Reductive Deuteration of Acyl Chlorides for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O
Abstract
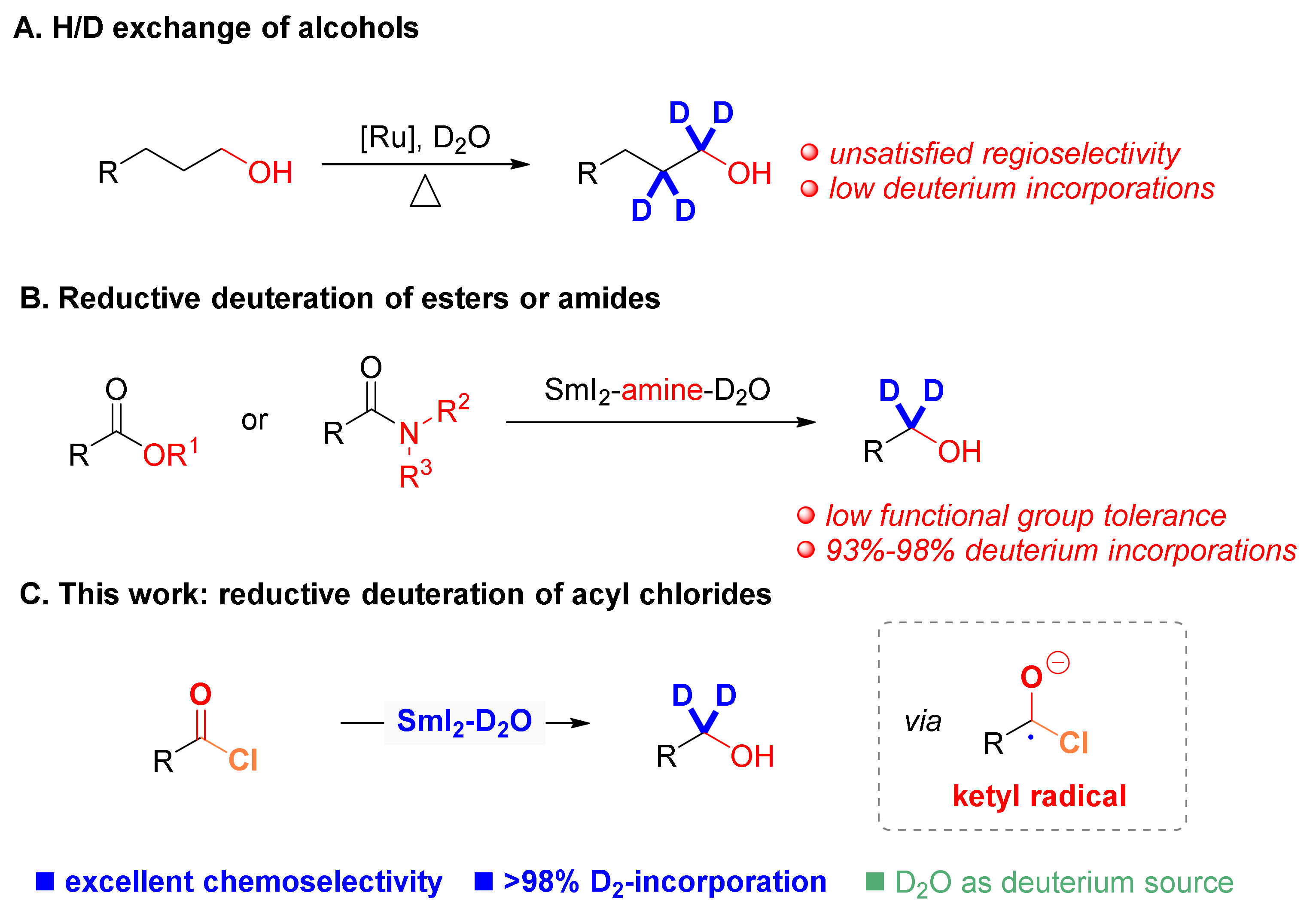
:1. Introduction
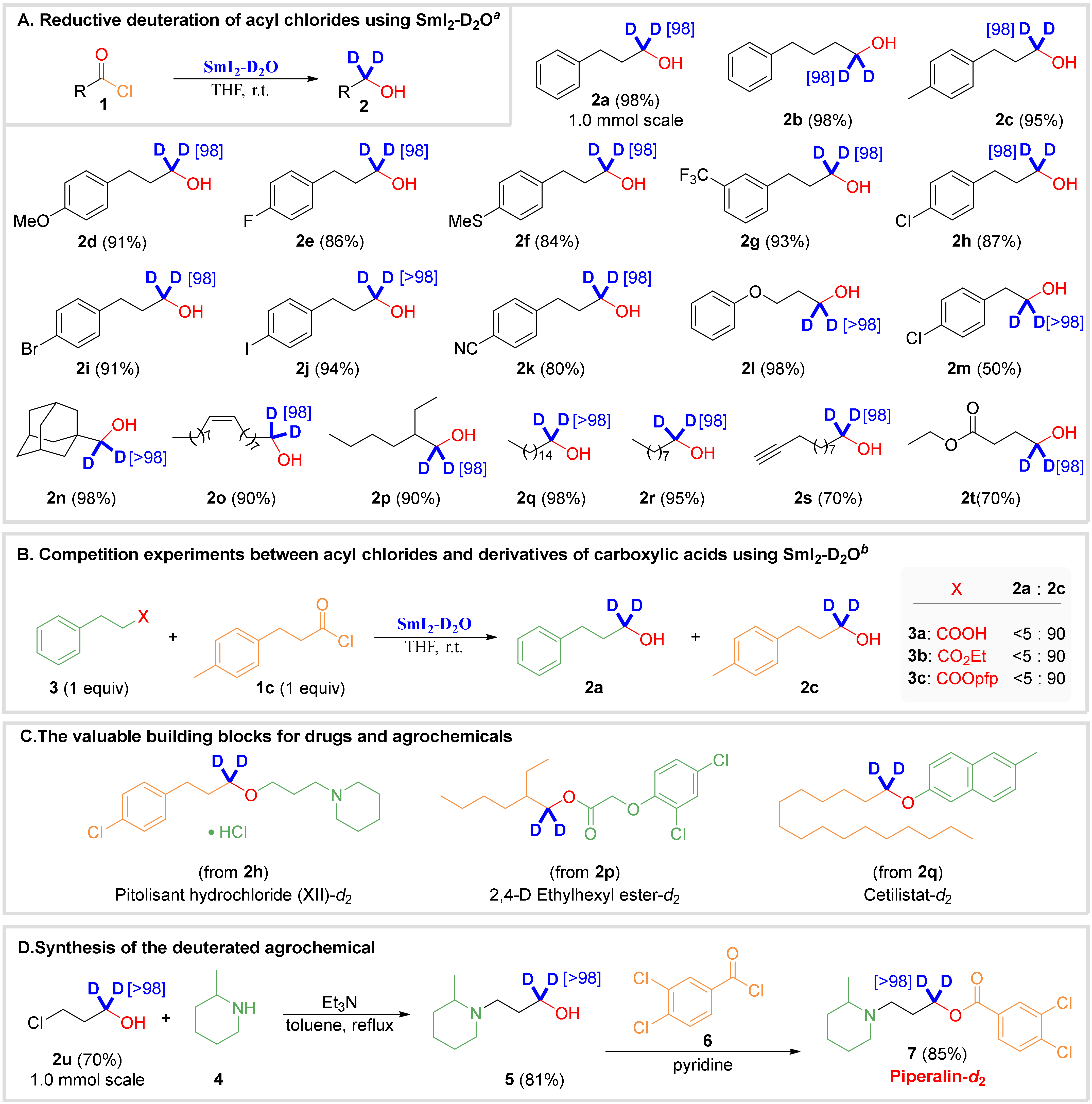
2. Results
3. Materials and Methods
3.1. General Information
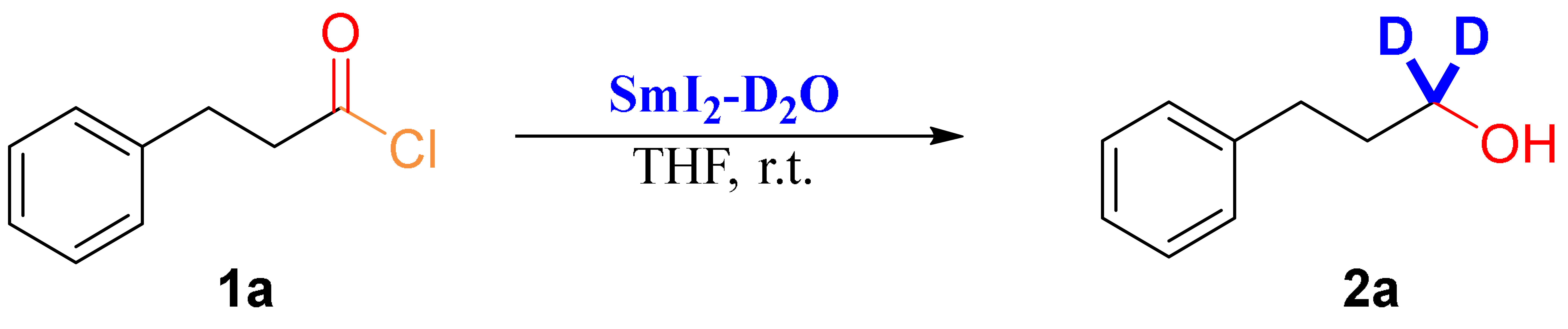
3.2. Optimization Studies (Table 1)
3.3. General Procedure for the Reductive Deuteration of Acyl Chlorides by SmI2-D2O (Scheme 2A)
3.4. Competition Experiments (Scheme 2B)
3.5. Synthesis of Piperalin-d2 (Scheme 2D)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzrodt, J.; Derdau, V. Pd- and Pt-Catalyzed H/D Exchange Methods and Their Application for Internal MS Standard Preparation from a Sanofi-Aventis Perspective. J. Label. Compd. Radiopharm. 2010, 53, 674–685. [Google Scholar] [CrossRef]
- Yang, J. Applications in Organic Chemistry. In Deuterium; Elsevier: Amsterdam, The Netherlands, 2016; pp. 31–97. ISBN 9780128110409. [Google Scholar]
- Schütze, A.; Morales-Agudelo, P.; Vidal, M.; Calafat, A.M.; Ospina, M. Quantification of Glyphosate and Other Organophosphorus Compounds in Human Urine via Ion Chromatography Isotope Dilution Tandem Mass Spectrometry. Chemosphere 2021, 274, 129427. [Google Scholar] [CrossRef] [PubMed]
- Nardin, T.; Barnaba, C.; Abballe, F.; Trenti, G.; Malacarne, M.; Larcher, R. Fast Analysis of Quaternary Ammonium Pesticides in Food and Beverages Using Cation-Exchange Chromatography Coupled with Isotope-Dilution High-Resolution Mass Spectrometry. J. Sep. Sci. 2017, 40, 3928–3937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.; Zhao, Y. Determination of Triazine Pesticides in Honey by Ultra High Performance Liquid Chromatography-High Resolution Isotope Dilution Mass Spectrometry Combined with Dispersive Micro-Solid Phase Extraction. Anal. Methods 2015, 7, 9867–9874. [Google Scholar] [CrossRef]
- Ahn, S.; Son, S.; Kim, B.; Choi, K. Development of an Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry Method for the Accurate Determination of Neonicotinoid Pesticides, Imidacloprid, Clothianidin, and Thiamethoxam in Kimchi Cabbage Reference Materials. J. Anal. Sci. Technol. 2022, 13, 9. [Google Scholar] [CrossRef]
- Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A.A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. [Google Scholar] [CrossRef]
- Mullard, A. Deuterated Drugs Draw Heavier Backing. Nat. Rev. Drug Discov. 2016, 15, 219–221. [Google Scholar] [CrossRef]
- Fan, X.; Han, M.; Ding, Y.; Zhang, X.; Li, H.; An, J. Synthesis and Fungicidal Activity of Deuterated Pefurazoate. Chin. J. Pestic. Sci. 2020, 22, 27–34. [Google Scholar] [CrossRef]
- Flick, A.C.; Leverett, C.A.; Ding, H.X.; McInturff, E.L.; Fink, S.J.; Mahapatra, S.; Carney, D.W.; Lindsey, E.A.; Deforest, J.C.; France, S.P.; et al. Synthetic Approaches to the New Drugs Approved during 2020. J. Med. Chem. 2022, 65, 9607–9661. [Google Scholar] [CrossRef]
- Flick, A.C.; Leverett, C.A.; Ding, H.X.; Mcinturff, E.; Fink, S.J.; Mahapatra, S.; Carney, D.W.; Lindsey, E.A.; Deforest, J.C.; France, S.P.; et al. Synthetic Approaches to the New Drugs Approved during 2019. J. Med. Chem. 2021, 64, 3604–3657. [Google Scholar] [CrossRef]
- Flick, A.C.; Leverett, C.A.; Ding, H.X.; McInturff, E.; Fink, S.J.; Helal, C.J.; Deforest, J.C.; Morse, P.D.; Mahapatra, S.; O’Donnell, C.J. Synthetic Approaches to New Drugs Approved during 2018. J. Med. Chem. 2020, 63, 10652–10704. [Google Scholar] [CrossRef]
- Ding, H.X.; Leverett, C.A.; Kyne, R.E.; Liu, K.K.C.; Fink, S.J.; Flick, A.C.; O’Donnell, C.J. Synthetic Approaches to the 2013 New Drugs. Bioorg. Med. Chem. 2015, 23, 1895–1922. [Google Scholar] [CrossRef]
- Flick, A.C.; Ding, H.X.; Leverett, C.A.; Fink, S.J.; O’Donnell, C.J. Synthetic Approaches to New Drugs Approved during 2016. J. Med. Chem. 2018, 61, 7004–7031. [Google Scholar] [CrossRef] [Green Version]
- Krämer, W.; Schirmer, U. Modern Crop Protection Compounds; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007; ISBN 9783527314966. [Google Scholar]
- Kopf, S.; Bourriquen, F.; Li, W.; Neumann, H.; Junge, K.; Beller, M. Recent Developments for the Deuterium and Tritium Labeling of Organic Molecules. Chem. Rev. 2022, 122, 6634–6718. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Fey, T.; Zimmermann, J. The Renaissance of H/D Exchange. Angew. Chem.-Int. Ed. 2007, 46, 7744–7765. [Google Scholar] [CrossRef]
- Michelotti, A.; Roche, M. 40 Years of Hydrogen-Deuterium Exchange Adjacent to Heteroatoms: A Survey. Synthesis 2019, 51, 1319–1328. [Google Scholar] [CrossRef]
- Chatterjee, B.; Gunanathan, C. Ruthenium Catalyzed Selective α- And α,β-Deuteration of Alcohols Using D2O. Org. Lett. 2015, 17, 4794–4797. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Sen, R.; Kothandaraman, J.; Surya Prakash, G.K. Regioselective Deuteration of Alcohols in D2O Catalysed by Homogeneous Manganese and Iron Pincer Complexes. Green Chem. 2018, 20, 2706–2710. [Google Scholar] [CrossRef]
- Khaskin, E.; Milstein, D. Simple and Efficient Catalytic Reaction for the Selective Deuteration of Alcohols. ACS Catal. 2013, 3, 448–452. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Peng, L.; Wu, D.; Zhu, L.; Yin, G. Nickel-Catalyzed Migratory Alkyl-Alkyl Cross-Coupling Reaction. Chem. Sci. 2020, 11, 10461–10464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Li, L.; Gong, T.-J.; Xiao, B.; Lu, X.; Fu, Y. Vicinal Diboration of Alkyl Bromides via Tandem Catalysis. Org. Lett. 2019, 21, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Weng, C.; Ding, Y.; Ling, C.; Szostak, M.; Ma, X.; An, J. Reductive Deuteration of Aromatic Esters for the Synthesis of α,α-Dideuterio Benzyl Alcohols Using D2O as Deuterium Source. Synlett 2021, 32, 51–56. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, S.; Weng, C.; An, J. Reductive Deuteration of Nitriles Using D2O as a Deuterium Source. J. Org. Chem. 2019, 84, 15098–15105. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Weng, C.; Qin, Z.; Li, K.; Zhao, T.; Ding, Y.; Ling, C.; Ma, Y.; An, J. Tandem H/D Exchange-SET Reductive Deuteration Strategy for the Synthesis of α,β-Deuterated Amines Using D2O. J. Org. Chem. 2021, 86, 11862–11870. [Google Scholar] [CrossRef]
- Molander, G.A.; Harris, C.R. Sequencing Reactions with Samarium(II) Iodide. Chem. Rev. 1996, 96, 307–338. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Procter, D.J. Selective Synthesis of α,α-Dideuterio Alcohols by the Reduction of Carboxylic Acids Using SmI2 and D2O as Deuterium Source under SET Conditions. Org. Lett. 2014, 16, 5052–5055. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Eberhart, A.J.; Procter, D.J. Mechanism of SmI2/Amine/H2O-Promoted Chemoselective Reductions of Carboxylic Acid Derivatives (Esters, Acids, and Amides) to Alcohols. J. Org. Chem. 2014, 79, 11988–12003. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Liu, C.; Lai, Z.; Ning, L.; Szostak, R.; Szostak, M.; An, J. Pentafluorophenyl Esters: Highly Chemoselective Ketyl Precursors for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O as a Deuterium Source. Org. Lett. 2020, 22, 1249–1253. [Google Scholar] [CrossRef]
- Li, H.; Peng, M.; Lai, Z.; Ning, L.; Chen, X.; Zhang, X.; Wang, P.; Szostak, R.; Szostak, M.; An, J. Acyl Fluorides as Direct Precursors to Fluoride Ketyl Radicals: Reductive Deuteration Using SmI2 and D2O. Chem. Commun. 2021, 57, 5195–5198. [Google Scholar] [CrossRef]
- Li, H.; Lai, Z.; Peng, M.; Ning, L.; Dong, Q.; Hou, Y.; An, J. One-Pot Sequential Hydrogen Isotope Exchange/Reductive Deuteration for the Preparation of α,β-Deuterated Alcohols Using Deuterium Oxide. Org. Lett. 2022, 24, 5319–5323. [Google Scholar] [CrossRef]
- Peng, M.; Li, H.; Qin, Z.; Li, J.; Sun, Y.; Zhang, X.; Jiang, L.; Do, H.; An, J. Pentafluorophenyl Group as Activating Group: Synthesis of α-Deuterio Carboxylic Acid Derivatives via Et3N Catalyzed H/D Exchange. Adv. Synth. Catal. 2022, 364, 2184–2189. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Lai, Z.; Ning, L.; Li, A.; Li, Y.; An, J. Synthesis of α-Deuterioalcohols by Single-Electron Umpolung Reductive Deuteration of Carbonyls Using D2O as Deuterium Source. Synlett 2021, 32, 1241–1245. [Google Scholar] [CrossRef]
- Ning, L.; Li, H.; Lai, Z.; Szostak, M.; Chen, X.; Dong, Y.; Jin, S.; An, J. Synthesis of α-Deuterated Primary Amines via Reductive Deuteration of Oximes Using D2O as a Deuterium Source. J. Org. Chem. 2021, 86, 2907–2916. [Google Scholar] [CrossRef]
- Praczyk, T.; Kardasz, P.; Jakubiak, E.; Syguda, A.; Materna, K.; Pernak, J. Herbicidal Ionic Liquids with 2,4-D. Weed Sci. 2012, 60, 189–192. [Google Scholar] [CrossRef]
- Schneegurt, M.A.; Henry, M.J. Effects of Piperalin and Fenpropidin on Sterol Biosynthesis in Ustilago Maydis. Pestic. Biochem. Physiol. 1992, 43, 45–52. [Google Scholar] [CrossRef]
- Szostak, M.; Spain, M.; Procter, D.J. Selective Synthesis of 3-Hydroxy Acids from Meldrum’s Acids Using SmI2-H2O. Nat. Protoc. 2012, 7, 970–977. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, X.; Zhang, P.; Yang, H.; Zhu, C.; Fu, H. Axially Chiral Cyclic Diphosphine Ligand-Enabled Palladium-Catalyzed Intramolecular Asymmetric Hydroarylation. iScience 2018, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Menche, D. Selective Deprotection of Silyl Ethers with Sodium Periodate. Synthesis 2009, 5, 1904–1908. [Google Scholar] [CrossRef]
- Sirichaiwat, C.; Intaraudom, C.; Kamchonwongpaisan, S.; Vanichtanankul, J.; Thebtaranonth, Y.; Yuthavong, Y. Target Guided Synthesis of 5-Benzyl-2,4-Diamonopyrimidines: Their Antimalarial Activities and Binding Affinities to Wild Type and Mutant Dihydrofolate Reductases from Plasmodium Falciparum. J. Med. Chem. 2004, 47, 345–354. [Google Scholar] [CrossRef]
- Ringstrand, B.; Oltmanns, M.; Batt, J.A.; Jankowiak, A.; Denicola, R.P.; Kaszynski, P. The Preparation of 3-Substituted-1,5-Dibromo-Pentanes as Precursors to Heteracyclohexanes. Beilstein J. Org. Chem. 2011, 7, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi Kantam, M.; Arundhathi, R.; Likhar, P.R.; Damodara, D. Reusable Copper-Aluminum Hydrotalcite/Rac-BINOL System for Room Temperature Selective Aerobic Oxidation of Alcohols. Adv. Synth. Catal. 2009, 351, 2633–2637. [Google Scholar] [CrossRef]
- Mori, K. New Syntheses of 1,7-Dimethylnonyl Propanoate, the Western Corn Rootworm Pheromone, in Four Different Ways via Cross Metathesis, Alkylation and Coupling Reactions. Biosci. Biotechnol. Biochem. 2010, 74, 595–600. [Google Scholar] [CrossRef] [PubMed]
 | |||||
|---|---|---|---|---|---|
| Entry | SmI2 (equiv.) | D2O (equiv.) | Time (min) | Yield b (%) | [D2] b (%) |
| 1 | 5 | 100 | 15 | 96 | 98 |
| 2 | 5 | 75 | 15 | >98 | 98 |
| 3 | 5 | 60 | 15 | >98 | 97 |
| 4 | 5 | 30 | 15 | >98 | 98 |
| 5 | 5 | 25 | 15 | 95 | 97 |
| 6 | 5 | 15 | 15 | 80 | 97 |
| 7 | 4 | 30 | 15 | 90 | 97 |
| 8 | 5 | 30 | 5 | 95 | 97 |
| 9 | 5 | 30 | 0.5 | 78 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hou, Y.; Peng, M.; Wang, L.; Li, J.; Ning, L.; Lai, Z.; Li, Y.; An, J. Reductive Deuteration of Acyl Chlorides for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O. Molecules 2023, 28, 416. https://doi.org/10.3390/molecules28010416
Li H, Hou Y, Peng M, Wang L, Li J, Ning L, Lai Z, Li Y, An J. Reductive Deuteration of Acyl Chlorides for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O. Molecules. 2023; 28(1):416. https://doi.org/10.3390/molecules28010416
Chicago/Turabian StyleLi, Hengzhao, Yuxia Hou, Mengqi Peng, Lijun Wang, Junyu Li, Lei Ning, Zemin Lai, Yixuan Li, and Jie An. 2023. "Reductive Deuteration of Acyl Chlorides for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O" Molecules 28, no. 1: 416. https://doi.org/10.3390/molecules28010416






