Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Solubility of GEF in Liquid Oils
2.2. Effect of Independent Variables on the Responses
2.2.1. PS
2.2.2. PDI
2.2.3. ZP
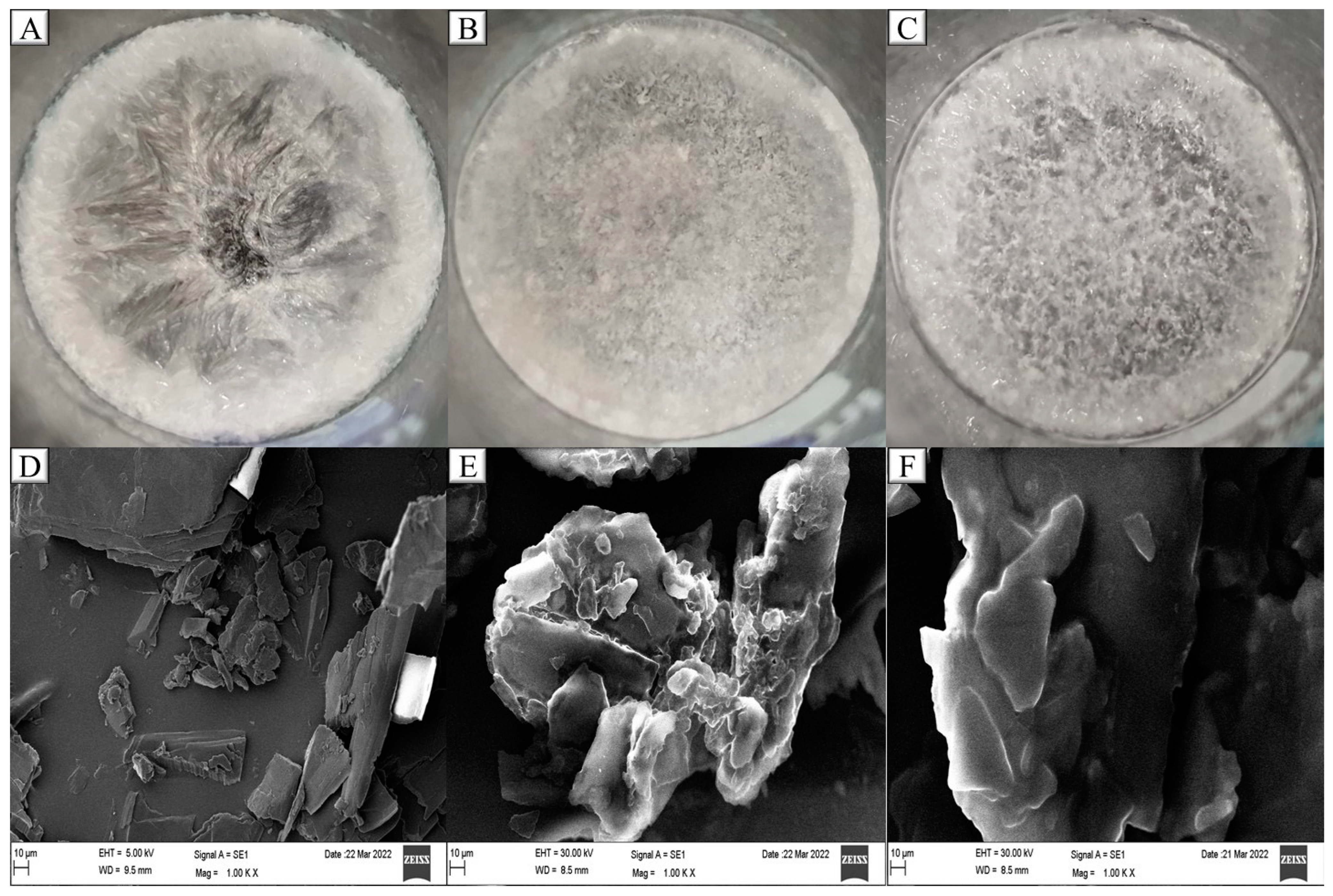
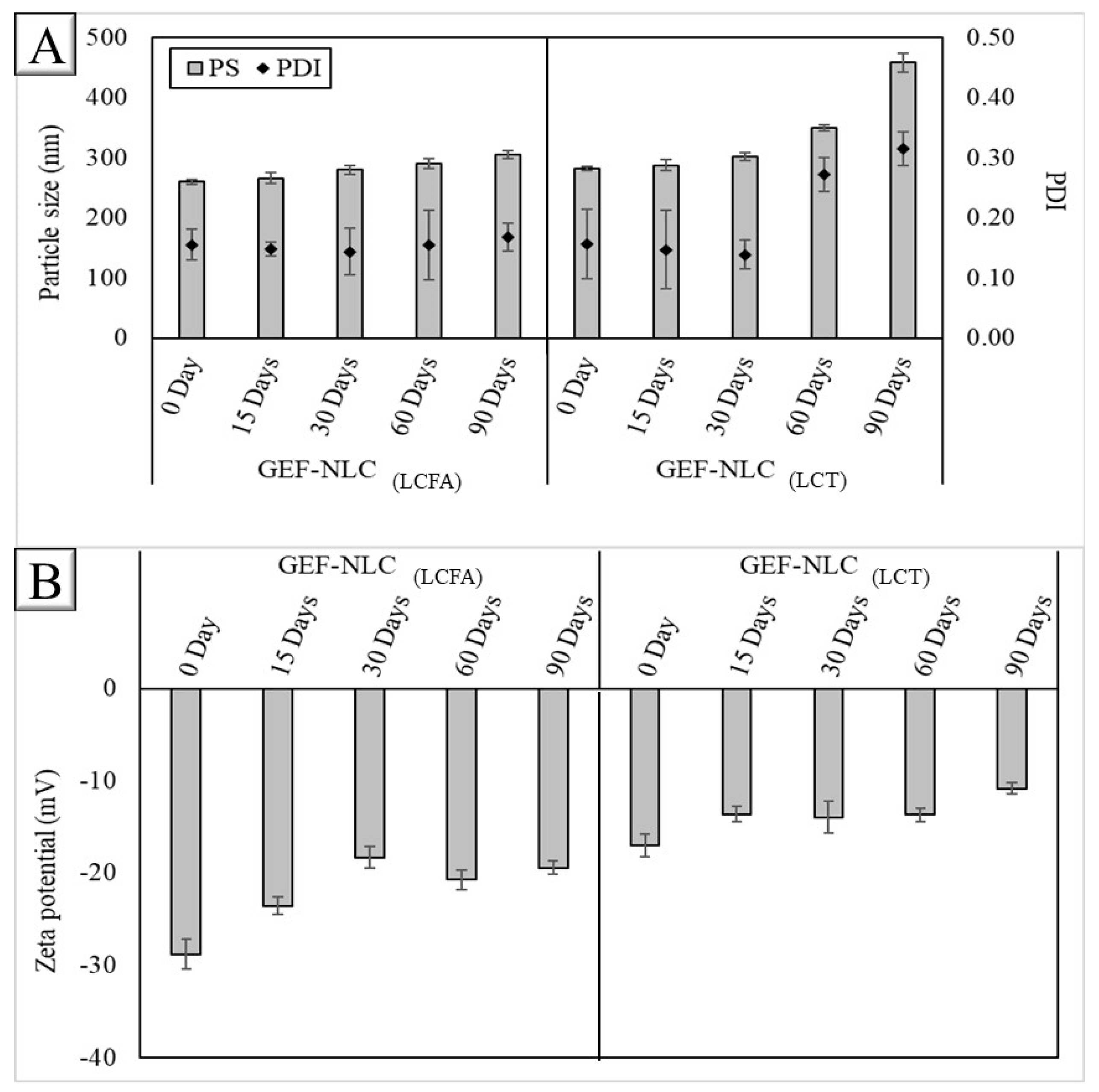
2.3. Stability of Plain NLC Formulations
2.4. Selection of the Optimum Formulation and Validation of DOE
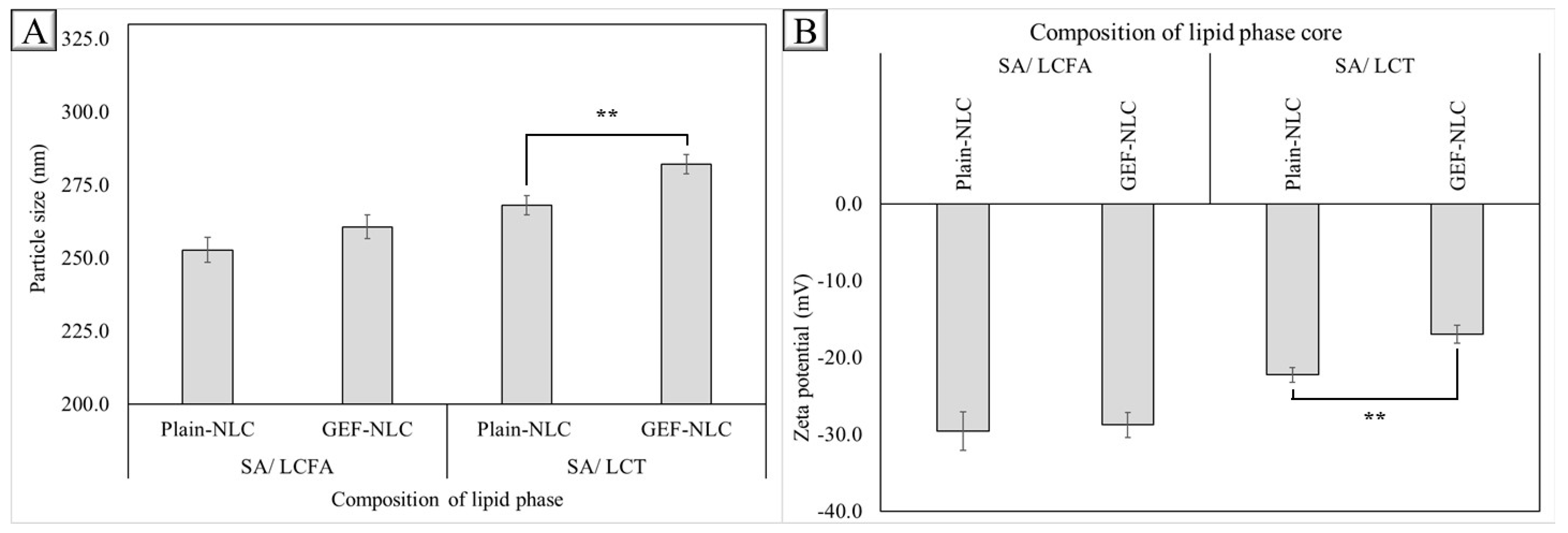
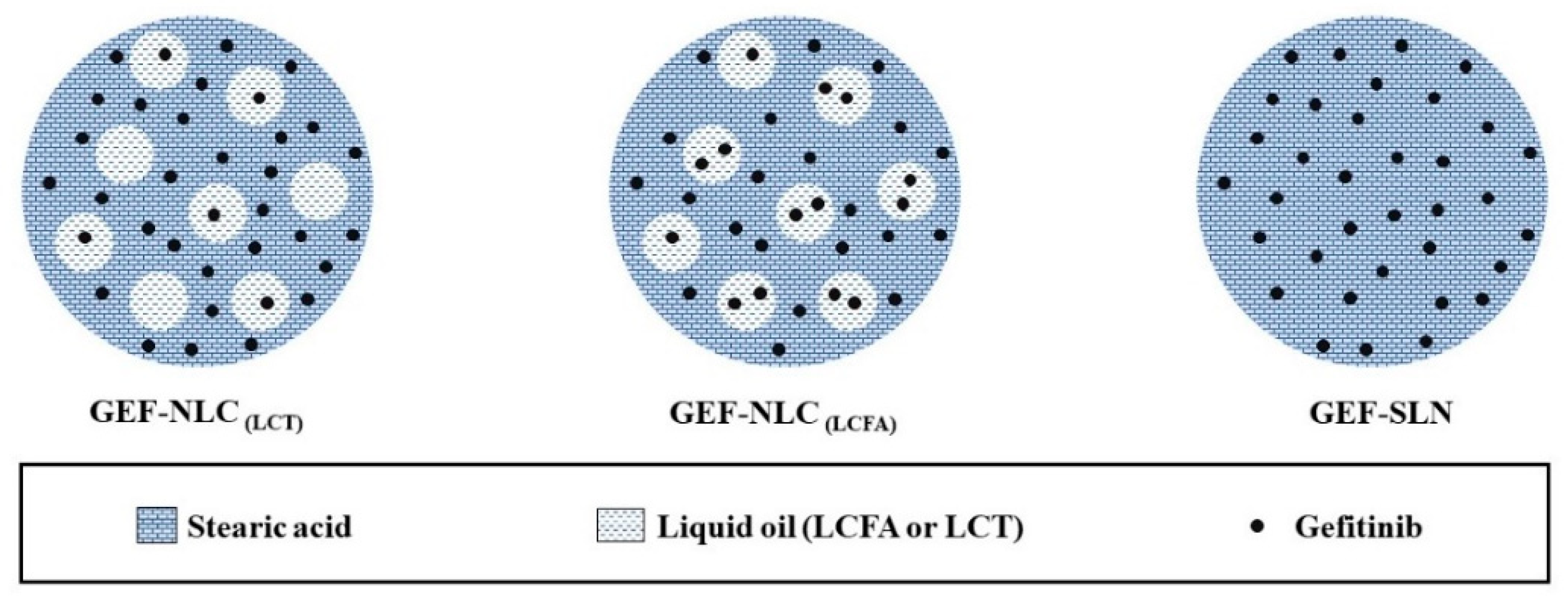
2.5. Effect of Drug Loading
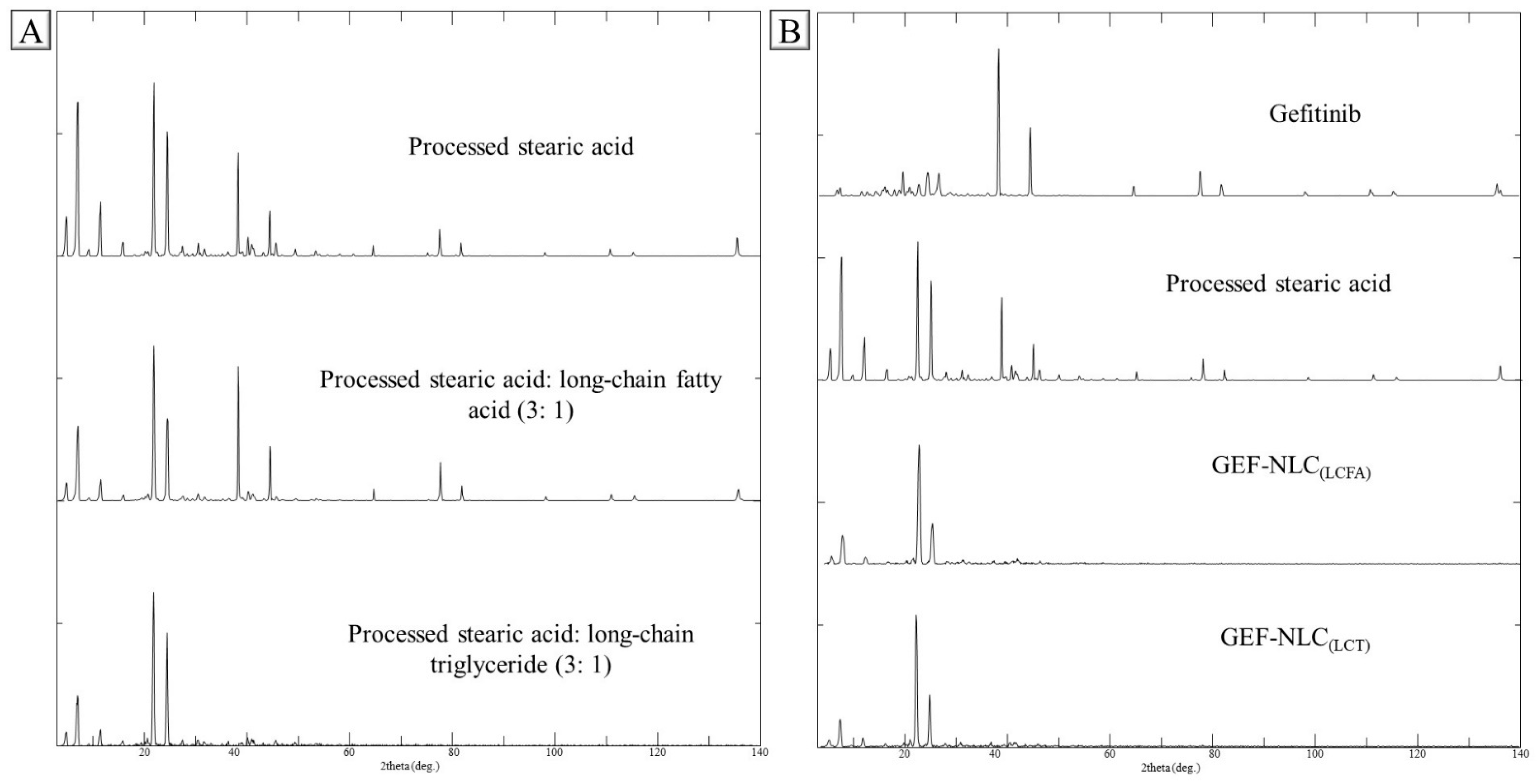
2.6. PXRD
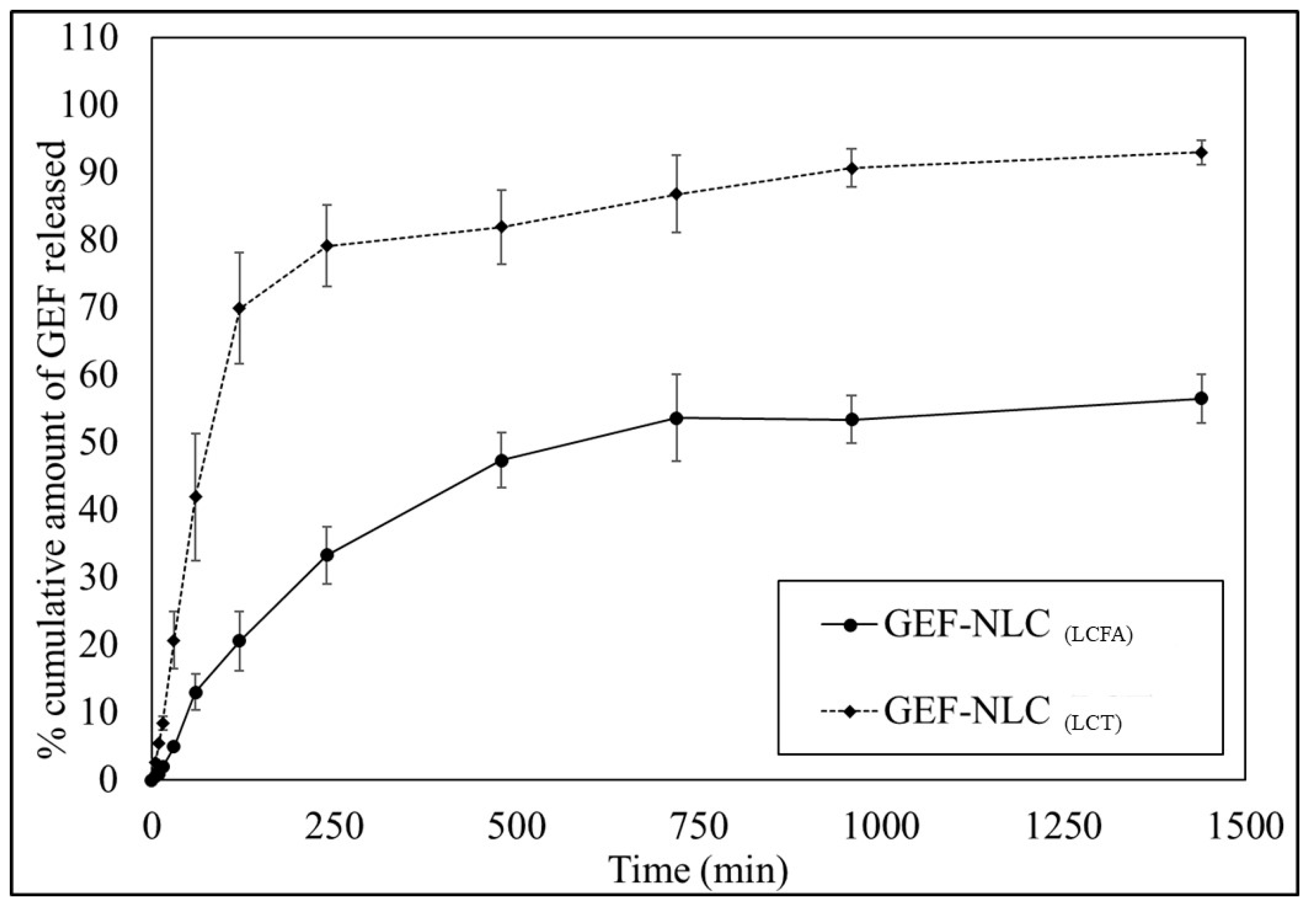
2.7. In Vitro Release
2.8. Stability of GEF-NLC Formulations
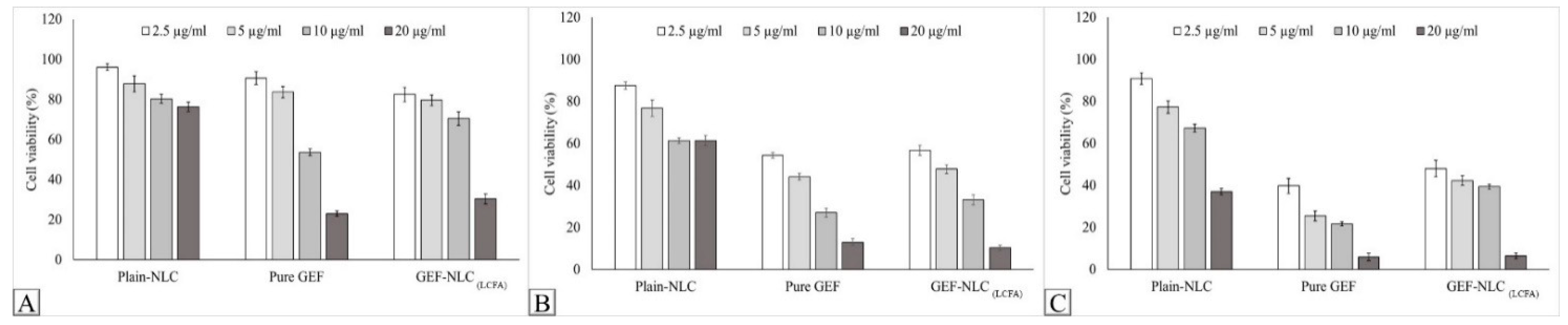
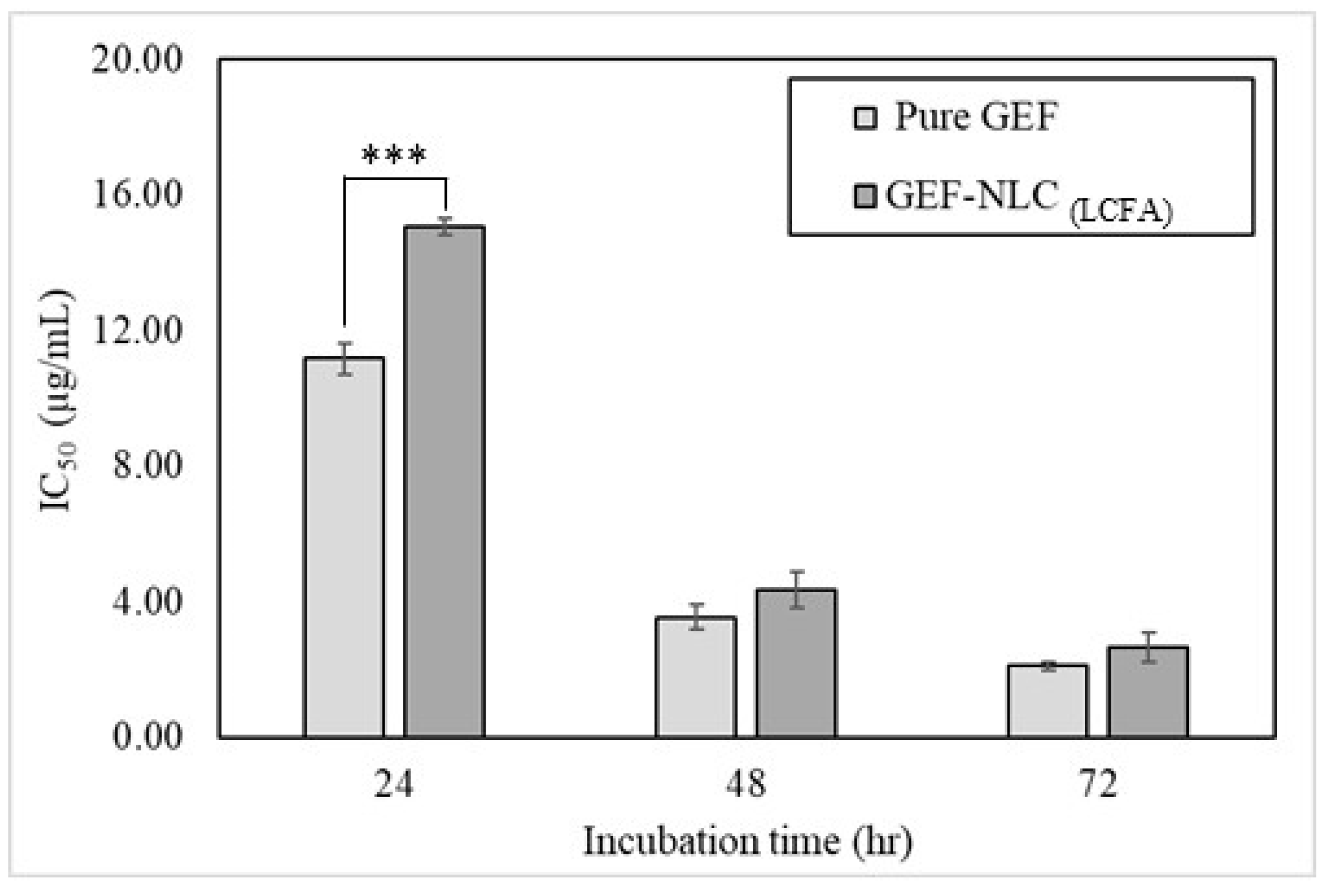
2.9. In Vitro Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Solubility Study of GEF in Liquid Oils
3.3. Design of Experiments (DOE)
3.4. Preparation of Plain NLC and GEF-NLC
3.5. Physicochemical Characterization
3.5.1. PS, PDI, and ZP
3.5.2. PXRD
3.5.3. Drug Content
3.5.4. Entrapment Efficiency (EE)
3.6. In Vitro Release
3.7. Stability Study
3.8. In Vitro Cytotoxicity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, Y.; Yuan, X.; Gao, H.; Yang, Q. Nanoformulations of small molecule protein tyrosine kinases inhibitors potentiate targeted cancer therapy. Int. J. Pharm. 2020, 573, 118785. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.Y.; Harisa, G.I.; Alanazi, F.K.; Nasr, F.A.; Alqahtani, A.S. PEGylated SLN as a Promising Approach for Lymphatic Delivery of Gefitinib to Lung Cancer. Int. J. Nanomed. 2022, 17, 3287. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines. J. Exp. Nanosci. 2022, 17, 214–246. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Li, X.; Zhou, Y.; Wang, H.; Zhang, Y. Uniform carboxymethyl chitosan-enveloped Pluronic F68/poly (lactic-co-glycolic acid) nano-vehicles for facilitated oral delivery of gefitinib, a poorly soluble antitumor compound. Colloids Surf. B Biointerfaces 2019, 177, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.Y.; Harisa, G.I.; Alanazi, F.K.; Youssof, A.M. Engineering of exosomes: Steps towards green production of drug delivery system. Curr. Drug Targets 2019, 20, 1537–1549. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Zhao, Q.; Pan, T.; Zhong, H.; Wang, W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics 2021, 13, 1151. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Verma, A.; Saharan, V.A. Lipid Drug Carriers for Cancer Therapeutics: An Insight into Lymphatic Targeting, P-gp, CYP3A4 Modulation and Bioavailability Enhancement. Adv. Pharm. Bull. 2020, 10, 524–541. [Google Scholar] [CrossRef]
- Patel, P.; Patel, M. Enhanced oral bioavailability of nintedanib esylate with nanostructured lipid carriers by lymphatic targeting: In vitro, cell line and in vivo evaluation. Eur. J. Pharm. Sci. 2021, 159, 105715. [Google Scholar] [CrossRef]
- Nayek, S.; Raghavendra, N.; Kumar, B.S. Development of novel S PC-3 gefitinib lipid nanoparticles for effective drug delivery in breast cancer. Tissue distribution studies and cell cytotoxicity analysis. J. Drug Deliv. Sci. Technol. 2021, 61, 102073. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, N.; Gupta, S. Implications of designing clarithromycin loaded solid lipid nanoparticles on their pharmacokinetics, antibacterial activity and safety. RSC Adv. 2016, 6, 76621–76631. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.-H.; Fayez, N.A.; Hohenwarter, L.; Li, S.-D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-based oral formulation strategies for lipophilic drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Shao, J.; Tan, B.; Guan, S.; Liu, Z.; Zhao, Z.; He, F.; Zhao, J. Targeted lung cancer therapy: Preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int. J. Nanomed. 2015, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Makoni, P.A.; Wa Kasongo, K.; Walker, R.B. Short term stability testing of efavirenz-loaded solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Fathi, H.A.; Allam, A.; Elsabahy, M.; Fetih, G.; El-Badry, M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf. B Biointerfaces 2018, 162, 236–245. [Google Scholar] [CrossRef]
- Moradpour, Z.; Barghi, L. Novel approaches for efficient delivery of tyrosine kinase inhibitors. J. Pharm. Pharm. Sci. 2019, 22, 37–48. [Google Scholar] [CrossRef]
- Dhairyasheel, G.; Adhikrao, Y.; Varsha, G. Design and development of solid self-microemulsifying drug delivery of gefitinib. Asian J. Pharm. Technol. 2018, 8, 193–199. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS PharmSciTech 2012, 13, 967–977. [Google Scholar] [CrossRef]
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2018, 55, 287–298. [Google Scholar] [CrossRef]
- Moghddam, S.M.M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Optimization of nanostructured lipid carriers for topical delivery of nimesulide using Box–Behnken design approach. Artif. Cells Nanomed. Biotechnol. 2017, 45, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, N.; Hingarh, P.K.; Arun, R.; Selvaraj, J.; Anbarasan, A.; Sathianarayanan, S.; Nagaraju, G. Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int. J. Biol. Macromol. 2018, 110, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.; Patil-Gadhe, A.; Kaur, G. Physicochemical and pharmacokinetic evaluation of rosuvastatin loaded nanostructured lipid carriers: Influence of long-and medium-chain fatty acid mixture. J. Pharm. Investig. 2018, 48, 465–476. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of food grade nanostructured lipid carrier (NLC) for potential applications in medicinal-functional foods. J. Drug Deliv. Sci. Technol. 2017, 39, 50–58. [Google Scholar] [CrossRef]
- Rigon, R.B.; Gonçalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef]
- Shahzadi, I.; Fürst, A.; Knoll, P.; Bernkop-Schnürch, A. Nanostructured Lipid Carriers (NLCs) for Oral Peptide Drug Delivery: About the Impact of Surface Decoration. Pharmaceutics 2021, 13, 1312. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int. J. Pharm. 2011, 415, 232–243. [Google Scholar] [CrossRef]
- Kraisit, P.; Sarisuta, N. Development of triamcinolone acetonide-loaded nanostructured lipid carriers (NLCs) for buccal drug delivery using the Box-Behnken design. Molecules 2018, 23, 982. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Badran, M.M.; Ali, H.M.; Abdel-Bakky, M.S.; Ibrahim, H.M. Multifunctional carbamazepine loaded nanostructured lipid carrier (NLC) formulation. Int. J. Pharm. 2018, 550, 359–371. [Google Scholar] [CrossRef]
- Yang, Y.; Corona, A., III; Schubert, B.; Reeder, R.; Henson, M.A. The effect of oil type on the aggregation stability of nanostructured lipid carriers. J. Colloid Interface Sci. 2014, 418, 261–272. [Google Scholar] [CrossRef]
- Shahba, A.A.; Tashish, A.Y.; Alanazi, F.K.; Kazi, M. Combined self-nanoemulsifying and solid dispersion systems showed enhanced cinnarizine release in hypochlorhydria/achlorhydria dissolution model. Pharmaceutics 2021, 13, 627. [Google Scholar] [CrossRef]
- Galvao, J.G.; Trindade, G.G.; Santos, A.J.; Santos, R.L.; Chaves Filho, A.B.; Lira, A.A.M.; Miyamoto, S.; Nunes, R.S. Effect of Ouratea sp. butter in the crystallinity of solid lipids used in nanostructured lipid carriers (NLCs). J. Therm. Anal. Calorim. 2016, 123, 941–948. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, W.; Li, Y.; Liu, G. Influence of different solid lipids on the properties of a novel nanostructured lipid carrier containing Antarctic krill oil. Int. J. Food Sci. Technol. 2022, 57, 2886–2895. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Fülöp, Z.; Ritthidej, G.C. Amphotericin B loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): Physicochemical and solid-solution state characterizations. Drug Dev. Ind. Pharm. 2019, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.S.; Patel, M.A.; Desai, D.T.; Patel, H.P.; Gupta, A.R.; Joshi, S.V.; Shah, D.O.; Maulvi, F.A. Bioavailability enhancement of repaglinide from transdermally applied nanostructured lipid carrier gel: Optimization, in vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101731. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, F.; Liu, W.; Wan, J.; Wan, C.; Xu, H.; Lam, C.W.; Yang, X. Nanostructured lipid carriers as a novel oral delivery system for triptolide: Induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int. J. Nanomed. 2014, 9, 1049. [Google Scholar]
- Thatipamula, R.; Palem, C.; Gannu, R.; Mudragada, S.; Yamsani, M. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 23–32. [Google Scholar]
- Rohilla, S.; Awasthi, R.; Mehta, M.; Chellappan, D.K.; Gupta, G.; Gulati, M.; Singh, S.K.; Anand, K.; Oliver, B.G.; Dua, K.; et al. Preparation and Evaluation of Gefitinib Containing Nanoliposomal Formulation for Lung Cancer Therapy. BioNanoScience 2022, 12, 241–255. [Google Scholar] [CrossRef]
- Ni, X.L.; Chen, L.X.; Zhang, H.; Yang, B.; Xu, S.; Wu, M.; Liu, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; et al. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017, 24, 1501–1512. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Sultan, M.H.; Albarraq, A.A.; Ahmed, R.A.; Alhazmi, H.A.; Alam, M.I. Preparation, Characterization, and Anti-Cancer Activity of Nanostructured Lipid Carriers Containing Imatinib. Pharmaceutics 2021, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater. Sci. Eng. C 2013, 33, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Goos, P. I-optimal versus D-optimal split-plot response surface designs. J. Qual. Technol. 2012, 44, 85–101. [Google Scholar] [CrossRef]
- Buya, A.B.; Terrasi, R.; Mbinze, J.K.; Muccioli, G.G.; Beloqui, A.; Memvanga, P.B.; Préat, V. Quality-by-Design-Based Development of a Voxelotor Self-Nanoemulsifying Drug-Delivery System with Improved Biopharmaceutical Attributes. Pharmaceutics 2021, 13, 1388. [Google Scholar] [CrossRef] [PubMed]
- Valicherla, G.R.; Dave, K.M.; Syed, A.A.; Riyazuddin, M.; Gupta, A.P.; Singh, A.; Wahajuddin, M.K.; Datta, D.; Gayen, J.R. Formulation optimization of Docetaxel loaded self-emulsifying drug delivery system to enhance bioavailability and anti-tumor activity. Sci. Rep. 2016, 6, 26895. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Plakogiannis, F.M. Development and oral bioavailability assessment of a supersaturated self-microemulsifying drug delivery system (SMEDDS) of albendazole. J. Pharm. Pharmacol. 2010, 62, 1112–1120. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Sherif, A.Y.; Elzayat, E.M.; Kazi, M. Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs). Pharmaceuticals 2022, 15, 1120. [Google Scholar] [CrossRef]
- Harisa, G.I.; Badran, M.M. Simvastatin nanolipid carriers decreased hypercholesterolemia induced cholesterol inclusion and phosphatidylserine exposure on human erythrocytes. J. Mol. Liq. 2015, 208, 202–210. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Harisa, G.I.; Alanazi, F.K.; Nasr, F.A.; Alqahtani, A.S. Engineered Nanoscale Lipid-Based Formulation as Potential Enhancer of Gefitinib Lymphatic Delivery: Cytotoxicity and Apoptotic Studies Against the A549 Cell Line. AAPS PharmSciTech 2022, 23, 183. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef]
- Srinivas, N.S.K.; Verma, R.; Kulyadi, G.P.; Kumar, L. A quality by design approach on polymeric nanocarrier delivery of gefitinib: Formulation, in vitro, and in vivo characterization. Int. J. Nanomed. 2017, 12, 15–28. [Google Scholar] [CrossRef] [PubMed]

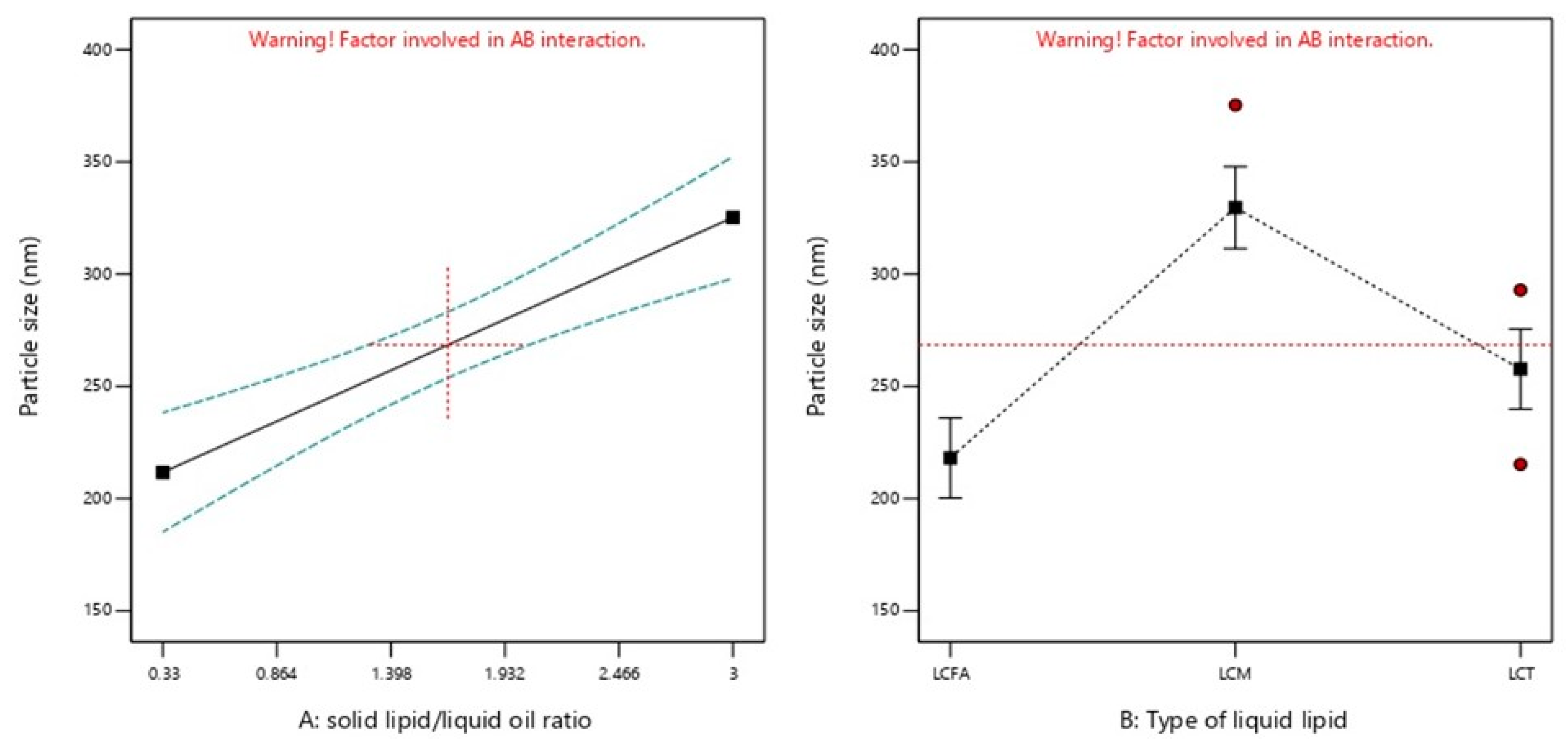
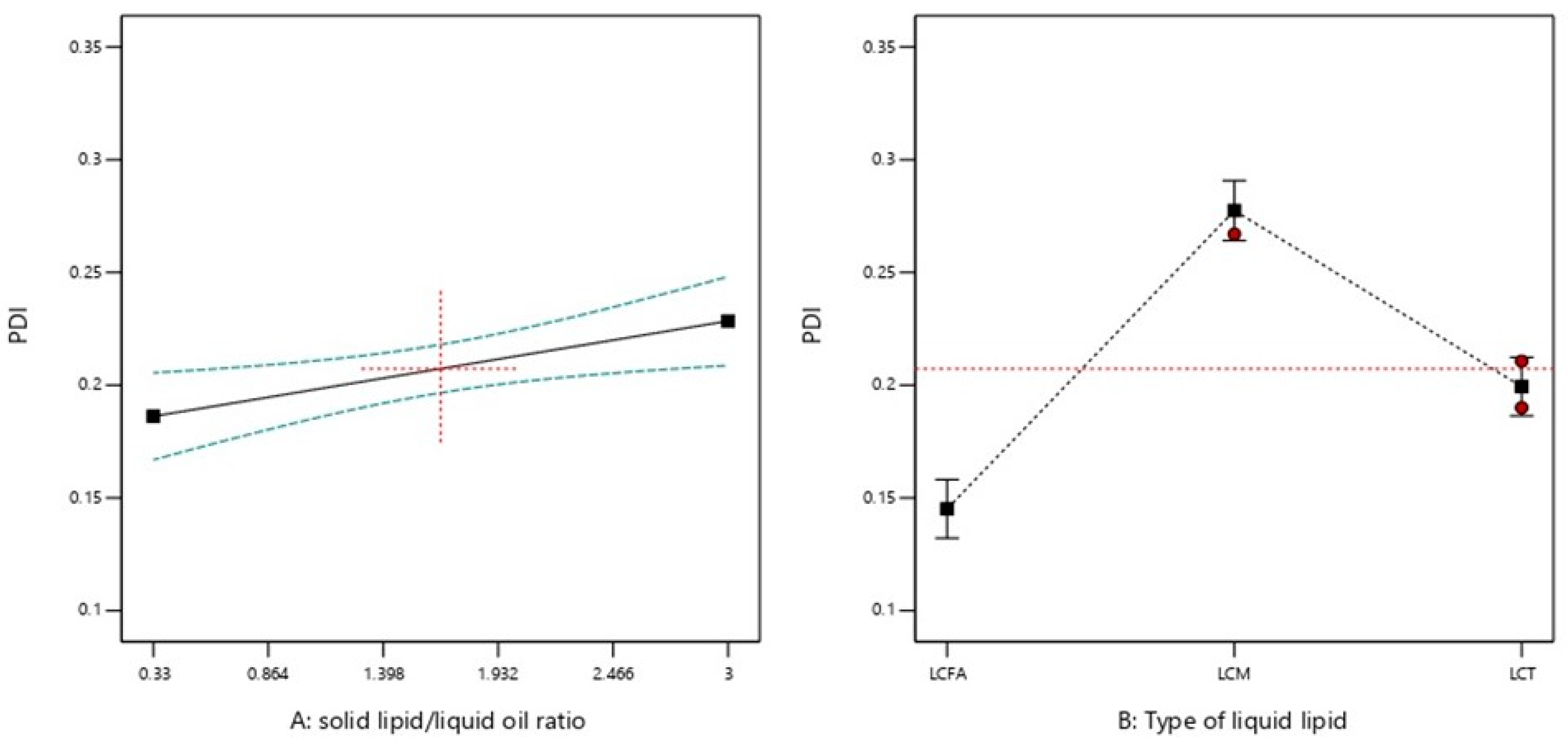
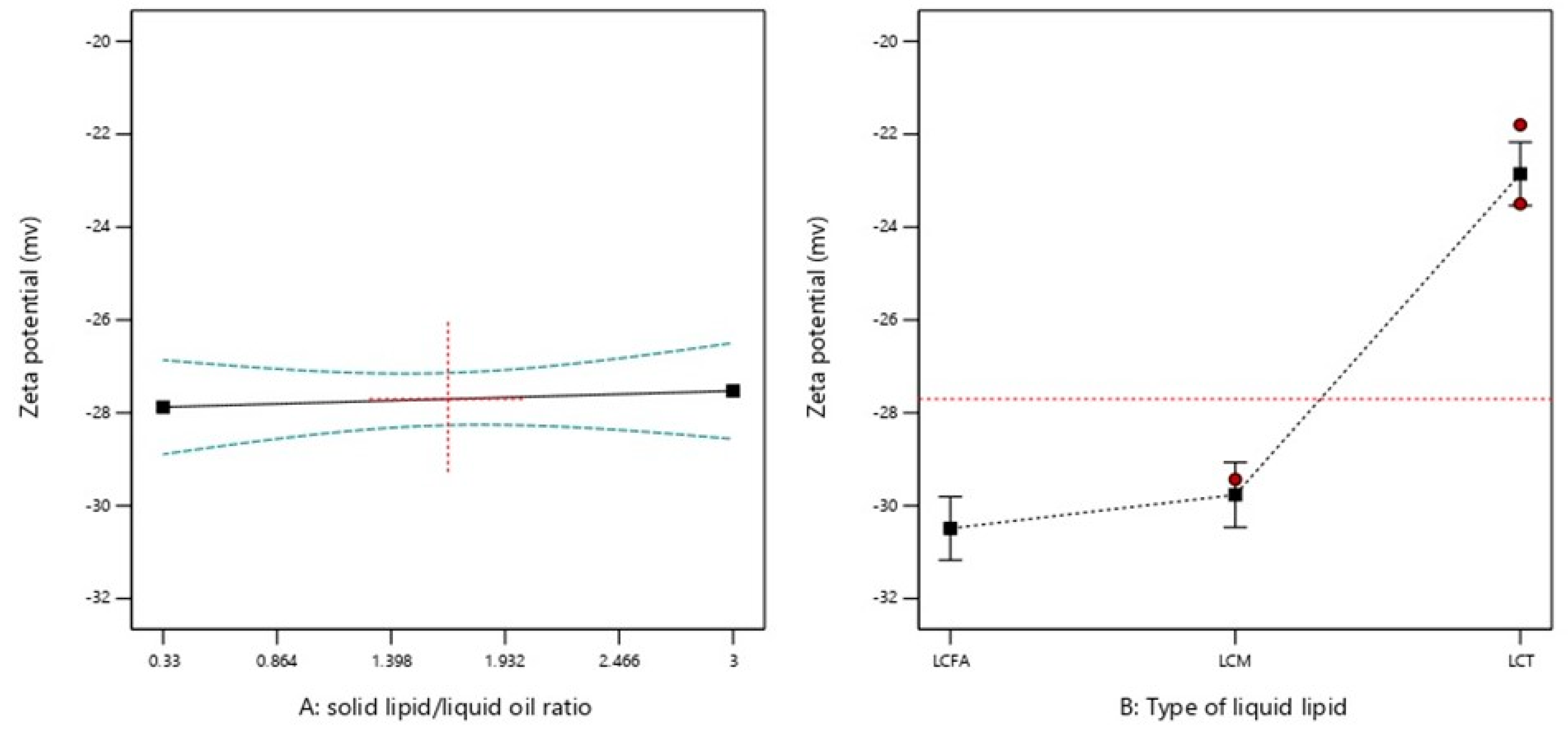
| Formulation Code | Factors | Responses | ||||
|---|---|---|---|---|---|---|
| X1: Solid Lipid: Liquid Lipid Ratio (SL/LO) | X2: Type of Liquid Lipid | Y1: PS (nm) | Y2: PDI | Y3: ZP (mv) | Y4: Aggregation upon Storage | |
| 9 | 0.33 | LCFA | 189.6 | 0.139 | −31.1 | Yes |
| 8 | 0.70 | LCFA | 207.7 | 0.129 | −29.8 | Yes |
| 5 | 1.68 | LCFA | 216.1 | 0.134 | −31.1 | No |
| 12 | 1.68 | LCFA | 229.6 | 0.152 | −28.9 | No |
| 4 | 2.65 | LCFA | 235.3 | 0.162 | −31.7 | No |
| 7 | 2.65 | LCFA | 225.6 | 0.15 | −30.4 | No |
| 1 | 0.68 | LCM | 235.1 | 0.262 | −31.4 | Yes |
| 14 | 0.68 | LCM | 244.5 | 0.248 | −29.5 | Yes |
| 17 | 1.67 | LCM | 375.3 | 0.267 | −29.4 | Yes |
| 3 | 2.63 | LCM | 390.6 | 0.261 | −30.4 | Yes |
| 6 | 2.63 | LCM | 399.1 | 0.349 | −28.1 | Yes |
| 13 | 0.33 | LCT | 220.7 | 0.175 | −22.7 | No |
| 10 | 1.00 | LCT | 219.5 | 0.195 | −23.8 | No |
| 15 | 1.67 | LCT | 292.9 | 0.211 | −23.5 | No |
| 16 | 1.67 | LCT | 215.2 | 0.19 | −21.8 | No |
| 11 | 2.33 | LCT | 318 | 0.215 | −21.9 | No |
| 2 | 3.00 | LCT | 279.9 | 0.211 | −23.4 | No |
| Response | Selected Model | Degree of Freedom | Adjusted R2 | Predicted R2 | F-Value | p-Value |
|---|---|---|---|---|---|---|
| PS | 2FI | 2 | 0.8346 | 0.7771 | 5.97 | 0.0175 |
| PDI | Linear | 3 | 0.8838 | 0.8246 | 35.91 | <0.0001 |
| ZP | Linear | 3 | 0.9184 | 0.8868 | 61.03 | <0.0001 |
| Response | X1: p-Value of Solid Lipid: Liquid Oil Ratio | X2: p-Value of Type of Liquid Lipid |
|---|---|---|
| PS | 0.0003 | 0.0001 |
| PDI | 0.0151 | <0.0001 |
| ZP | 0.6680 | <0.0001 |
| Response | n | SD | Predicted Mean | SE Pred | 95% PI Low | Data Mean | 95% PI High |
|---|---|---|---|---|---|---|---|
| PS | 3 | 27.40 | 238.89 | 26.2 | 181.3 | 252.7 | 296.5 |
| PDI | 3 | 0.023 | 0.162634 | 0.018 | 0.123 | 0.183 | 0.202 |
| ZP | 3 | 1.068 | −30.314 | 0.86 | −32.2 | −29.6 | −28.5 |
| Response | n | SD | Predicted Mean | SE Pred | 95% PI Low | Data Mean | 95% PI High |
|---|---|---|---|---|---|---|---|
| PS | 3 | 27.40 | 301.1 | 26.0 | 243.8 | 268.1 | 358.3 |
| PDI | 3 | 0.023 | 0.220 | 0.016 | 0.185 | 0.193 | 0.256 |
| ZP | 3 | 1.068 | −22.68 | 0.85 | −24.5 | −22.3 | −20.8 |
| Formulation | Drug Content (mg/mL) | EE (%) |
|---|---|---|
| GEF-NLC(LCFA) | 2.13 ± 0.48 | 94.48 ± 2.14 |
| GEF-NLC(LCT) | 2.09 ± 0.75 | 91.94 ± 3.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherif, A.Y.; Harisa, G.I.; Shahba, A.A.; Alanazi, F.K.; Qamar, W. Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer. Molecules 2023, 28, 448. https://doi.org/10.3390/molecules28010448
Sherif AY, Harisa GI, Shahba AA, Alanazi FK, Qamar W. Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer. Molecules. 2023; 28(1):448. https://doi.org/10.3390/molecules28010448
Chicago/Turabian StyleSherif, Abdelrahman Y., Gamaleldin I. Harisa, Ahmad A. Shahba, Fars K. Alanazi, and Wajhul Qamar. 2023. "Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer" Molecules 28, no. 1: 448. https://doi.org/10.3390/molecules28010448
APA StyleSherif, A. Y., Harisa, G. I., Shahba, A. A., Alanazi, F. K., & Qamar, W. (2023). Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer. Molecules, 28(1), 448. https://doi.org/10.3390/molecules28010448






