Effect of Loblolly Pine (Pinus taeda L.) Hemicellulose Structure on the Properties of Hemicellulose-Polyvinyl Alcohol Composite Film
Abstract
1. Introduction
2. Results and Discussion
2.1. Yields of Hemicellulosic Fractions Precipitated by Different Ethanol Concentrations
2.2. Chemical Composition of Alkali-Soluble Hemicellulosic Fractions
2.3. Molecular Weight Analysis
2.4. FT-IR Spectra Analysis
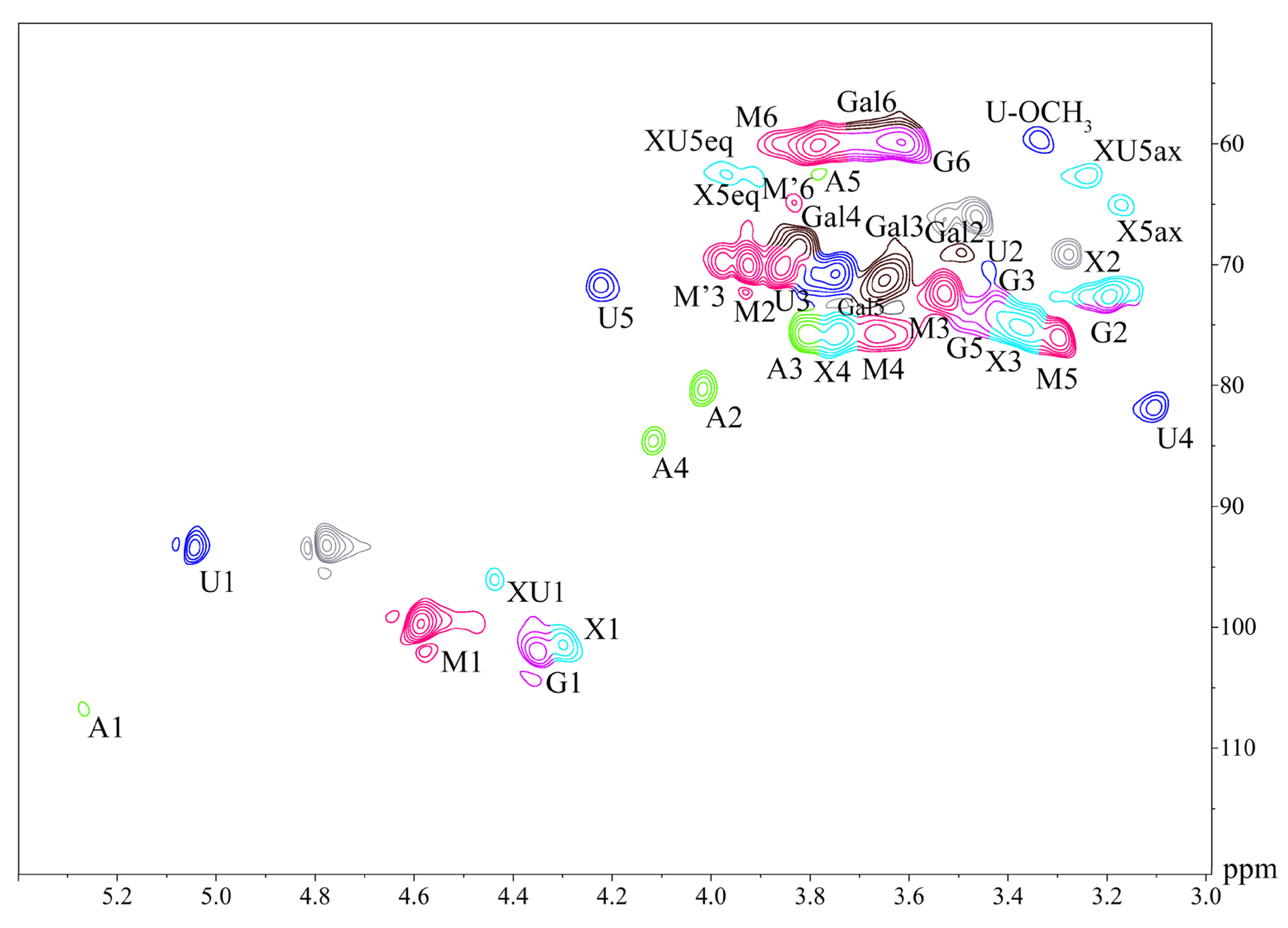
2.5. HSQC NMR Analysis
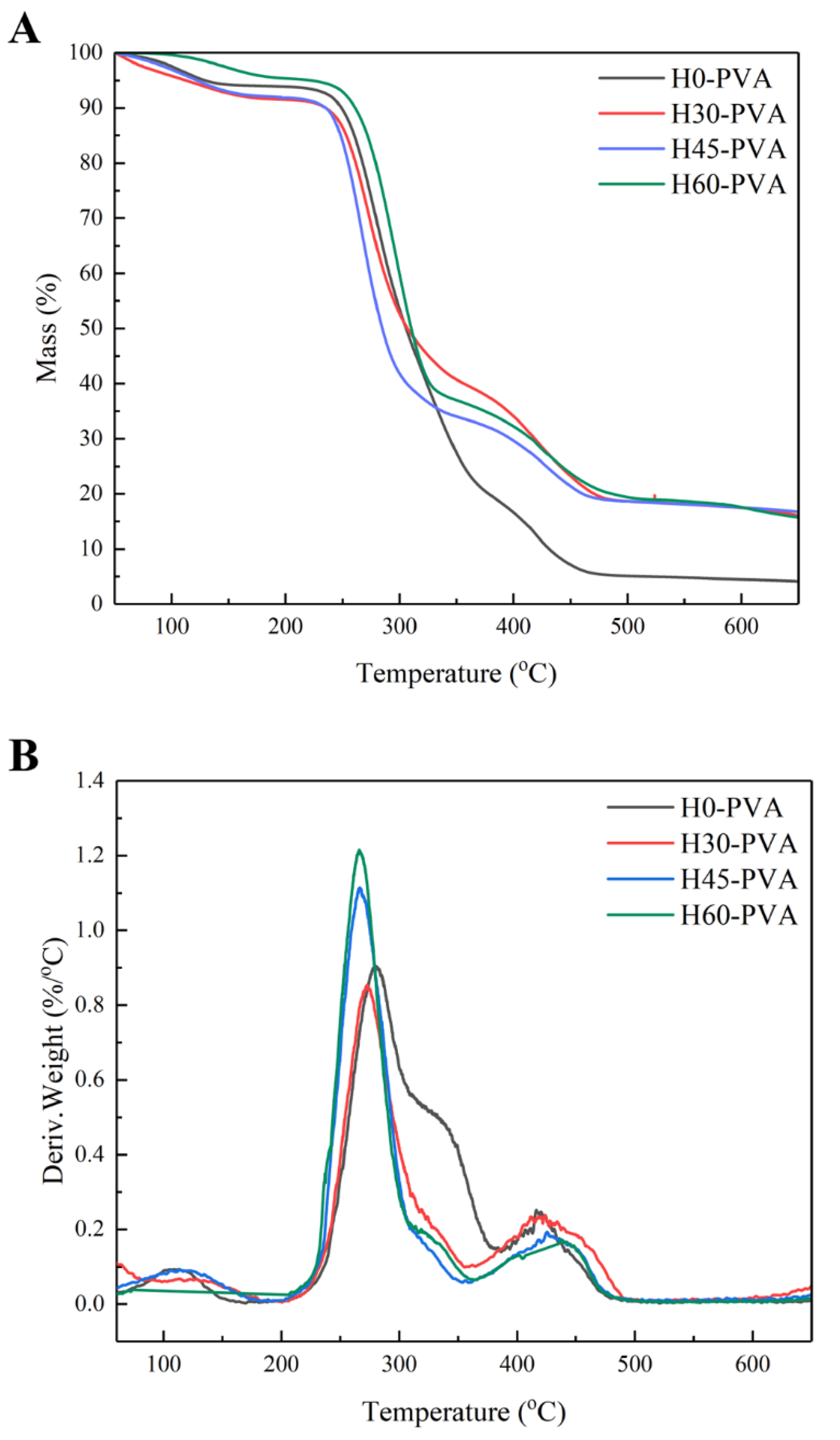
2.6. Thermal Stability Analysis of Hemicellulose
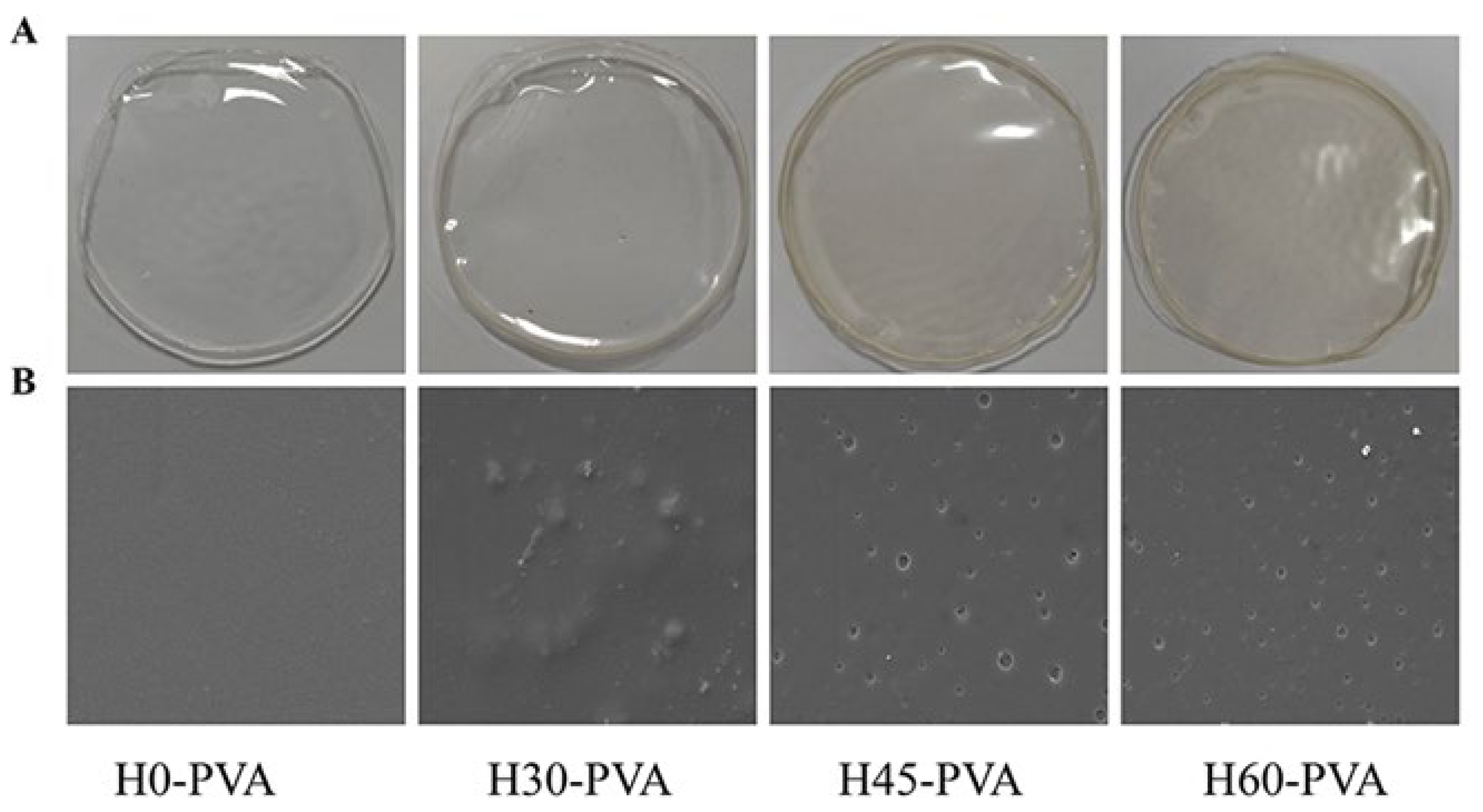
2.7. Morphological Observation of Composite Film
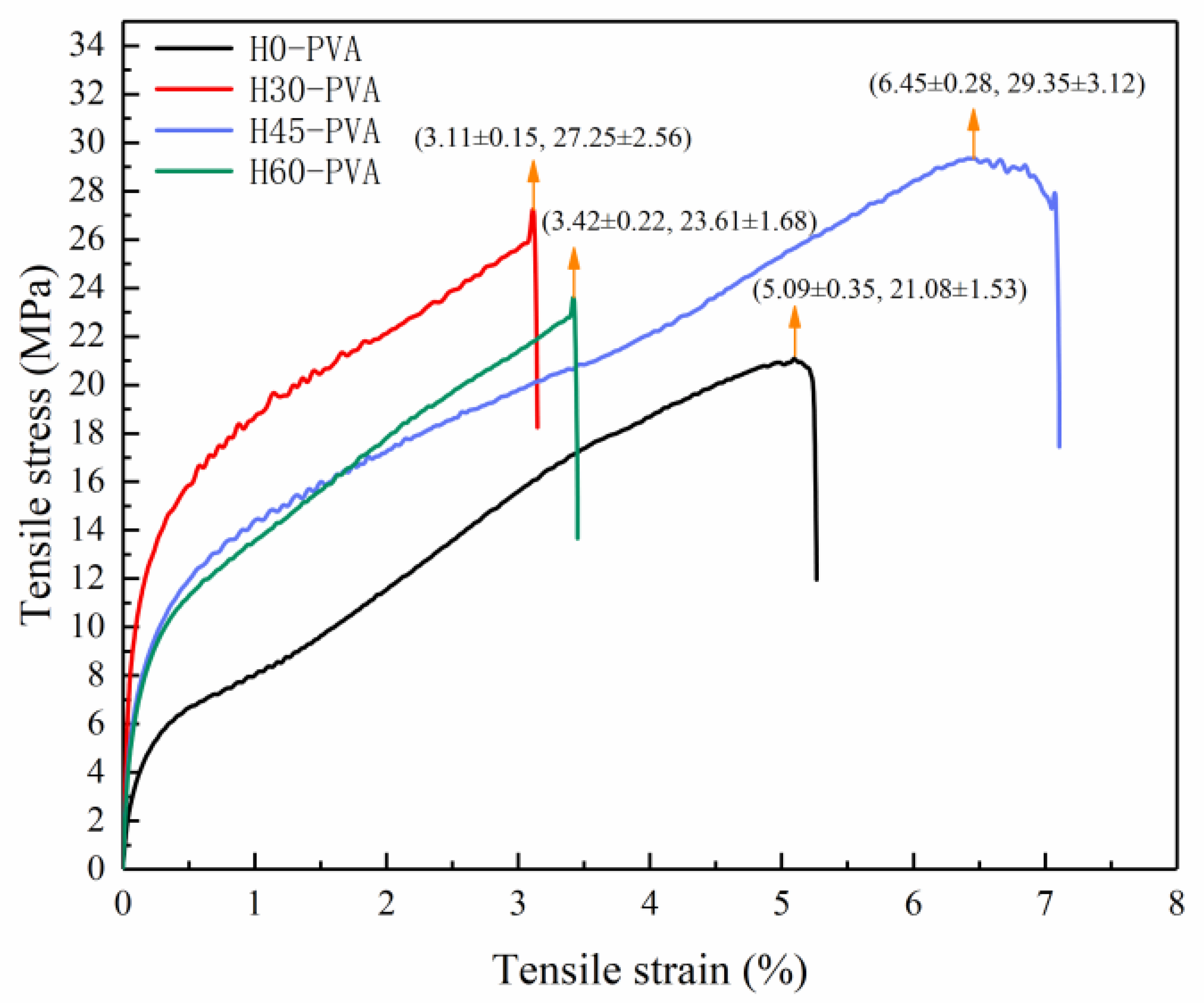
2.8. Mechanical Properties of Composite Film
2.9. Transparency of Composite Film
2.10. Thermal Stability of Composite Films
3. Materials and Methods
3.1. Materials
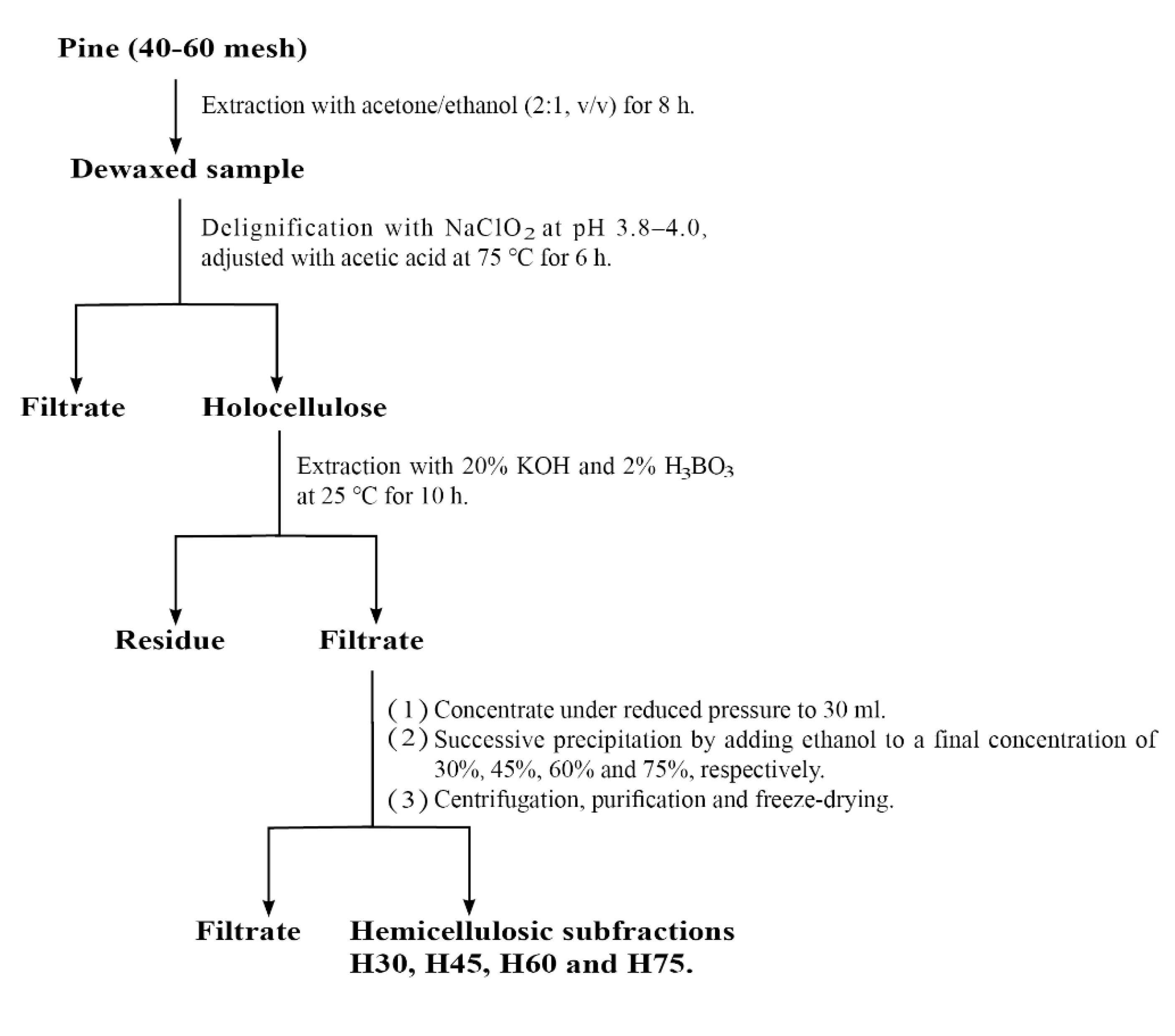
3.2. Extraction and Fractionation of Hemicellulose
3.3. Characteristics of Alkali-Soluble Hemicellulosic Fractions
3.4. Preparation of Hemicellulose-PVA Composite Film
3.5. Characteristics of Composite Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T. Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef]
- Wu, A.; Zhao, X.; Xie, Q.; Xie, X. Research progress in glucuronoxylan biosynthesis. J. South China Agric. Univ. 2015, 36, 1–10. [Google Scholar]
- Gille, S.; Pauly, M. O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 2012, 3, 12. [Google Scholar] [CrossRef]
- Vázquez, M.; Garrote, G.; Alonso, J.; Domínguez, H.; Parajó, J. Refining of autohydrolysis liquors for manufacturing xylooligosaccharides: Evaluation of operational strategies. Bioresour. Technol. 2005, 96, 889–896. [Google Scholar] [CrossRef]
- Bhattarai, M.; Pitkänen, L.; Kitunen, V.; Korpinen, R.; Ilvesniemi, H.; Kilpeläinen, P.O.; Lehtonen, M.; Mikkonen, K.S. Functionality of spruce galactoglucomannans in oil-in-water emulsions. Food Hydrocoll. 2019, 86, 154–161. [Google Scholar] [CrossRef]
- Chadni, M.; Bals, O.; Ziegler-Devin, I.; Brosse, N.; Grimi, N. Microwave-assisted extraction of high-molecular-weight hemicelluloses from spruce wood. Comptes Rendus Chim. 2019, 22, 574–584. [Google Scholar] [CrossRef]
- Edlund, U.; Ryberg, Y.Z.; Albertsson, A.-C. Barrier films from renewable forestry waste. Biomacromolecules 2010, 11, 2532–2538. [Google Scholar] [CrossRef]
- Escalante, A.; Gonçalves, A.; Bodin, A.; Stepan, A.; Sandström, C.; Toriz, G.; Gatenholm, P. Flexible oxygen barrier films from spruce xylan. Carbohydr. Polym. 2012, 87, 2381–2387. [Google Scholar] [CrossRef]
- Pereira, P.H.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; de Brito, E.S.; Rodrigues, T.H.; Rosa, M.F.; Azeredo, H.M. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Jin, X.; Huang, C.; Li, L.; Wu, W.; Qi, H.; Nishiyama, Y. Water cast film formability of sugarcane bagasse xylans favored by side groups. Cellulose 2020, 27, 7307–7320. [Google Scholar] [CrossRef]
- Sternemalm, E.; Höije, A.; Gatenholm, P. Effect of arabinose substitution on the material properties of arabinoxylan films. Carbohydr. Res. 2008, 343, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, U.R.; Smith, S.; Pingali, S.V.; Yang, H.; Zahran, M.; Breunig, L.; Wilson, L.A.; Kowali, M.; Kubicki, J.D.; Cosgrove, D.J. Arabinose substitution effect on xylan rigidity and self-aggregation. Cellulose 2019, 26, 2267–2278. [Google Scholar] [CrossRef]
- Jin, X.; Hu, Z.; Wu, S.; Song, T.; Yue, F.; Xiang, Z. Promoting the material properties of xylan-type hemicelluloses from the extraction step. Carbohydr. Polym. 2019, 215, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Heikkilä, M.I.; Willför, S.M.; Tenkanen, M. Films from glyoxal-crosslinked spruce galactoglucomannans plasticized with sorbitol. Int. J. Polym. Sci. 2012, 2012, 482810. [Google Scholar] [CrossRef]
- Xu, J.; Xia, R.; Zheng, L.; Yuan, T.; Sun, R. Plasticized hemicelluloses/chitosan-based edible films reinforced by cellulose nanofiber with enhanced mechanical properties. Carbohydr. Polym. 2019, 224, 115164. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.C.; Lindblad, M.S.; Sjöberg, J. Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films. J. Appl. Polym. Sci. 2006, 100, 2985–2991. [Google Scholar] [CrossRef]
- Chen, G.G.; Qi, X.M.; Li, M.P.; Guan, Y.; Bian, J.; Peng, F.; Yao, C.L.; Sun, R.C. Hemicelluloses/montmorillonite hybrid films with improved mechanical and barrier properties. Sci. Rep. 2015, 5, 16405. [Google Scholar] [CrossRef]
- Mondal, A.K.; Qin, C.; Ragauskas, A.J.; Ni, Y.; Huang, F. Conversion of Loblolly pine biomass residues to bio-oil in a two-step process: Fast pyrolysis in the presence of zeolite and catalytic hydrogenation. Ind. Crops Prod. 2020, 148, 112318. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, P.; Xu, F.; Sun, R.C. Isolation and fractionation of hemicelluloses by graded ethanol precipitation from Caragana korshinskii. Carbohydr. Res. 2010, 345, 802–809. [Google Scholar] [CrossRef]
- Yue, Z.; Sun, L.L.; Sun, S.N.; Cao, X.F.; Wen, J.L.; Zhu, M.Q. Structure of corn bran hemicelluloses isolated with aqueous ethanol solutions and their potential to produce furfural. Carbohydr. Polym. 2022, 288, 119420. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the water-and alkali-soluble hemicelluloses fractionated by sequential acidification and graded-ethanol from sweet maize stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Tomkinson, J. Characterization of hemicelluloses isolated with tetraacetylethylenediamine activated peroxide from ultrasound irradiated and alkali pre-treated wheat straw. Eur. Polym. J. 2003, 39, 751–759. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Geng, Z.; Liu, C.; Ren, J.; Sun, R.; Fowler, D.P.; Baird, M. Comparative study of water-soluble and alkali-soluble hemicelluloses from perennial ryegrass leaves (Lolium peree). Carbohydr. Polym. 2007, 67, 56–65. [Google Scholar] [CrossRef]
- Yang, H.; Yi, N.; Zhao, S.; Qaseem, M.F.; Zheng, B.; Li, H.; Feng, J.X.; Wu, A.M. Characterization of hemicelluloses in sugarcane (Saccharum spp. hybrids) culm during xylogenesis. Int. J. Biol. Macromol. 2020, 165, 1119–1128. [Google Scholar] [CrossRef]
- Li, H.; Dai, Q.; Ren, J.; Jian, L.; Peng, F.; Sun, R.; Liu, G. Effect of structural characteristics of corncob hemicelluloses fractionated by graded ethanol precipitation on furfural production. Carbohydr. Polym. 2016, 136, 203–209. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef]
- Xu, J.K.; Sun, Y.C.; Sun, R.C. Structural and hydrolysis characteristics of cypress pretreated by ionic liquids in a microwave irradiation environment. BioEnergy Res. 2014, 7, 1305–1316. [Google Scholar] [CrossRef]
- Ma, X.; Yang, X.; Zheng, X.; Lin, L.; Chen, L.; Huang, L.; Cao, S. Degradation and dissolution of hemicelluloses during bamboo hydrothermal pretreatment. Bioresour. Technol. 2014, 161, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ru, B.; Lin, H.; Sun, W. Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods. Fuel 2015, 150, 243–251. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Barbieri, S.F.; Ruthes, A.C.; de Oliveira Petkowicz, C.L.; de Godoy, R.C.B.; Sassaki, G.L.; Santana Filho, A.P.; Silveira, J.L.M. Extraction, purification and structural characterization of a galactoglucomannan from the gabiroba fruit (Campomanesia xanthocarpa Berg), Myrtaceae family. Carbohydr. Polym. 2017, 174, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Buriti, F.C.; dos Santos, K.M.; Sombra, V.G.; Maciel, J.S.; Sá, D.M.T.; Salles, H.O.; Oliveira, G.; de Paula, R.C.; Feitosa, J.P.; Moreira, A.C.M. Characterisation of partially hydrolysed galactomannan from Caesalpinia pulcherrima seeds as a potential dietary fibre. Food Hydrocoll. 2014, 35, 512–521. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Hu, X.; Kang, J.; Yada, R.Y. Structural characterization of a low-molecular-weight heteropolysaccharide (glucomannan) isolated from Artemisia sphaerocephala Krasch. Carbohydr. Res. 2012, 350, 31–39. [Google Scholar] [CrossRef]
- Hannuksela, T.; du Penhoat, C.H. NMR structural determination of dissolved O-acetylated galactoglucomannan isolated from spruce thermomechanical pulp. Carbohydr. Res. 2004, 339, 301–312. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, S.N.; Zhou, X.; Peng, F.; Sun, R. Structural characterization of hemicelluloses and topochemical changes in Eucalyptus cell wall during alkali ethanol treatment. Carbohydr. Polym. 2015, 123, 17–26. [Google Scholar] [CrossRef]
- Wang, K.; Wang, B.; Hu, R.; Zhao, X.; Li, H.; Zhou, G.; Song, L.; Wu, A.M. Characterization of hemicelluloses in Phyllostachys edulis (moso bamboo) culm during xylogenesis. Carbohydr. Polym. 2019, 221, 127–136. [Google Scholar] [CrossRef]
- Yang, H.; Yi, N.; Zhao, S.; Xiang, Z.; Qaseem, M.F.; Zheng, B.; Li, H.; Feng, J.X.; Wu, A.M. Characterization of hemicellulose in Cassava (Manihot esculenta Crantz) stem during xylogenesis. Carbohydr. Polym. 2021, 264, 118038. [Google Scholar] [CrossRef]
- Sun, S.N.; Li, M.F.; Yuan, T.Q.; Xu, F.; Sun, R.C. Effect of ionic liquid pretreatment on the structure of hemicelluloses from corncob. J. Agric. Food Chem. 2012, 60, 11120–11127. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Sun, X.; Sun, R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym. Degrad. Stab. 2001, 74, 307–319. [Google Scholar] [CrossRef]
- Weerasooriya, P.; Nadhilah, R.; Owolabi, F.; Hashim, R.; Khalil, H.A.; Syahariza, Z.; Hussin, M.; Hiziroglu, S.; Haafiz, M. Exploring the properties of hemicellulose based carboxymethyl cellulose film as a potential green packaging. Curr. Res. Green Sustain. Chem. 2020, 1, 20–28. [Google Scholar] [CrossRef]
- Dornath, P.; Cho, H.J.; Paulsen, A.; Dauenhauer, P.; Fan, W. Efficient mechano-catalytic depolymerization of crystalline cellulose by formation of branched glucan chains. Green Chem. 2015, 17, 769–775. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Promhuad, K.; Srisa, A.; San, H.; Laorenza, Y.; Wongphan, P.; Sodsai, J.; Tansin, K.; Phromphen, P.; Chartvivatpornchai, N.; Ngoenchai, P.; et al. Applications of Hemp Polymers and Extracts in Food, Textile and Packaging: A Review. Polymers 2022, 14, 4274. [Google Scholar] [CrossRef]
- Laorenza, Y.; Chonhenchob, V.; Bumbudsanpharoke, N.; Jittanit, W.; Sae-Tan, S.; Rachtanapun, C.; Chanput, W.P.; Charoensiddhi, S.; Srisa, A.; Promhuad, K.; et al. Polymeric packaging applications for seafood products: Packaging-deterioration relevance, technology and trends. Polymers 2022, 14, 3706. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Q.; Zhu, R.; Liu, Y.; Xu, F.; Zhang, X. Facile fabrication of transparent lignin sphere/PVA nanocomposite films with excellent UV-shielding and high strength performance. Int. J. Biol. Macromol. 2021, 189, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Rodembusch, F.S.; Leusin, F.P.; Campo, L.F.; Stefani, V. Excited state intramolecular proton transfer in amino 2-(2′-hydroxyphenyl) benzazole derivatives: Effects of the solvent and the amino group position. J. Lumin. 2007, 126, 728–734. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Hamedeyaz, S. Floating drug delivery systems for eradication of helicobacter pylori in treatment of peptic ulcer disease. Trends Helicobacter Pylori Infect. 2014, 1, 13. [Google Scholar]
- Zheng, B.; Xiang, Z.; Qaseem, M.F.; Zhao, S.; Li, H.; Feng, J.X.; Zhang, W.; Stolarski, M.J. Characterization of hemicellulose during xylogenesis in rare tree species Castanopsis hystrix. Int. J. Biol. Macromol. 2022, 212, 348–357. [Google Scholar]
- Xue, B.; Wen, J.; Xu, F.; Sun, R. Structural characterization of hemicelluloses fractionated by graded ethanol precipitation from Pinus yunnanensis. Carbohydr. Res. 2012, 352, 159–165. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Liu, T.; Sun, S.; Ren, J.; Wu, A.; Li, H. A novel wet-mechanochemical pretreatment for the efficient enzymatic saccharification of lignocelluloses: Small dosage dilute alkali assisted ball milling. Energy Convers. Manag. 2019, 194, 46–54. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, Y.; Zheng, S.; Mo, Y.; Sun, S.; Ren, J.; Li, Y.; Wu, A.; Li, H. Upgrade the torrefaction process of bamboo based on autohydrolysis pretreatment. Ind. Crops Prod. 2021, 166, 113470. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, T.; Li, H.; Lu, H.; Ren, J.; Zhang, A.; Deng, X.; Chen, X.; Wu, A.-M. Characterization of hemicelluloses from Neolamarckia cadamba (Rubiaceae) during xylogenesis. Carbohydr. Polym. 2017, 156, 333–339. [Google Scholar] [CrossRef]



| Fraction | H30 | H45 | H60 | H75 |
|---|---|---|---|---|
| Yield (%) | 8.86 ± 0.68 a | 6.87 ± 0.24 b | 2.22 ± 0.42 c | 1.07 ± 0.12 d |
| Composition | H30 | H45 | H60 | H75 |
|---|---|---|---|---|
| Lignin | 2.56 ± 0.13 d | 3.77 ± 0.22 c | 5.01 ± 0.43 b | 12.45 ± 1.60 a |
| Arabinose | 3.42 ± 0.02 a | 3.42 ± 0.32 a | 3.57 ± 0.20 a | 2.97 ± 0.31 b |
| Galactose | 7.66 ± 0.14 d | 10.08 ± 0.07 b | 11.02 ± 0.30 a | 8.58 ± 0.26 c |
| Glucose | 15.81 ± 0.15 a | 12.69 ± 0.14 b | 10.00 ± 0.21 c | 9.19 ± 0.09 d |
| Xylose | 23.97 ± 0.23 a | 21.03 ± 0.39 b | 17.10 ± 0.42 c | 14.9 ± 0.50 d |
| Mannose | 46.58 ± 0.47 d | 49.01 ± 0.05 c | 53.30 ± 0.57 a | 51.91 ± 0.48 b |
| DS1 | 0.14 | 0.16 | 0.21 | 0.19 |
| DS2 | 0.12 | 0.16 | 0.17 | 0.14 |
| Hemicellulosic Fractions | Mw (g/mol) | Mn (g/mol) | Mw/Mn |
|---|---|---|---|
| H30 | 71,295 | 42,072 | 1.69 |
| H45 | 72,619 | 49,702 | 1.46 |
| H60 | 53,771 | 33,675 | 1.60 |
| H75 | 25,182 | 13,422 | 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Zheng, B.; Yang, H.; Guan, Y.; Zhang, L.; Xu, X.; Wu, A.; Li, H. Effect of Loblolly Pine (Pinus taeda L.) Hemicellulose Structure on the Properties of Hemicellulose-Polyvinyl Alcohol Composite Film. Molecules 2023, 28, 46. https://doi.org/10.3390/molecules28010046
Pan H, Zheng B, Yang H, Guan Y, Zhang L, Xu X, Wu A, Li H. Effect of Loblolly Pine (Pinus taeda L.) Hemicellulose Structure on the Properties of Hemicellulose-Polyvinyl Alcohol Composite Film. Molecules. 2023; 28(1):46. https://doi.org/10.3390/molecules28010046
Chicago/Turabian StylePan, Huaizhi, Biao Zheng, Hui Yang, Yingying Guan, Liuyang Zhang, Xiaoli Xu, Aimin Wu, and Huiling Li. 2023. "Effect of Loblolly Pine (Pinus taeda L.) Hemicellulose Structure on the Properties of Hemicellulose-Polyvinyl Alcohol Composite Film" Molecules 28, no. 1: 46. https://doi.org/10.3390/molecules28010046
APA StylePan, H., Zheng, B., Yang, H., Guan, Y., Zhang, L., Xu, X., Wu, A., & Li, H. (2023). Effect of Loblolly Pine (Pinus taeda L.) Hemicellulose Structure on the Properties of Hemicellulose-Polyvinyl Alcohol Composite Film. Molecules, 28(1), 46. https://doi.org/10.3390/molecules28010046







