Effects of a Semisynthetic Catechin on Phosphatidylglycerol Membranes: A Mixed Experimental and Simulation Study
Abstract
1. Introduction
2. Results and Discussion
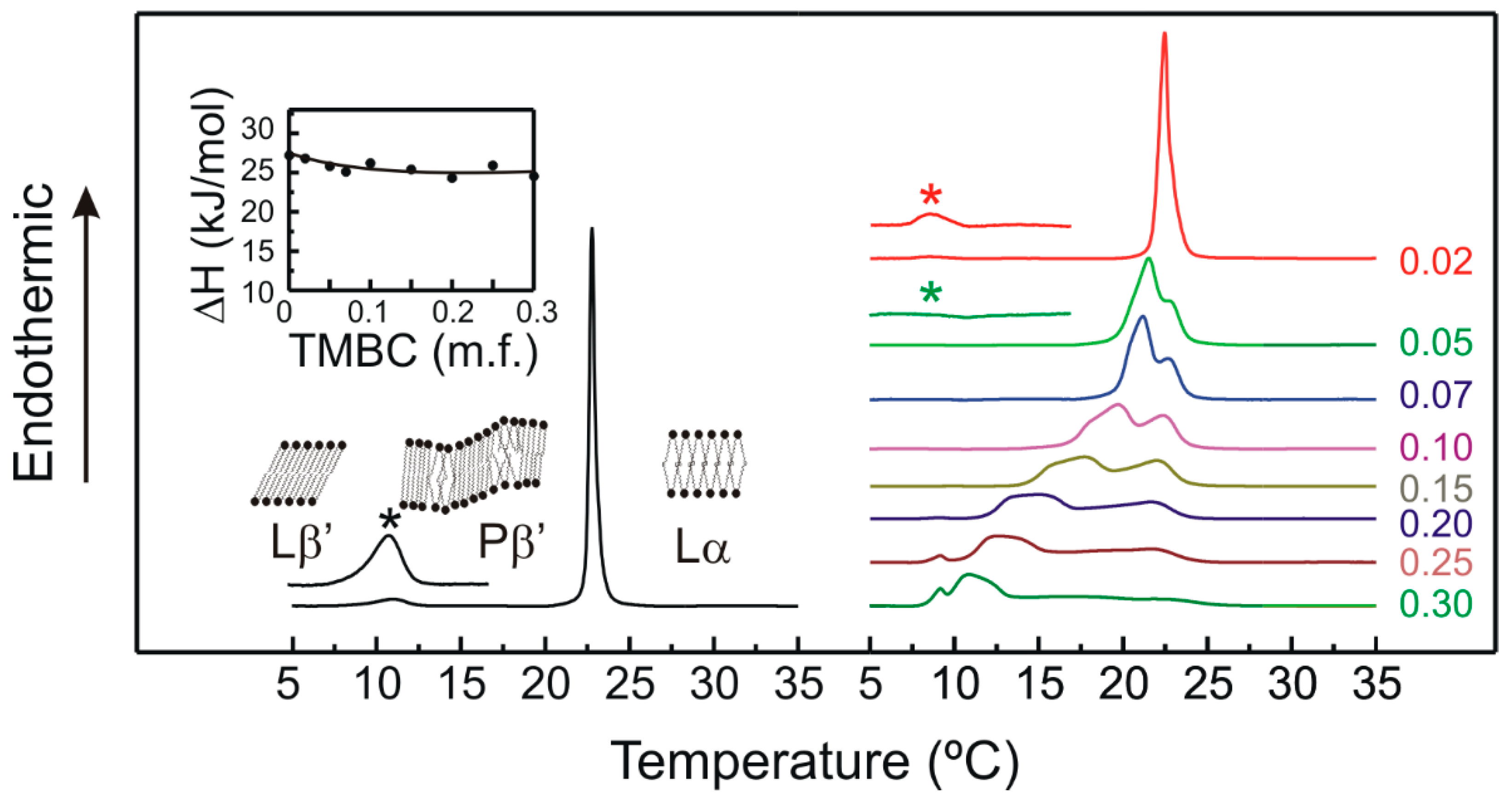
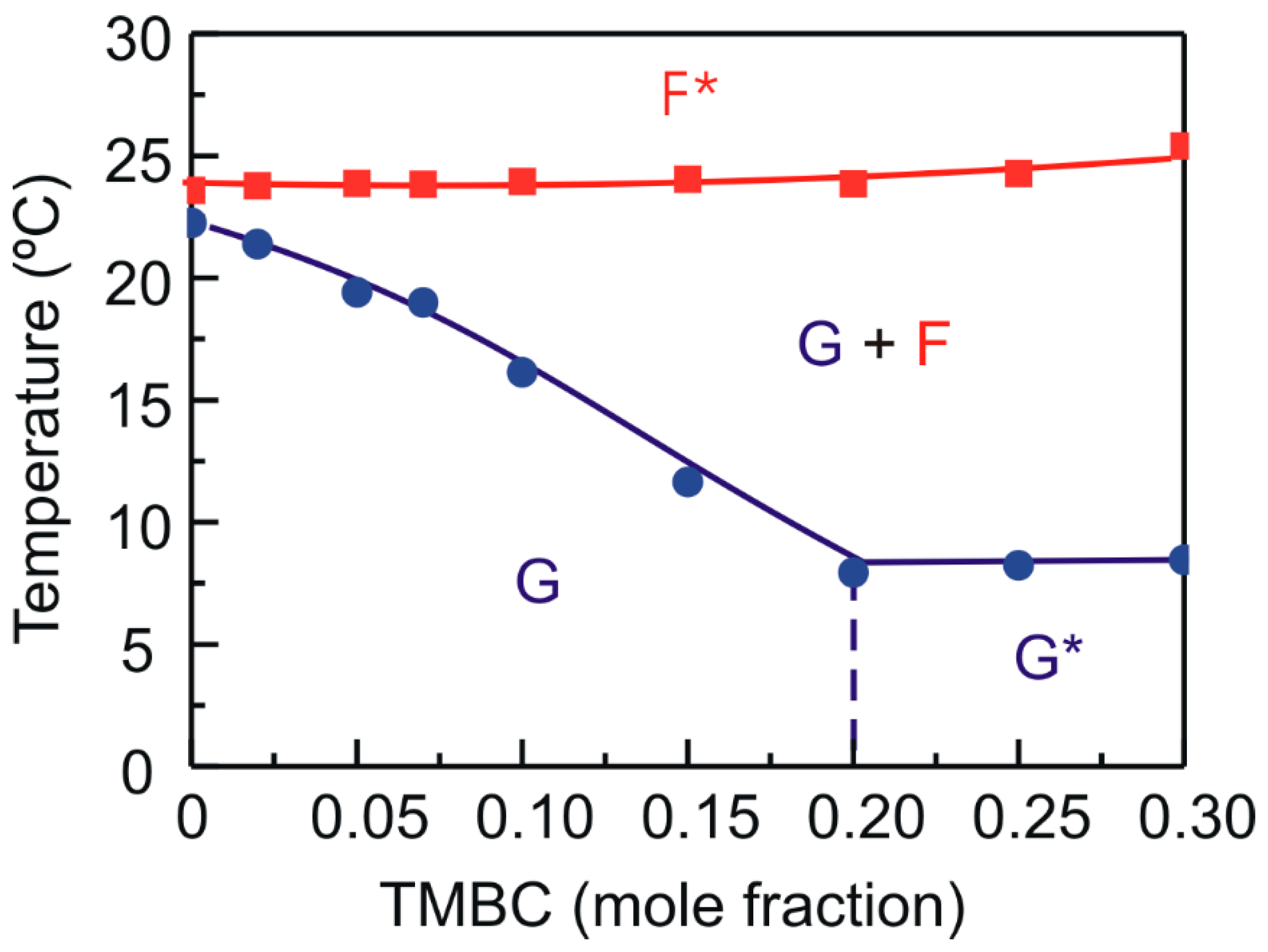
2.1. Differential Scanning Calorimetry
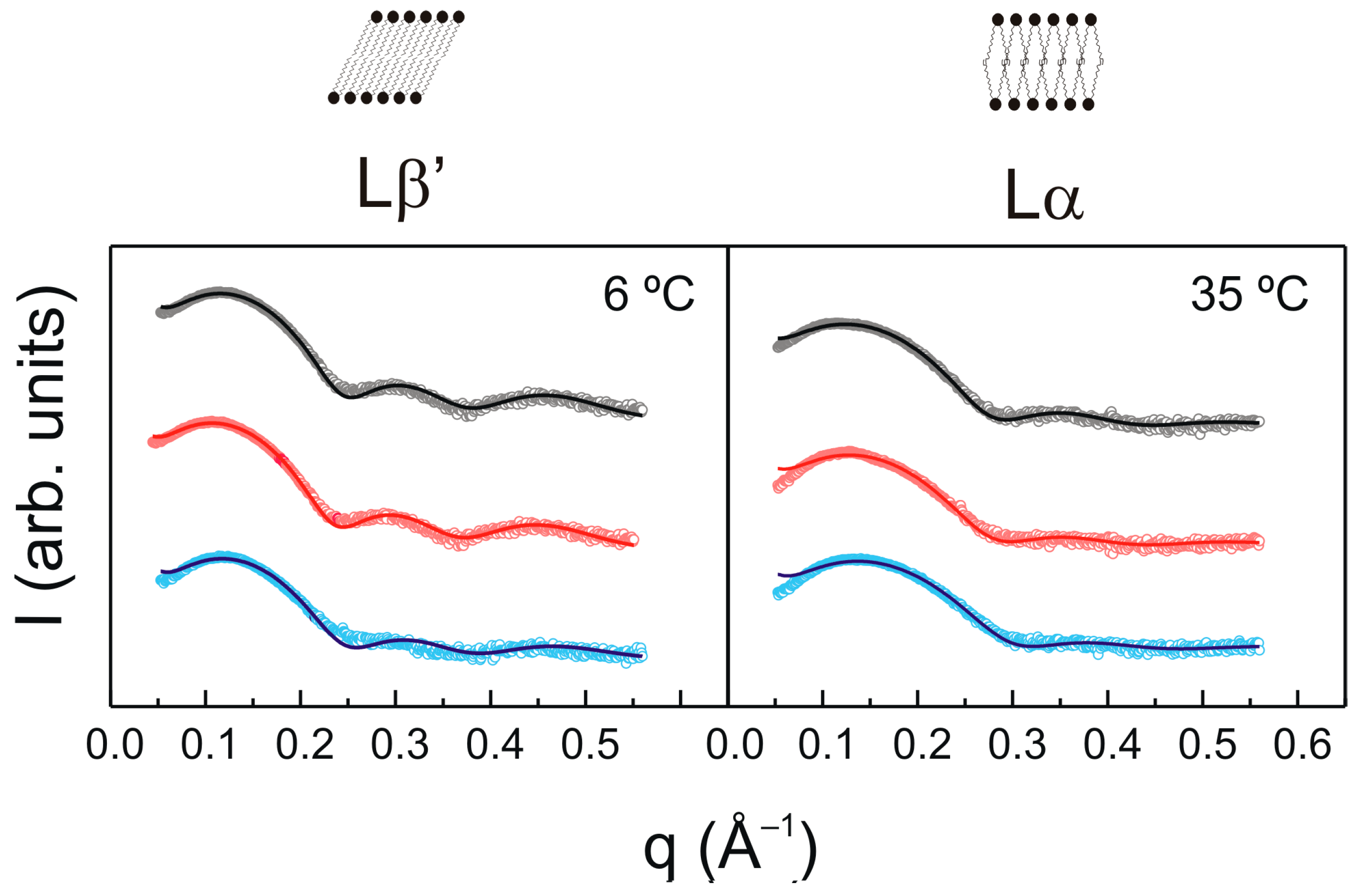
2.2. X-ray Diffraction
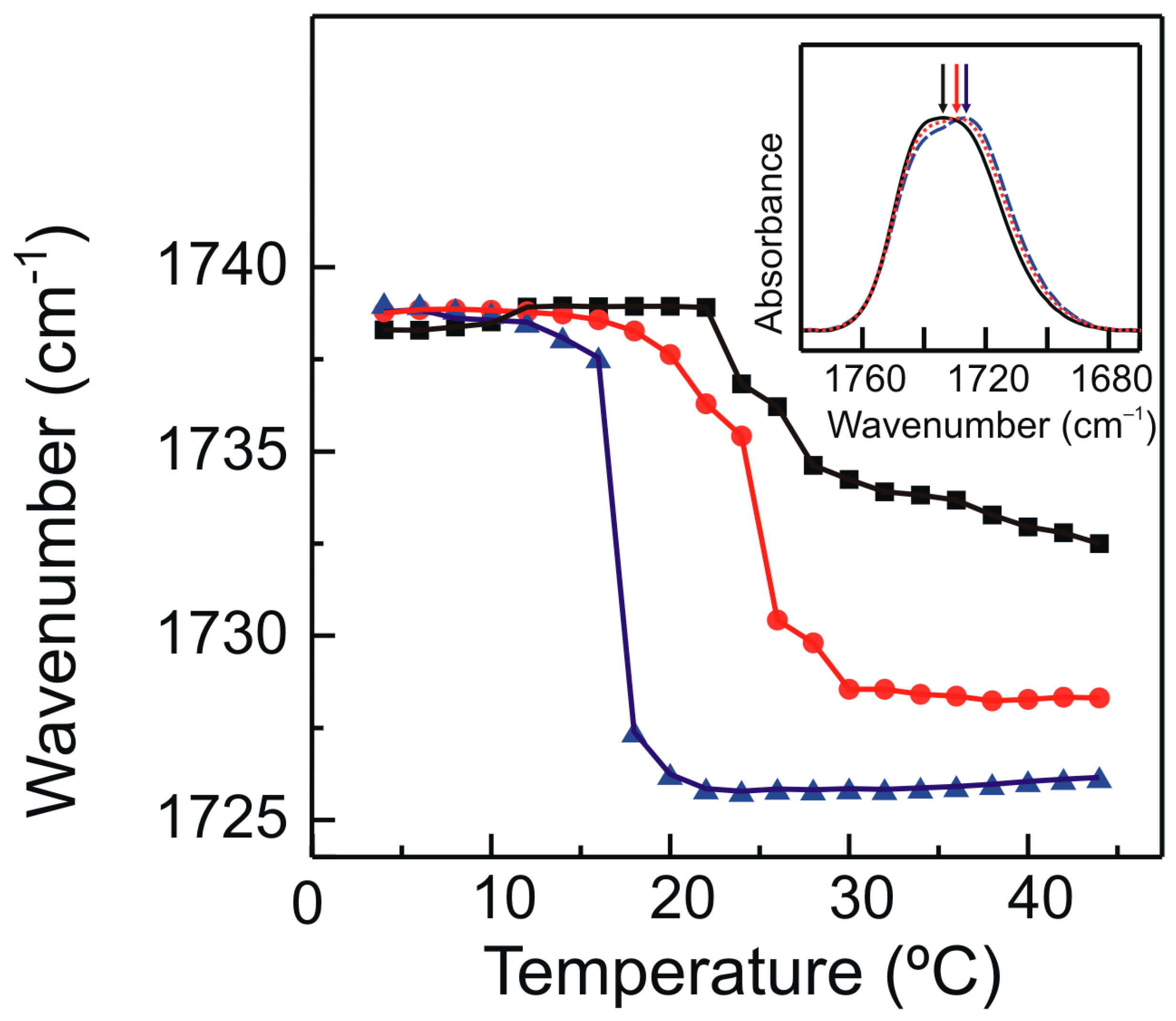
2.3. FTIR Spectroscopy
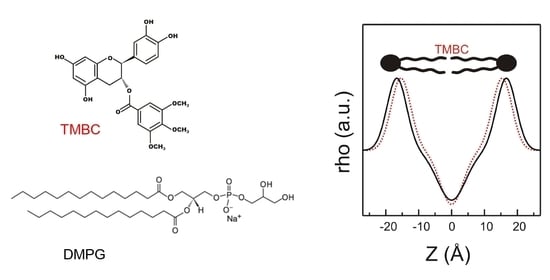
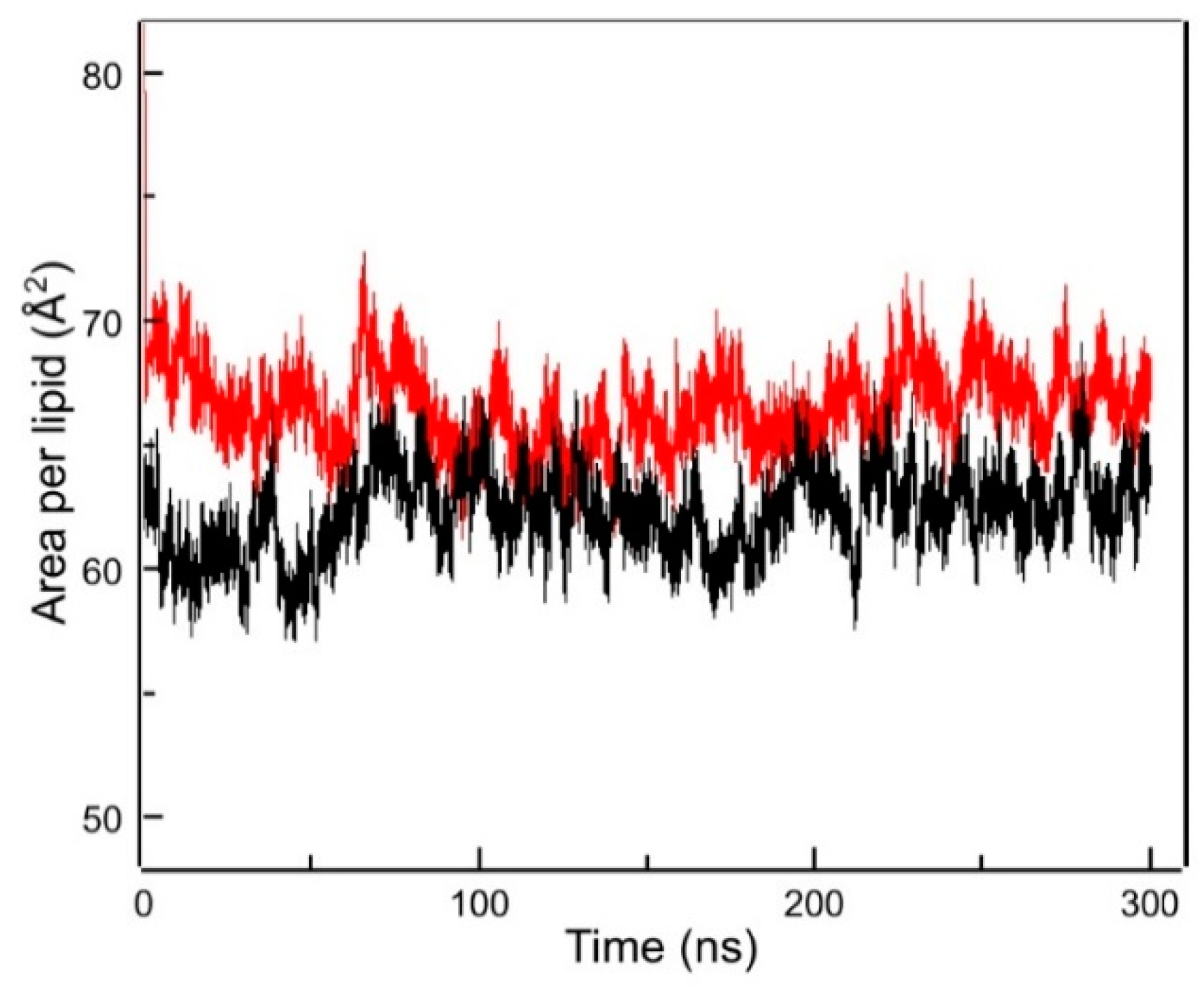
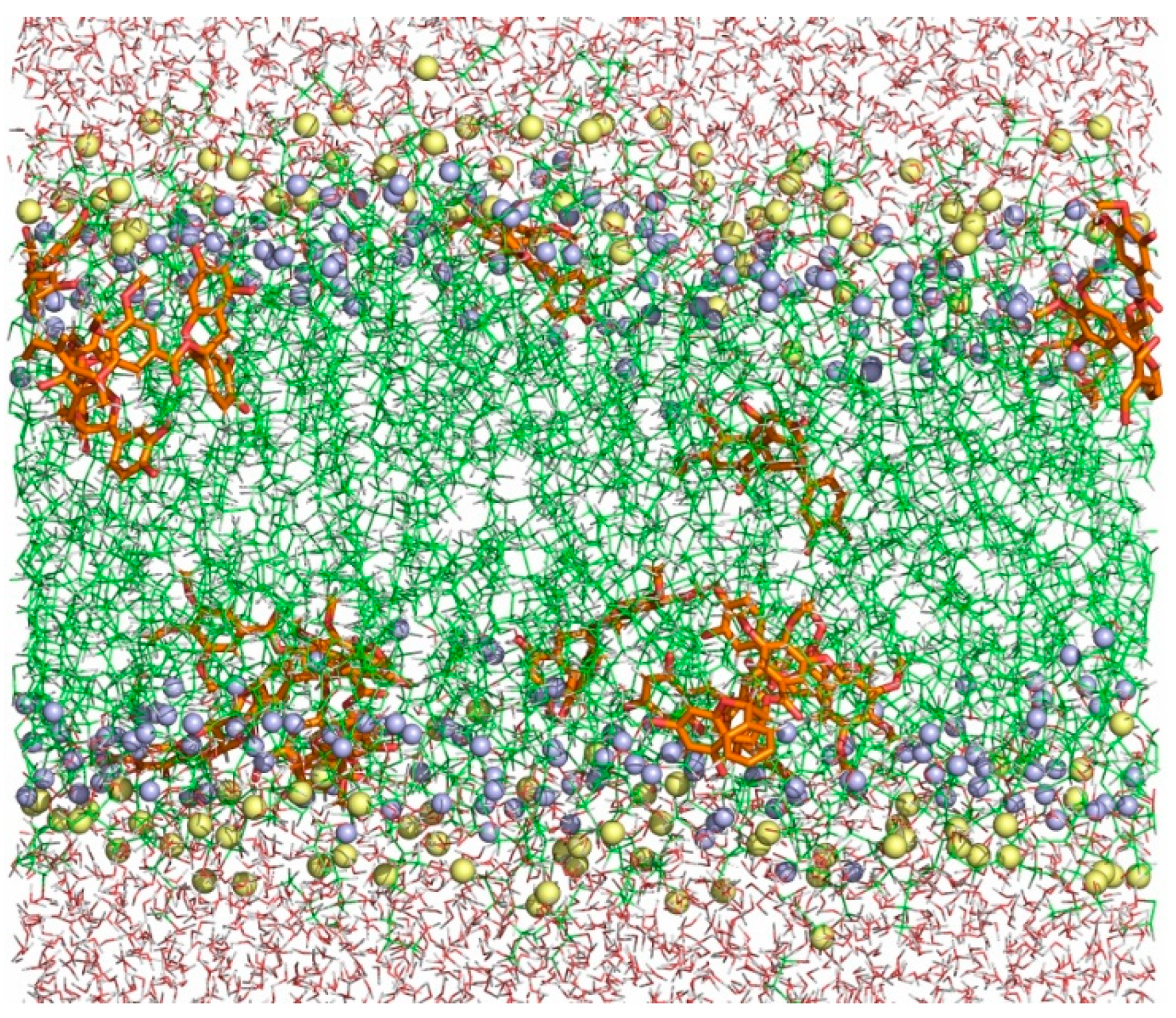
2.4. Molecular Dynamics
3. Materials and Methods
3.1. Materials
3.2. Differential Scanning Calorimetry
3.3. X-ray Diffraction
3.4. Infrared Spectroscopy
3.5. Molecular Dynamics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Brown, A.C. Applications of catechins in the treatment of bacterial infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research progress of the antiviral bioactivities of natural flavonoids. Nat. Products Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Cadoná, F.C.; Dantas, R.F.; de Mello, G.H.; Silva, F.P., Jr. Natural products targeting into cancer hallmarks: An update on caffeine, theobromine, and (+)-catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M. Possible mechanisms of green tea and its constituents against cancer. Molecules 2018, 23, 2284. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin—Gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (2)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef]
- Takahashi, A.; Watanabe, T.; Mondal, A.; Suzuki, K.; Kurusu-Kanno, M.; Li, Z.; Yamazaki, T.; Fujiki, H.; Suganuma, M. Mechanism-based inhibition of cancer metastasis with (-)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2014, 443, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duhon, D.; Bigelow, R.L.H.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-met receptor in prostate cancer cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; ten Hagen, T.L.M. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Koeppe, R.E.; Andersen, O.S. Effects of green tea catechins on gramicidin channel function and inferred changes in bilayer properties. FEBS Lett. 2011, 585, 3101–3105. [Google Scholar] [CrossRef]
- Stillwell, W. An Introduction to Biological Membranes: Composition, Structure and Function, 2nd ed.; Academic Press: London, UK, 2016. [Google Scholar] [CrossRef]
- Furse, S. Is phosphatidylglycerol essential for terrestrial life? J. Chem. Biol. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.C.; Zavodnik, I.B. Flavonoids modulate liposomal membrane structure, regulate mitochondrial membrane permeability and prevent erythrocyte oxidative damage. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183442. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and mitochondria: Activation of cytoprotective pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef]
- Bollag, W.B.; Xie, D.; Zheng, X.; Zhong, X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a phosphatidylglycerol signaling lipid. J. Investig. Dermatol. 2007, 127, 2823–2831. [Google Scholar] [CrossRef]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol inhibits toll-like receptor–mediated inflammation by danger-associated molecular patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef]
- Kuronuma, K.; Mitsuzawa, H.; Takeda, K.; Nishitani, C.; Chan, E.D.; Kuroki, Y.; Nakamura, M.; Voelker, D.R. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 2009, 284, 25488–25500. [Google Scholar] [CrossRef]
- Chen, W.W.; Chao, Y.J.; Chang, W.H.; Chan, J.F.; Hsu, Y.H.H. Phosphatidylglycerol incorporates into cardiolipin to improve mitochondrial activity and inhibits inflammation. Sci. Rep. 2018, 8, 4949. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Flynn, J.D.; Teague, W.E.; Gawrisch, K.; Lee, J.C. Stimulation of α-synuclein amyloid formation by phosphatidylglycerol micellar tubules. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Kandasamy, P.; Nagashima, Y.; Posey, J.; Hartshorn, K.; Woodland, D.; Voelker, D.R. Phosphatidylglycerol suppresses influenza a virus infection. Am. J. Respir. Cell Mol. Biol. 2012, 46, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Annu. Rev. Biochem. 1997, 66, 199–232. [Google Scholar] [CrossRef]
- Elmore, D.E. Molecular dynamics simulation of a phosphatidylglycerol membrane. FEBS Lett. 2006, 580, 144–148. [Google Scholar] [CrossRef]
- Sosa Morales, M.C.; Álvarez, R.M.S. Structural characterization of phosphatidylglycerol model membranes containing the antibiotic target lipid II molecule: A raman microspectroscopy study. J. Raman Spectrosc. 2017, 48, 170–179. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure-activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Taylor, P.W. Interactions of tea-derived catechin gallates with bacterial pathogens. Molecules 2020, 25, 1986. [Google Scholar] [CrossRef]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (−)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. App. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef]
- Sugita-Konishi, Y.; Hara-Kudo, Y.; Amano, F.; Okubo, T.; Aoi, N.; Iwaki, M.; Kumagai, S. Epigallocatechin gallate and gallocatechin gallate in green tea catechins inhibit extracellular release of Vero toxin from enterohemorrhagic Escherichia coli O157:H7. Biochim. Biophys. Acta 1999, 1472, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Targeting bacterial biofilms by the green tea polyphenol EGCG. Molecules 2019, 24, 2403. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Shigemune, N.; Nakayama, M.; Tsugukuni, T.; Hitomi, J.; Yoshizawa, C.; Mekada, Y.; Kurahachi, M.; Miyamoto, T. The mechanisms and effect of epigallocatechin gallate (EGCg) on the germination and proliferation of bacterial spores. Food Control 2012, 27, 269–274. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial mechanism of flavonoids against Escherichia coli ATCC 25922 by model membrane study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Mahmood, H.Y.; Jamshidi, S.; Mark Sutton, J.M.; Rahman, K.M. Current advances in developing inhibitors of bacterial multidrug efflux pumps. Curr. Med. Chem. 2016, 23, 1062–1081. [Google Scholar] [CrossRef]
- Mehmood, S.; Maqsood, M.; Mahtab, N.; Khan, M.I.; Sahar, A.; Zaib, S.; Gul, S. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J. Food Biochem. 2022, 46, 14189. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Sáez-Ayala, M.; Sánchez-Del-Campo, L.; Montenegro, M.F.; Chazarra, S.; Tárraga, A.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Comparison of a pair of synthetic tea-catechin-derived epimers: Synthesis, antifolate activity, and tyrosinase-mediated activation in melanoma. Chem. Med. Chem. 2011, 6, 440–449. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Sáez-Ayala, M.; Piñero-Madrona, A.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Reactivation of the tumour suppressor RASSF1A in breast cancer by simultaneous targeting of DNA and E2F1 methylation. PLoS ONE 2012, 7, e52231. [Google Scholar] [CrossRef]
- How, C.W.; Teruel, J.A.; Ortiz, A.; Montenegro, M.F.; Rodríguez-López, J.N.; Aranda, F.J. Effects of a synthetic antitumoral catechin and its tyrosinase-processed product on the structural properties of phosphatidylcholine membranes. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Casado, F.; Teruel, J.A.; Casado, S.; Ortiz, A.; Rodríguez-López, J.N.; Aranda, F.J. Location and effects of an antitumoral catechin on the structural properties of phosphatidylethanolamine membranes. Molecules 2016, 21, 829. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; Teruel, J.A.; Ortiz, A.; Pérez-Cárceles, M.-D.; Rodríguez-López, J.N.; Aranda, F.J. 3,4,5-trimethoxybenzoate of catechin, an anticarcinogenic semisynthetic catechin, modulates the physical properties of anionic phospholipid membranes. Molecules 2022, 27, 2910. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; Mannock, D.A.; McElhaney, R.N. Differential scanning calorimetry in the study of lipid phase transitions in model and biological membranes. Methods Mol. Biol. 2007, 400, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Lewis, R.N.A.H.; McElhaney, R.N. Calorimetric and spectroscopic studies of the thermotropic phase behavior of the N-saturated 1,2-diacylphosphatidylglycerols. Biophys. J. 1997, 72, 779–793. [Google Scholar] [CrossRef]
- Pabst, G.; Danner, S.; Karmakar, S.; Deutsch, G.; Raghunathany, V.A. On the propensity of phosphatidylglycerols to form interdigitated phases. Biophys. J. 2007, 93, 513–525. [Google Scholar] [CrossRef]
- Semeraro, E.F.; Marx, L.; Frewein, M.P.K.; Pabst, G. Increasing complexity in small-angle X-ray and neutron scattering experiments: From biological membrane mimics to live cells. Soft Matter. 2021, 17, 222–232. [Google Scholar] [CrossRef]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin-water phases. J. Mol. Biol. 1973, 75, 711–733. [Google Scholar] [CrossRef]
- Pabst, G.; Grage, S.L.; Danner-Pongratz, S.; Jing, W.; Ulrich, A.S.; Watts, A.; Lohner, K.; Hickel, A. Membrane thickening by the antimicrobial peptide PGLa. Biophys. J. 2008, 95, 5779–5788. [Google Scholar] [CrossRef]
- Lohner, K.; Latal, A.; Degovics, G.; Garidel, P. Packing characteristics of a model system mimicking cytoplasmic bacterial membranes. Chem. Phys. Lipids 2001, 111, 177–192. [Google Scholar] [CrossRef]
- Ortiz, A.; Teruel, J.A.; Manresa, A.; Espuny, M.J.; Marqués, A.; Aranda, F.J. Effects of a bacterial trehalose lipid on phosphatidylglycerol membranes. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaum, M.; Laggner, P. States of phase transitions in biological structures. Prog. Surf. Sci. 1996, 51, 233–261. [Google Scholar] [CrossRef]
- Riske, K.A.; Amaral, L.Q.; Lamy-Freund, M.T. Thermal transitions of DMPG bilayers in aqueous solution: SAXS structural studies. Biochim. Biophys. Acta-Biomembr. 2001, 1511, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.M.; Riske, K.A.; Amaral, L.Q.; Itri, R.; Lamy, M.T. Influence of salt on the structure of DMPG studied by SAXS and optical microscopy. Biochim. Biophys. Acta-Biomembr. 2008, 1778, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.A. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta-Rev. Biomembr. 1998, 1376, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, L.E.; de Mendoza, D. Bilayer Hydrophobic thickness and integral membrane protein function. Curr. Protein Pept. Sci. 2011, 12, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Mantsch, H.H.; McElhaney, R. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids 1991, 57, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; McElhaney, R.N. Fourier transform infrared spectroscopy in the study of lipid phase transitions in model and biological membranes: Practical considerations. Methods Mol. Biol. 2007, 400, 207–226. [Google Scholar] [CrossRef]
- Blume, A.; Hiibner, W.; Messner, G. Fourier transform infrared spectroscopy of 13C=0-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry 1988, 27, 8239–8249. [Google Scholar] [CrossRef]
- Friedman, R.; Khalid, S.; Aponte-Santamaría, C.; Arutyunova, E.; Becker, M.; Boyd, K.J.; Christensen, M.; Coimbra, J.T.; Daday, C.; van Eerden, F.J.; et al. Understanding conformational dynamics of complex lipid mixtures relevant to biology. J. Membr. Biol. 2018, 251, 609–631. [Google Scholar] [CrossRef]
- Venable, R.M.; Krämer, A.; Pastor, R.W. Molecular dynamics simulations of membrane permeability. Chem. Rev. 2019, 119, 5954–5997. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Heberle, F.A.; Tristram-Nagle, S.; Szymanski, M.; Koepfinger, M.; Katsaras, J.; Kučerka, N. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small angle neutron and X-ray scattering. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Gent, C.; Pries, C. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 1961, 24, 203–204. [Google Scholar] [CrossRef]
- Sun, Y.; Hung, W.-C.; Chen, F.-Y.; Lee, C.-C.; Huang, H.W. Interaction of tea catechin (−)-epigallocatechin gallate with lipid bilayers. Biophys. J. 2009, 96, 1026–1035. [Google Scholar] [CrossRef]
- Casali, C.I.; Weber, K.; Favale, N.O.; Fernández Tome, M.C. Environmental hyperosmolality regulates phospholipid biosynthesis in the renal epithelial cell line MDCK. J. Lipid Res. 2013, 54, 677–691. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural Information from multilamellar liposomes at full hydration: Full q-range fitting with high quality X-ray data. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 4000–4009. [Google Scholar] [CrossRef]
- Pabst, G.; Koschuch, R.; Pozo-Navas, B.; Rappolt, M.; Lohner, K.; Laggner, P. Structural analysis of weakly ordered membrane stacks. J. Appl. Crystallogr. 2003, 36, 1378–1388. [Google Scholar] [CrossRef]
- Pabst, G. Global properties of biomimetic membranes: Perspectives on molecular features. Biophys. Rev. Lett. 2006, 01, 57–84. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System, version 2.3; Schrödinger, Inc.: New York, NY, USA, 2010. [Google Scholar]


| Hydrogen Bonds | S.E. (n = 3) | ||
|---|---|---|---|
| DMPS | DMPS-DMPS | 193.59 | 5.21 |
| DMPS + TMBC | DMPS-DMPS | 185.13 | 6.26 |
| TMBC-DMPS | 13.51 | 0.88 | |
| TMBC-TMBC | 1.26 | 0.48 | |
| DMPG | DMPG-DMPG | 147.89 | 4.36 |
| DMPG + TMBC | DMPG-DMPG | 145.49 | 4.39 |
| TMBC-DMPG | 16.55 | 1.13 | |
| TMBC-TMBC | 4.95 | 0.83 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda, E.; Teruel, J.A.; Ortiz, A.; Pérez-Cárceles, M.D.; Rodríguez-López, J.N.; Aranda, F.J. Effects of a Semisynthetic Catechin on Phosphatidylglycerol Membranes: A Mixed Experimental and Simulation Study. Molecules 2023, 28, 422. https://doi.org/10.3390/molecules28010422
Aranda E, Teruel JA, Ortiz A, Pérez-Cárceles MD, Rodríguez-López JN, Aranda FJ. Effects of a Semisynthetic Catechin on Phosphatidylglycerol Membranes: A Mixed Experimental and Simulation Study. Molecules. 2023; 28(1):422. https://doi.org/10.3390/molecules28010422
Chicago/Turabian StyleAranda, Elisa, José A. Teruel, Antonio Ortiz, María Dolores Pérez-Cárceles, José N. Rodríguez-López, and Francisco J. Aranda. 2023. "Effects of a Semisynthetic Catechin on Phosphatidylglycerol Membranes: A Mixed Experimental and Simulation Study" Molecules 28, no. 1: 422. https://doi.org/10.3390/molecules28010422
APA StyleAranda, E., Teruel, J. A., Ortiz, A., Pérez-Cárceles, M. D., Rodríguez-López, J. N., & Aranda, F. J. (2023). Effects of a Semisynthetic Catechin on Phosphatidylglycerol Membranes: A Mixed Experimental and Simulation Study. Molecules, 28(1), 422. https://doi.org/10.3390/molecules28010422