Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance
Abstract
:1. Introduction
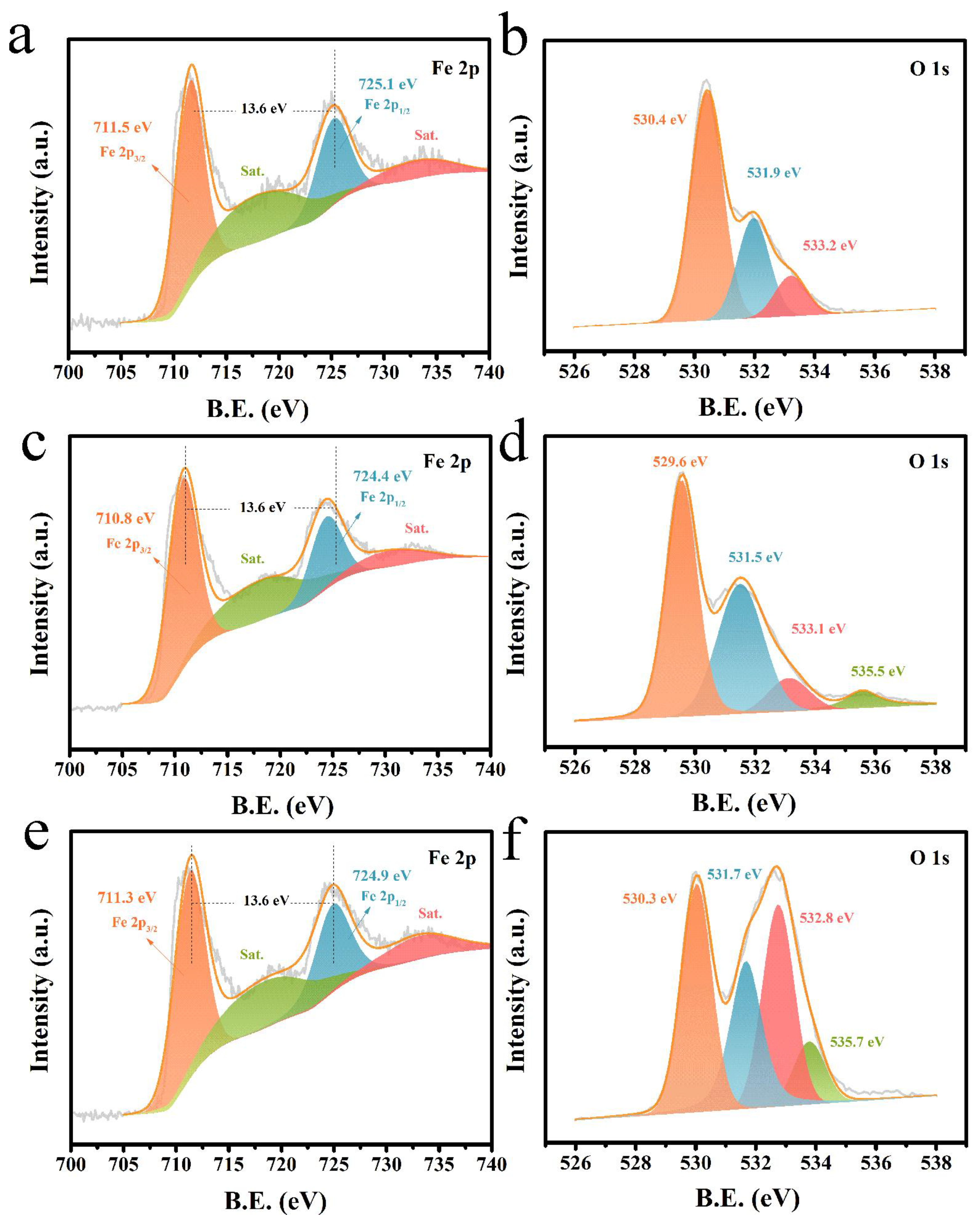
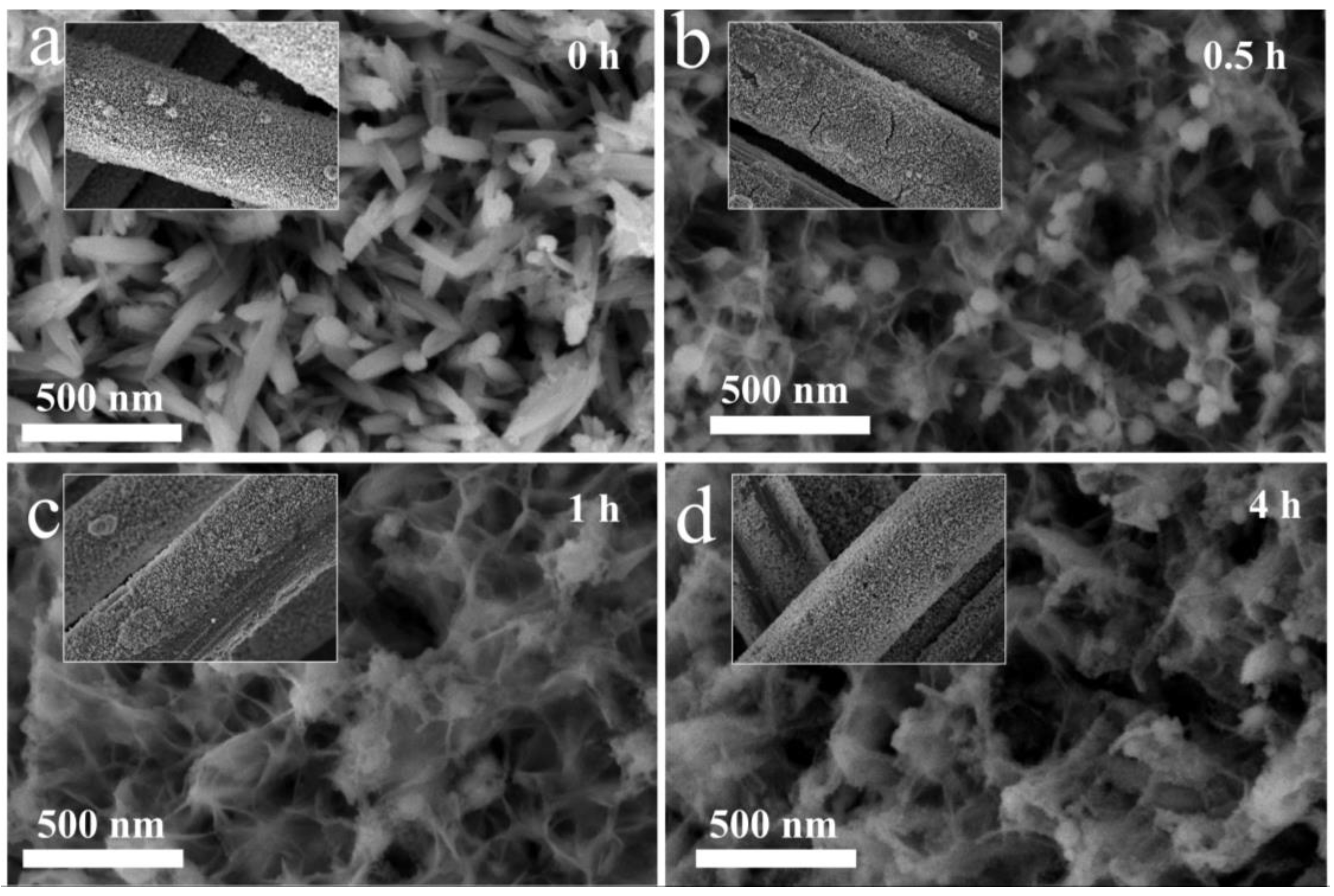
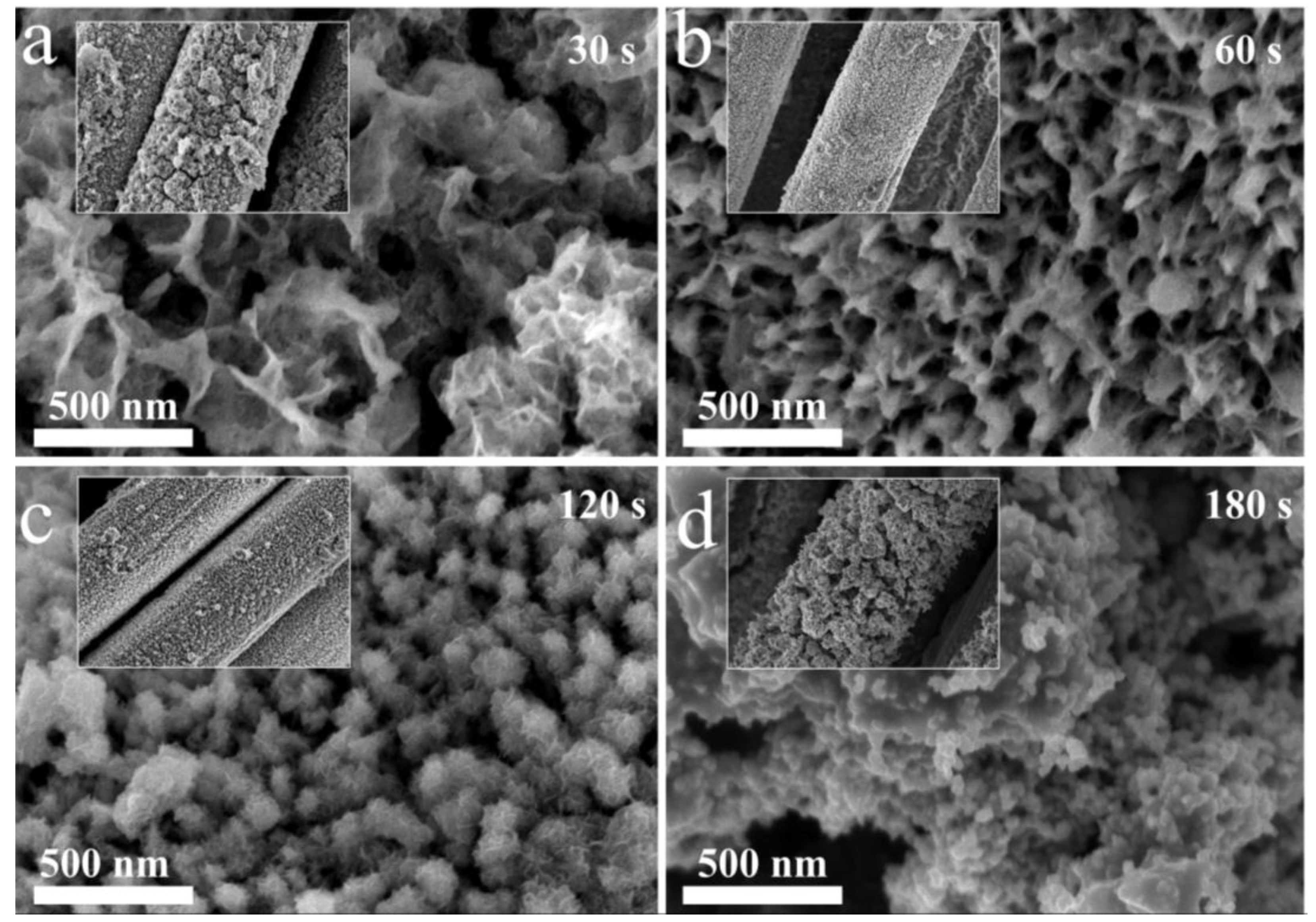
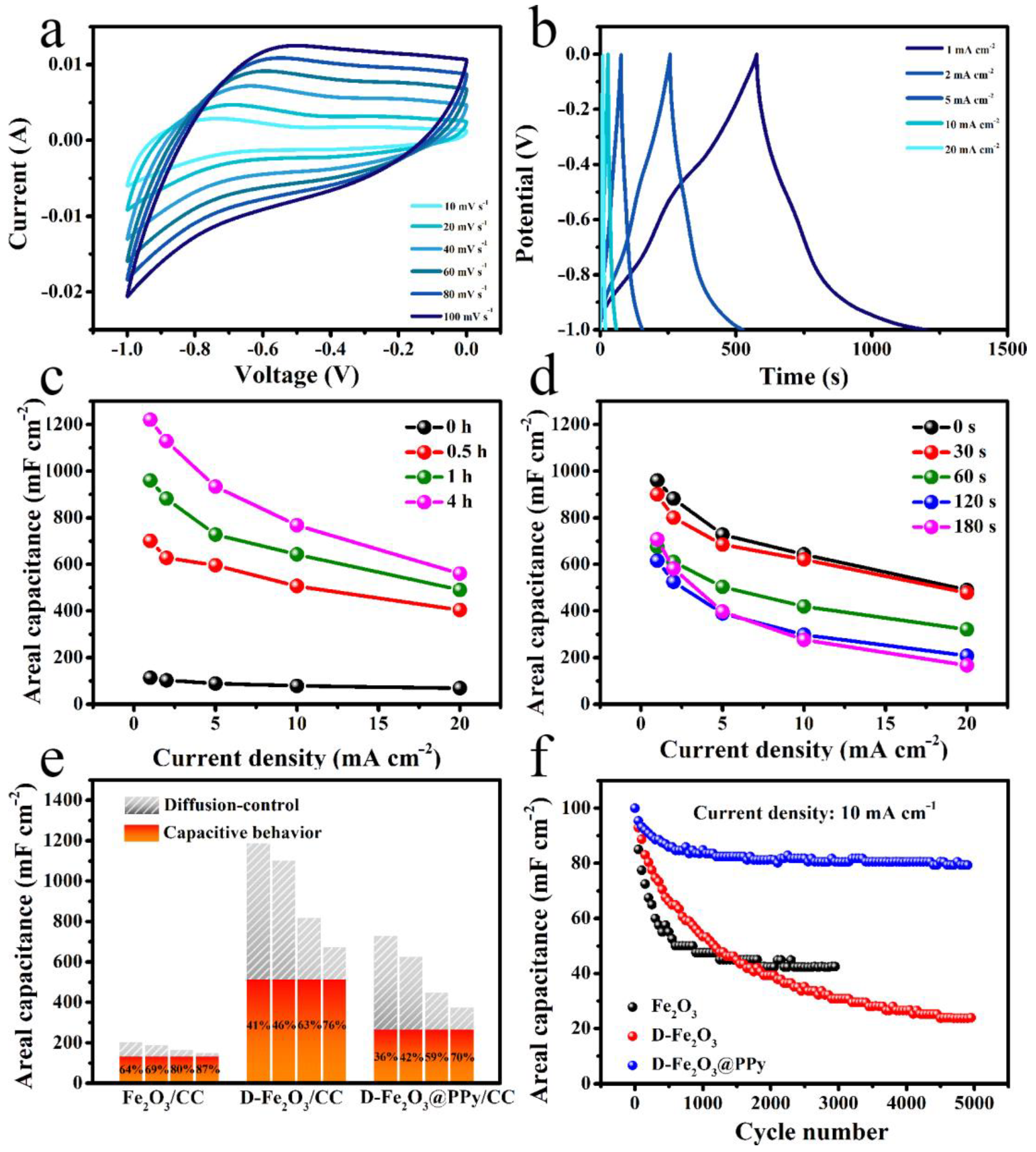
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Fe2O3/CC
3.3. Synthesis of D-Fe2O3/CC
3.4. Synthesis of D-Fe2O3@PPy/CC
3.5. Material Characterization
3.6. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, H.; Zhang, T.C.; Yuan, S.; Liang, B. N-doped porous carbon derived from rGO-Incorporated polyphenylenediamine composites for CO2 adsorption and supercapacitors. J. Power Sources 2020, 472, 228610. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. N-doped porous carbon derived from solvent-free synthesis of cross-linked triazine polymers for simultaneously achieving CO2 capture and supercapacitors. Chem. Eur. J. 2021, 27, 7908–7914. [Google Scholar] [CrossRef]
- Mourad, E.; Coustan, L.; Lannelongue, P.; Zigah, D.; Mehdi, A.; Vioux, A.; Freunberger, S.A.; Favier, F.; Fontaine, O. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 2017, 16, 446–453. [Google Scholar] [CrossRef]
- Sun, B.; Xiong, P.; Maitra, U.; Langsdorf, D.; Yan, K.; Wang, C.; Janek, J.; Schröder, D.; Wang, G. Design strategies to enable the efficient use of sodium metal anodes in high-energy batteries. Adv. Mater. 2020, 32, 1903891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xiao, J.; Wang, H.; Zhang, T.C.; Yuan, S. Binary doping of nitrogen and phosphorus into porous carbon: A novel di-functional material for enhancing CO2 capture and super-capacitance. J. Mater. Sci. Tech. 2022, 99, 73–81. [Google Scholar] [CrossRef]
- Zhi, J.; Zhou, M.; Zhang, Z.; Reiser, O.; Huang, F. Interstitial boron-doped mesoporous semiconductor oxides for ultratransparent energy storage. Nat. Commun. 2021, 12, 445. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Yin, Y.; Yuan, Y.; Meng, Y.; Jiang, T.; Peng, Q.; Wang, W.; Chen, W. Production of a hybrid capacitive storage device via hydrogen gas and carbon electrodes coupling. Nat. Commun. 2022, 13, 2805. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Xiao, J.; Tian, X.; Yuan, S. Enhancing electrochemical performance of ultrasmall Fe2O3-embedded carbon nanotubes via combusting-induced high-valence dopants. J. Mater. Sci. Technol. 2023, 134, 142–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, Z.; Lin, T.; Zhu, X.; Luo, B.; Hu, H.; Xing, W.; Yan, Z.; Wang, L. Lithiation-induced vacancy engineering of Co3O4 with improved faradic reactivity for high-performance supercapacitor. Adv. Funct. Mater. 2020, 30, 2004172. [Google Scholar] [CrossRef]
- Mane, S.A.; Kashale, A.A.; Kamble, G.P.; Kolekar, S.S.; Dhas, S.D.; Patil, M.D.; Moholkar, A.V.; Sathe, B.R.; Ghule, A.V. Facile synthesis of flower-like Bi2O3 as an efficient electrode for high performance asymmetric supercapacitor. J. Alloys Compd. 2022, 926, 166722. [Google Scholar] [CrossRef]
- Huang, Z.; Song, Y.; Feng, D.; Sun, Z.; Sun, X.; Liu, X. High mass loading MnO2 with hierarchical nanostructures for supercapacitors. ACS Nano 2018, 12, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.A.; Das, H.T.; Guddeti, P.R.; Nallapureddy, R.R.; Pallavolu, M.R.; Alzahmi, S.; Obaidat, I.M. Self-supported Co3O4@Mo-Co3O4 needle-like nanosheet heterostructured architectures of battery-type electrodes for high-performance asymmetric supercapacitors. Nanomaterials 2022, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Anil Kumar, Y.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-dimensional core-shell structure of cobalt-doped@MnO2 nanosheets grown on nickel foam as a binder-free battery-type electrode for supercapacitor application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Zeng, Y.; Han, Y.; Zhao, Y.; Zeng, Y.; Yu, M.; Liu, Y.; Tang, H.; Tong, Y.; Lu, X. Advanced Ti-Doped Fe2O3@PEDOT core/shell anode for high-energy asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1402176. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Zhang, T.; Ouyang, L.; Yuan, S. Single-step preparation of ultrasmall iron oxide-embedded carbon nanotubes on carbon cloth with excellent superhydrophilicity and enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2021, 13, 45670–45678. [Google Scholar] [CrossRef]
- Sun, S.; Zhai, T.; Liang, C.; Savilov, S.V.; Xia, H. Boosted crystalline/amorphous Fe2O3-δ core/shell heterostructure for flexible solid-state pseudocapacitors in large scale. Nano Energy 2018, 45, 390–397. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Wu, Z.; Cui, G.; Xie, F.; Guo, X.; Sun, X. FeP nanorod arrays on carbon cloth: A high-performance anode for sodium-ion batteries. Chem. Commun. 2018, 54, 9341–9344. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Q.; Jia, K.; Wang, H.; Gao, J.; Xu, C.; Zhong, Y.; Alshehri, A.A.; Alzahrani, K.A.; Guo, X.; et al. Ni2P nanosheets on carbon cloth: An efficient flexible electrode for sodium-ion batteries. Inorg. Chem. 2019, 58, 6579–6583. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Sun, S.; Liu, X.; Liang, C.; Wang, G.; Xia, H. Achieving insertion-like capacity at ultrahigh rate via tunable surface pseudocapacitance. Adv. Mater. 2018, 30, 1706640. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, H.; Duan, Q.; Liu, Y.; Wang, Y.; Yuan, S. Phosphorus-functionalized low-crystallinity transition-metal oxide nanorod arrays grown on carbon cloth for high-performance asymmetric supercapacitors. Colloids Surf. A 2022, 654, 130189. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.; Tong, Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, J.; Wang, L.; Xiong, X.; Shao, Y.; Wang, G.; Wang, J.; Zhong, S.; Wu, M. PPy-encapsulated SnS2 nanosheets stabilized by defects on a TiO2 support as a durable anode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2019, 58, 811–815. [Google Scholar] [CrossRef]
- Shimoga, G.; Palem, R.; Choi, D.; Shin, E.; Ganesh, P.; Saratale, G.; Saratale, R.; Lee, S.; Kim, S. Polypyrrole-based metal nanocomposite electrode materials for high-performance supercapacitors. Metals 2021, 11, 905. [Google Scholar] [CrossRef]
- Yuan, L.; Yao, B.; Hu, B.; Huo, K.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Lai, L.; Yang, H.; Hua Lim, S.; Zhen, Y.; Yu, T.; Shen, Z.; Lin, J. Three dimensionals α-Fe2O3/polypyrrole (Ppy) nanoarray as anode for micro lithium ion batteries. Nano Energy 2013, 2, 726–732. [Google Scholar] [CrossRef]
- Zuo, W.; Zang, L.; Wang, X.; Liu, Q.; Qiu, J.; Liang, C.; Liu, X.; Yang, C. Flexible Polypyrrole@Fe2O3@stainless steel yarn composite electrode for symmetric thread-like supercapacitor with extended operating voltage window in Li2SO4-based aqueous electrolyte. Adv. Sustain. Syst. 2020, 4, 2000173. [Google Scholar] [CrossRef]
- Arbi, H.; Yadav, A.; Anil Kumar, Y.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I. Polypyrrole-assisted Ag doping strategy to boost Co(OH)2 nanosheets on Ni foam as a novel electrode for high-performance hybrid supercapacitors. Nanomaterials 2022, 12, 3982. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, M.; Pei, Z.; Huang, Y.; Geng, H.; Zhi, C. Extremely stable polypyrrole achieved via molecular ordering for highly flexible supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, Z.; Xiao, J.; Cen, W.; Yuan, S. Polypyrrole-encapsulated Fe2O3 nanotube arrays on a carbon cloth support: Achieving synergistic effect for enhanced supercapacitor performance. Electrochim. Acta 2021, 386, 138486. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Liu, X.; Zeng, R.; Li, M.; Huang, Y.; Hu, X. Constructing hierarchical tectorum-like α-Fe2O3/PPy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 2017, 56, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Zhang, T.C.; Xiang, G.; Wang, X.; Yuan, S. Removal of trace arsenite through simultaneous photocatalytic oxidation and adsorption by magnetic Fe3O4@PpPDA@TiO2 core–shell nanoparticles. ACS Appl. Nano Mater. 2020, 3, 8495–8504. [Google Scholar] [CrossRef]
- Wu, X.; Liu, R.; Zhao, J.; Fan, Z. Advanced carbon materials with different spatial dimensions for supercapacitors. Nano Mater. Sci. 2021, 3, 241–267. [Google Scholar] [CrossRef]
- Liu, H.; Huo, W.; Zhang, T.C.; Ouyang, L.; Yuan, S. Photocatalytic removal of tetracycline by a Z-scheme heterojunction of bismuth oxyiodide/exfoliated g-C3N4: Performance, mechanism, and degradation pathway. Mater. Today Chem. 2022, 23, 100729. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Zhang, T.C.; Xiang, G.; Wang, X.; Pehkonen, S.; Yuan, S. A magnetic γ-Fe2O3@PANI@TiO2 core–shell nanocomposite for arsenic removal via a coupled visible-light-induced photocatalytic oxidation–adsorption process. Nanoscale Adv. 2020, 2, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, X.; Chen, Q.; Zhang, T.C.; Ouyang, L.; Yuan, S. Simultaneous photocatalytic oxidation and adsorption for efficient As(III) removal by magnetic BiOI/γ-Fe2O3 core–shell nanoparticles. Mater. Today Chem. 2022, 24, 100823. [Google Scholar] [CrossRef]
- Ji, W.; Xiong, Y.; Wang, Y.; Zhang, T.C.; Yuan, S. Multilayered TNAs/SnO2/PPy/β-PbO2 anode achieving boosted electrocatalytic oxidation of As(III). J. Hazard. Mater. 2022, 430, 128449. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Yuan, S. N,S-containing polycondensate-derived porous carbon materials for superior CO2 adsorption and supercapacitor. Appl. Surf. Sci. 2021, 562, 150128. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Ouyang, L.; Yuan, S. Phytic acid-induced self-assembled chitosan gel-derived N, P–co-doped porous carbon for high-performance CO2 capture and supercapacitor. J. Power Sources 2022, 517, 230727. [Google Scholar] [CrossRef]
- Sui, S.; Sha, J.; Deng, X.; Zhu, S.; Ma, L.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Boosting the charge transfer efficiency of metal oxides/carbon nanotubes composites through interfaces control. J. Power Sources 2021, 489, 229501. [Google Scholar] [CrossRef]
- Cai, D.; Du, J.; Zhu, C.; Cao, Q.; Huang, L.; Wu, J.; Zhou, D.; Xia, Q.; Chen, T.; Guan, C.; et al. Iron oxide nanoneedles anchored on N-doped carbon nanoarrays as an electrode for high-performance hybrid supercapacitor. ACS Appl. Energy Mater. 2020, 3, 12162–12171. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Ko, T.; Kim, B. Rational design of binder-free ZnCo2O4 and Fe2O3 decorated porous 3D Ni as high-performance electrodes for asymmetric supercapacitor. Ceram. Int. 2018, 44, 10635–10645. [Google Scholar] [CrossRef]
- Liang, H.; Xia, C.; Emwas, A.; Anjum, D.; Miao, X.; Alshareef, H. Phosphine plasma activation of α-Fe2O3 for high energy asymmetric supercapacitors. Nano Energy 2018, 49, 155–162. [Google Scholar] [CrossRef]
- Sun, J.; Huang, Y.; Fu, C.; Huang, Y.; Zhu, M.; Tao, X.; Zhi, C.; Hu, H. A high performance fiber-shaped PEDOT@MnO2//C@Fe3O4 asymmetric supercapacitor for wearable electronics. J. Mater. Chem. A 2016, 4, 14877–14883. [Google Scholar] [CrossRef]
- Lu, X.; Chen, X.; Zhou, W.; Tong, Y.; Li, G. α-Fe2O3@PANI Core–shell nanowire arrays as negative electrodes for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar] [CrossRef]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.; Wang, Z. Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Let. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Pei, Z.; Lv, M.; Huang, D.; Wang, Y.; Yuan, S. Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance. Molecules 2023, 28, 434. https://doi.org/10.3390/molecules28010434
Wu C, Pei Z, Lv M, Huang D, Wang Y, Yuan S. Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance. Molecules. 2023; 28(1):434. https://doi.org/10.3390/molecules28010434
Chicago/Turabian StyleWu, Chunhui, Zifan Pei, Menglin Lv, Duchen Huang, Yuan Wang, and Shaojun Yuan. 2023. "Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance" Molecules 28, no. 1: 434. https://doi.org/10.3390/molecules28010434
APA StyleWu, C., Pei, Z., Lv, M., Huang, D., Wang, Y., & Yuan, S. (2023). Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance. Molecules, 28(1), 434. https://doi.org/10.3390/molecules28010434






