Stem Cells or G-CSF per Se Accelerate Recovery and Improve Survival after Liver Injury, Predominantly by Promoting Endogenous Repair Programs. Exp. Hematol. 2005, 33, 108–119. [Google Scholar] [CrossRef]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant Properties of Proanthocyanidins Attenuate Carbon Tetrachloride (CCl4)-Induced Steatosis and Liver Injury in Rats via CYP2E1 Regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Wang, X.; Chung, T.Y.; Ye, W.; Hodge, L.; Zhang, L.; Chng, K.; Xiao, Y.F.; Wang, Y.J. Carbon Tetrachloride (CCl4) Accelerated Development of Non-Alcoholic Fatty Liver Disease (NAFLD)/Steatohepatitis (NASH) in MS-NASH Mice Fed Western Diet Supplemented with Fructose (WDF). BMC Gastroenterol. 2020, 20, 339. [Google Scholar] [CrossRef]
- Sturgill, M.G.; Lambert, G.H. Xenobiotic-Induced Hepatotoxicity: Mechanisms of Liver Injury and Methods of Monitoring Hepatic Function. Clin. Chem. 1997, 43, 1512–1526. [Google Scholar] [CrossRef] [Green Version]
- Osadebe, P.O.; Okoye, F.B.C.; Uzor, P.F.; Nnamani, N.R.; Adiele, I.E.; Obiano, N.C. Phytochemical Analysis, Hepatoprotective and Antioxidant Activity of Alchornea Cordifolia Methanol Leaf Extract on Carbon Tetrachloride-Induced Hepatic Damage in Rats. Asian Pac J. Trop Med. 2012, 5, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, C.Z.; Ye, J.Z.; Li, H.T. Hepatoprotective Effects of Polyprenols from Ginkgo Biloba L. Leaves on CCl4-Induced Hepatotoxicity in Rats. Fitoterapia 2011, 82, 834–840. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.; González-Rubio, M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective Effect of Silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef]
- Staels, B.; Rubenstrunk, A.; Noel, B.; Rigou, G.; Delataille, P.; Millatt, L.J.; Baron, M.; Lucas, A.; Tailleux, A.; Hum, D.W.; et al. Hepatoprotective Effects of the Dual Peroxisome Proliferator-Activated Receptor Alpha/Delta Agonist, GFT505, in Rodent Models of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Hepatology 2013, 58, 1941–1952. [Google Scholar] [CrossRef]
- Bril, F.; Cusi, K. Nonalcoholic Fatty Liver Disease: The New Complication of Type 2 Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2016, 45, 765–781. [Google Scholar] [CrossRef]
- Maier, M.T.; Vilhelmsson, A.; Louie, S.M.; Vagena, E.; Nomura, D.K.; Koliwad, S.K.; Xu, A.W. Regulation of Hepatic Lipid Accumulation and Distribution by Agouti-Related Protein in Male Mice. Endocrinology 2018, 159, 2408–2420. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine Improves Glucose and Lipid Metabolism in HepG2 Cells Through AMPKα1 Activation. Front. Pharmacol. 2020, 11, 647. [Google Scholar] [CrossRef]
- Sahgal, G.; Ramanathan, S.; Sasidharan, S.; Mordi, M.N.; Ismail, S.; Mansor, S.M. Brine Shrimp Lethality and Acute Oral Toxicity Studies on Swietenia Mahagoni (Linn.) Jacq. Seed Methanolic Extract. Pharmacogn. Res. 2010, 2, 215–220. [Google Scholar] [CrossRef]
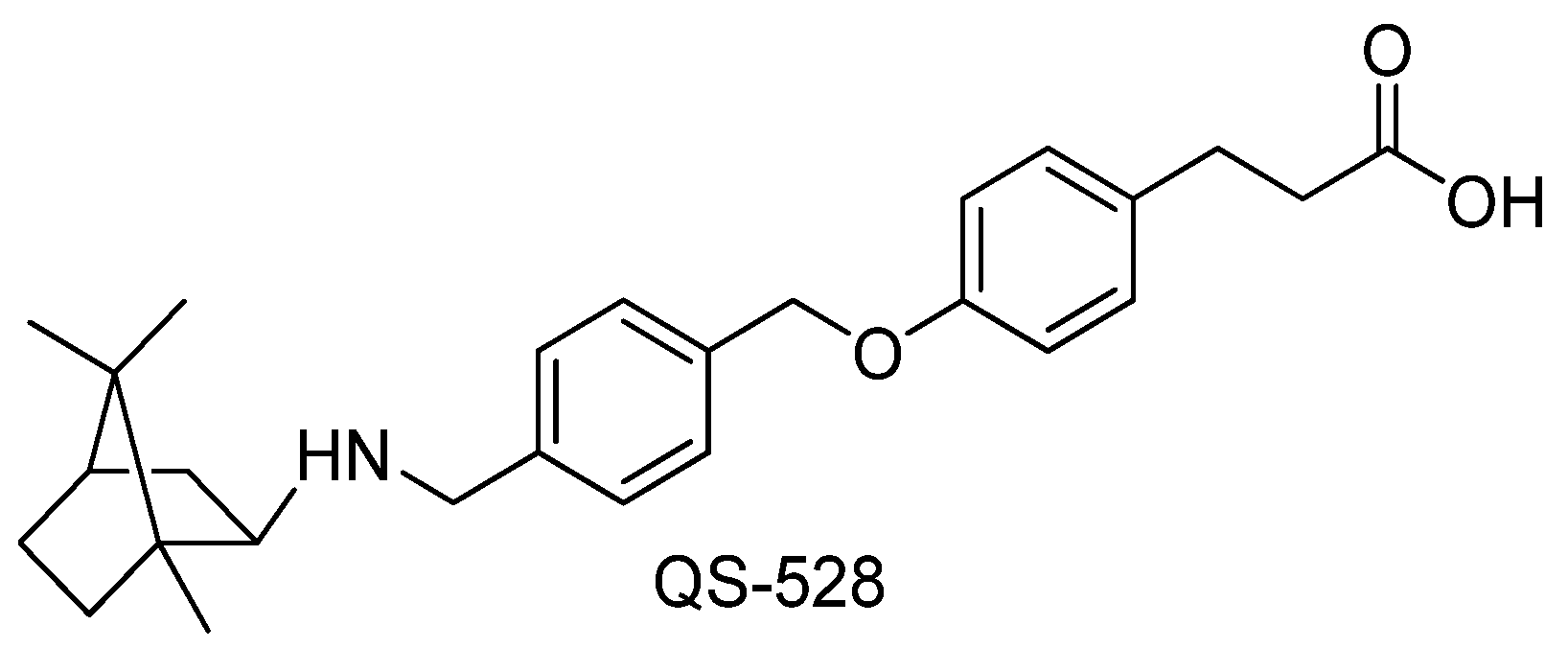
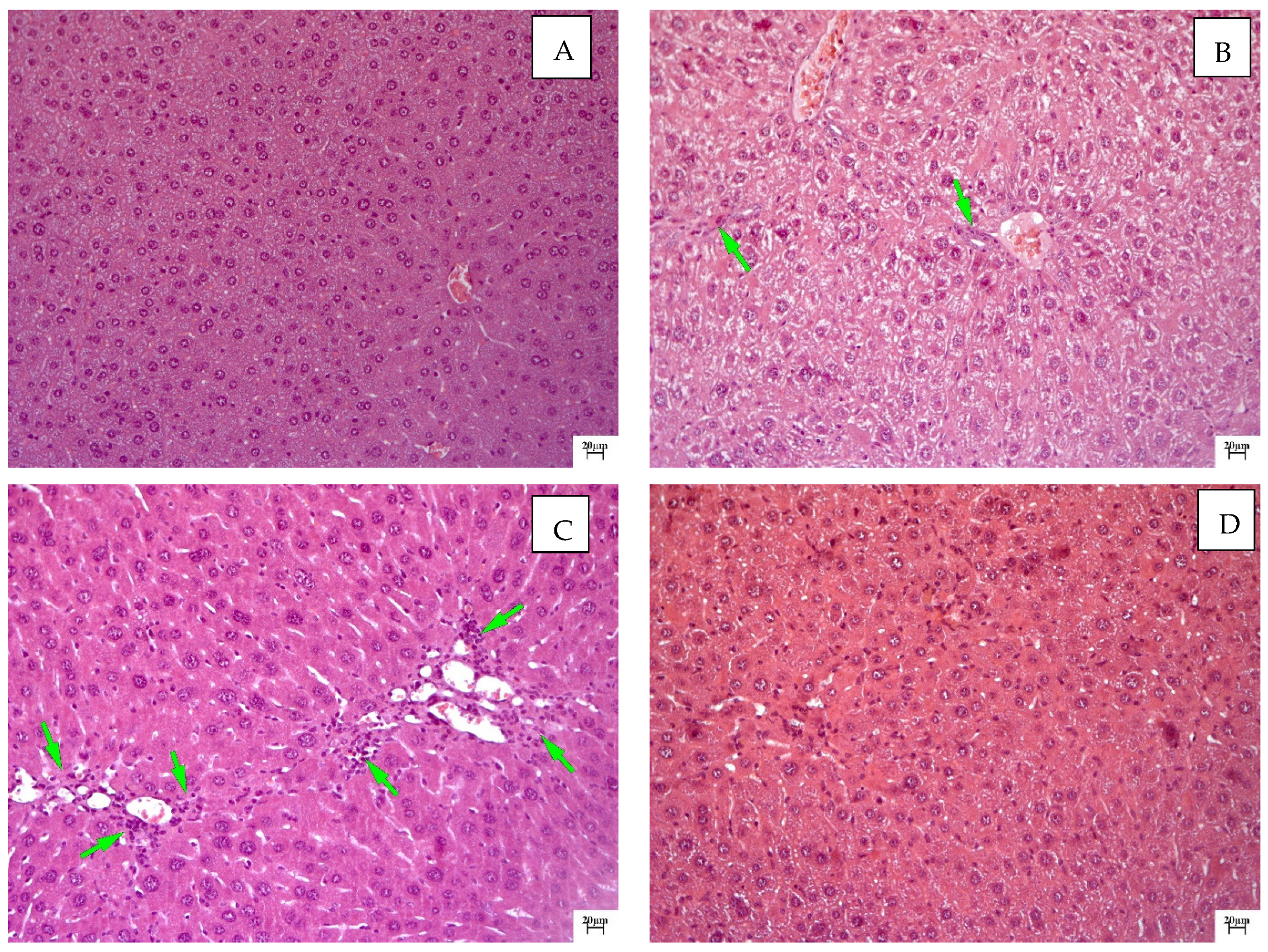

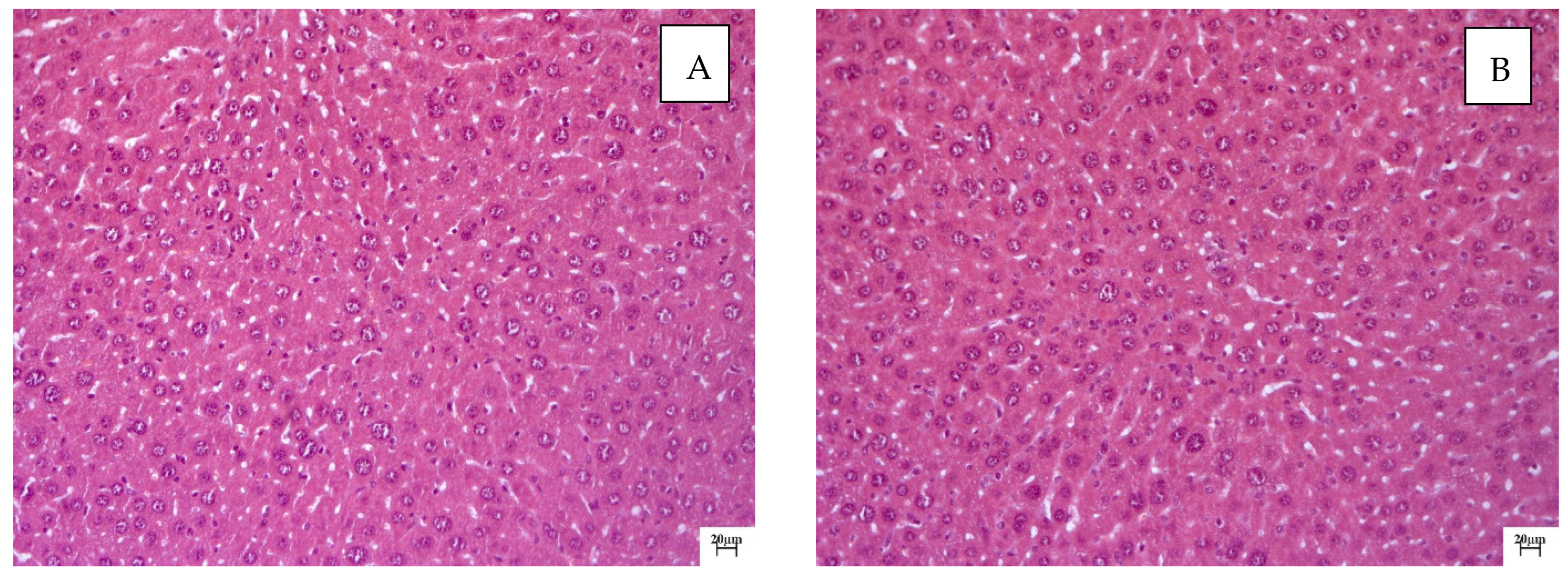
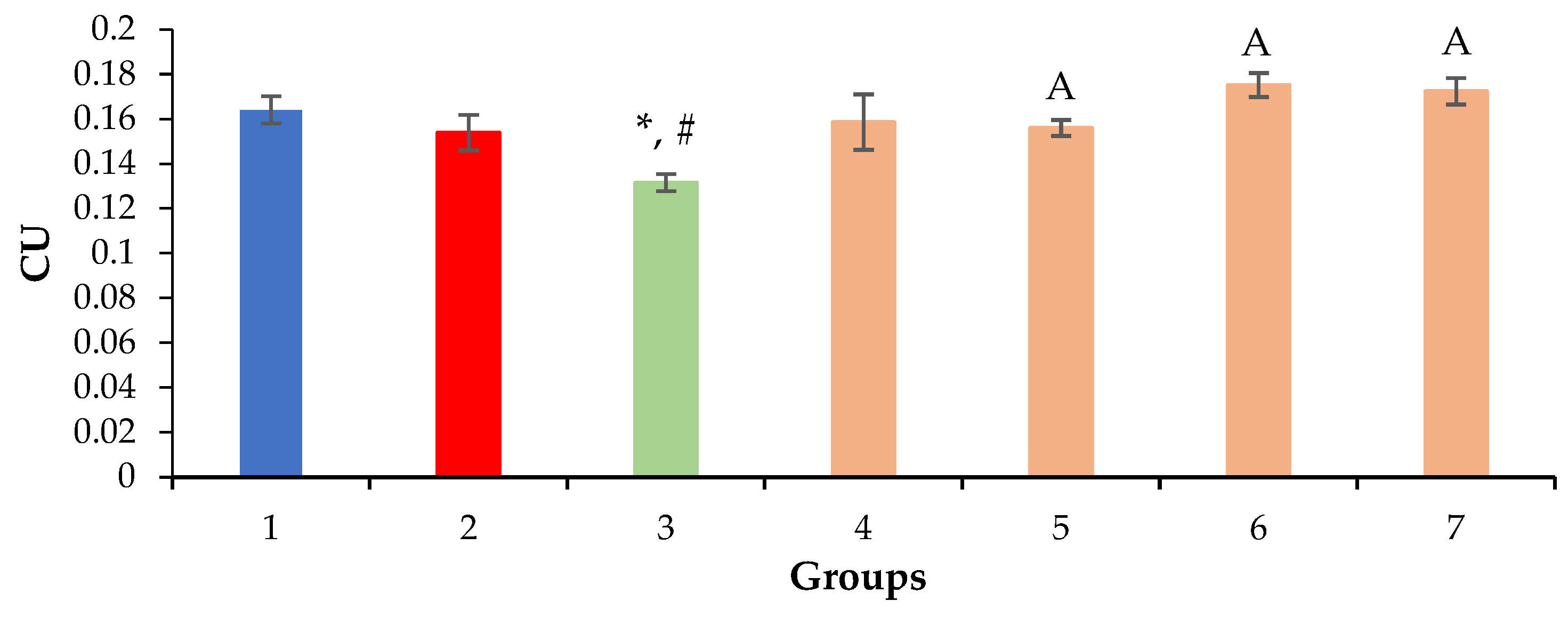
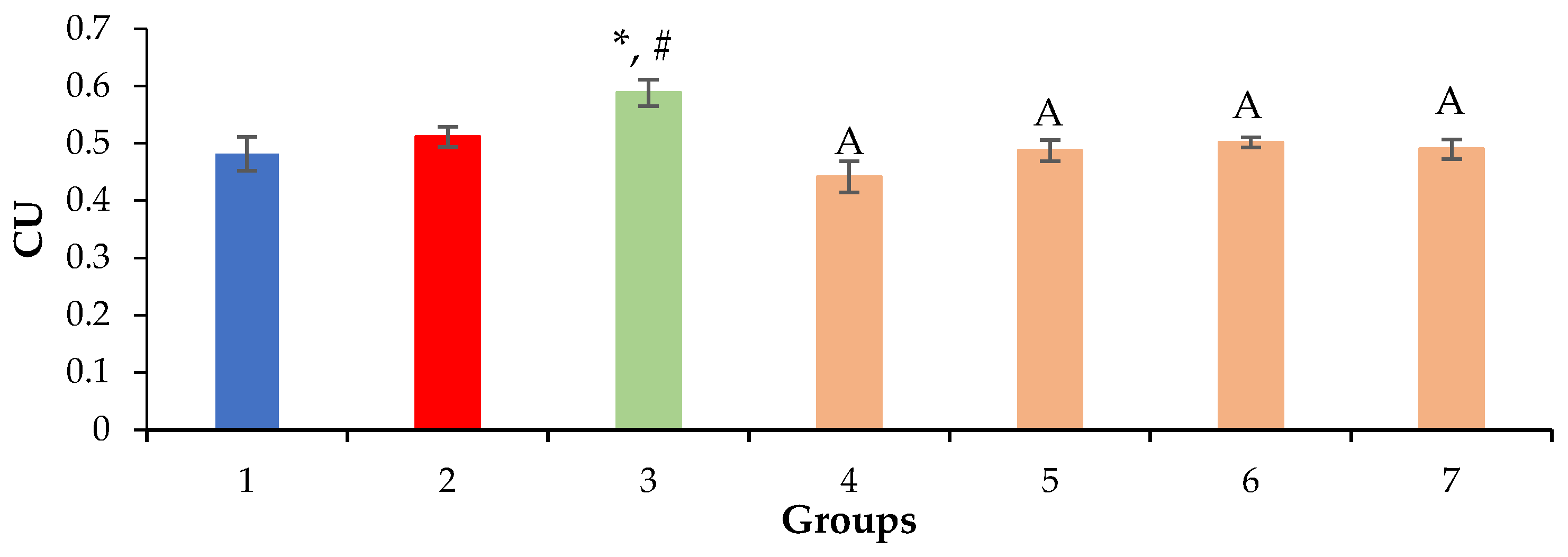

| Group | ALT, U/L | AST, U/L | ALKP, U/L | TP, g/dL |
|---|---|---|---|---|
| Intact control | 15.29 ± 1.28 * | 41.49 ± 3.26 * | 40.96 ± 5.63 * | 85.30 ± 0.84 |
| Negative control | 26.5 ± 1.68 | 52.06 ± 2.56 | 68.01 ± 5.03 | 85.76 ± 0.74 |
| Silymarin 100 mg/kg | 20.75 ± 1.35 * | 39.59 ± 2.71 * | 43.08 ± 3.57 * | 83.7 ± 1.00 |
| QS-528 60 mg/kg | 21.07 ± 1.95 * | 44.48 ± 3.53 | 82.71 ± 14.25 | 84.23 ± 1.69 |
| QS-528 90 mg/kg | 25.82 ± 1.72 | 52.93 ± 4.41 | 75.62 ± 4.73 | 85.05 ± 1.43 |
| QS-528 120 mg/kg | 20.97 ± 1.39 * | 40.83 ± 3.34 * | 60.44 ± 6.02 | 84.98 ± 0.75 |
| QS-528 150 mg/kg | 14.95 ± 1.36 *, # | 45.78 ± 6.93 | 54.35 ± 6.33 | 83.19 ± 1.18 |
| Group | Glucose Consumption, % | Lactate Release, % | Cell Viability, % |
|---|---|---|---|
| Control | 24.98 ± 1.68 | 15.03 ± 0.59 | 100.00 ± 3.07 |
| Metf. 1 mM | 48.52 ± 2.87 *** | 30.03 ± 3.04 *** | 101.51 ± 1.90 |
| Metf. 2.5 mM | 62.32 ± 0.72 *** | 46.08 ± 3.20 *** | 91.11 ± 3.40 |
| QS-528 2.5 μM | 39.80 ± 2.75 ** | 25.58 ± 1.35 *** | 146.31 ± 6.16 *** |
| QS-528 5 μM | 37.79 ± 4.46 ** | 27.65 ± 1.15 *** | 135.77 ± 4.19 *** |
| QS-528 10 μM | 44.91 ± 3.00 *** | 42.62 ± 1.20 *** | 114.54 ± 3.95 |
| QS-528 25 μM | 85.52 ± 1.99 *** | 61.61 ± 0.49 *** | 109.57 ± 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pon`kina, D.; Kuranov, S.; Khvostov, M.; Zhukova, N.; Meshkova, Y.; Marenina, M.; Luzina, O.; Tolstikova, T.; Salakhutdinov, N. Hepatoprotective Effect of a New FFAR1 Agonist—N-Alkylated Isobornylamine. Molecules 2023, 28, 396. https://doi.org/10.3390/molecules28010396
Pon`kina D, Kuranov S, Khvostov M, Zhukova N, Meshkova Y, Marenina M, Luzina O, Tolstikova T, Salakhutdinov N. Hepatoprotective Effect of a New FFAR1 Agonist—N-Alkylated Isobornylamine. Molecules. 2023; 28(1):396. https://doi.org/10.3390/molecules28010396
Chicago/Turabian StylePon`kina, Darya, Sergey Kuranov, Mikhail Khvostov, Nataliya Zhukova, Yulia Meshkova, Mariya Marenina, Olga Luzina, Tatyana Tolstikova, and Nariman Salakhutdinov. 2023. "Hepatoprotective Effect of a New FFAR1 Agonist—N-Alkylated Isobornylamine" Molecules 28, no. 1: 396. https://doi.org/10.3390/molecules28010396







