Experimental and Theoretical Studies on Acid Corrosion Inhibition of API 5L X70 Steel with Novel 1-N-α-d-Glucopyranosyl-1H-1,2,3-Triazole Xanthines
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Corrosion Inhibition Studies
2.2.1. Open Potential Circuit of Carbohydrate-Xanthine Conjugates
2.2.2. Effect of Concentration by EIS
2.2.3. Xanthine Triazole Concentration by CP
2.2.4. Adsorption Process
2.2.5. Mechanism of Corrosion Inhibition by the New Carbohydrate-Xanthine
2.2.6. Surface Morphology by SEM-EDS
2.2.7. AFM Analysis
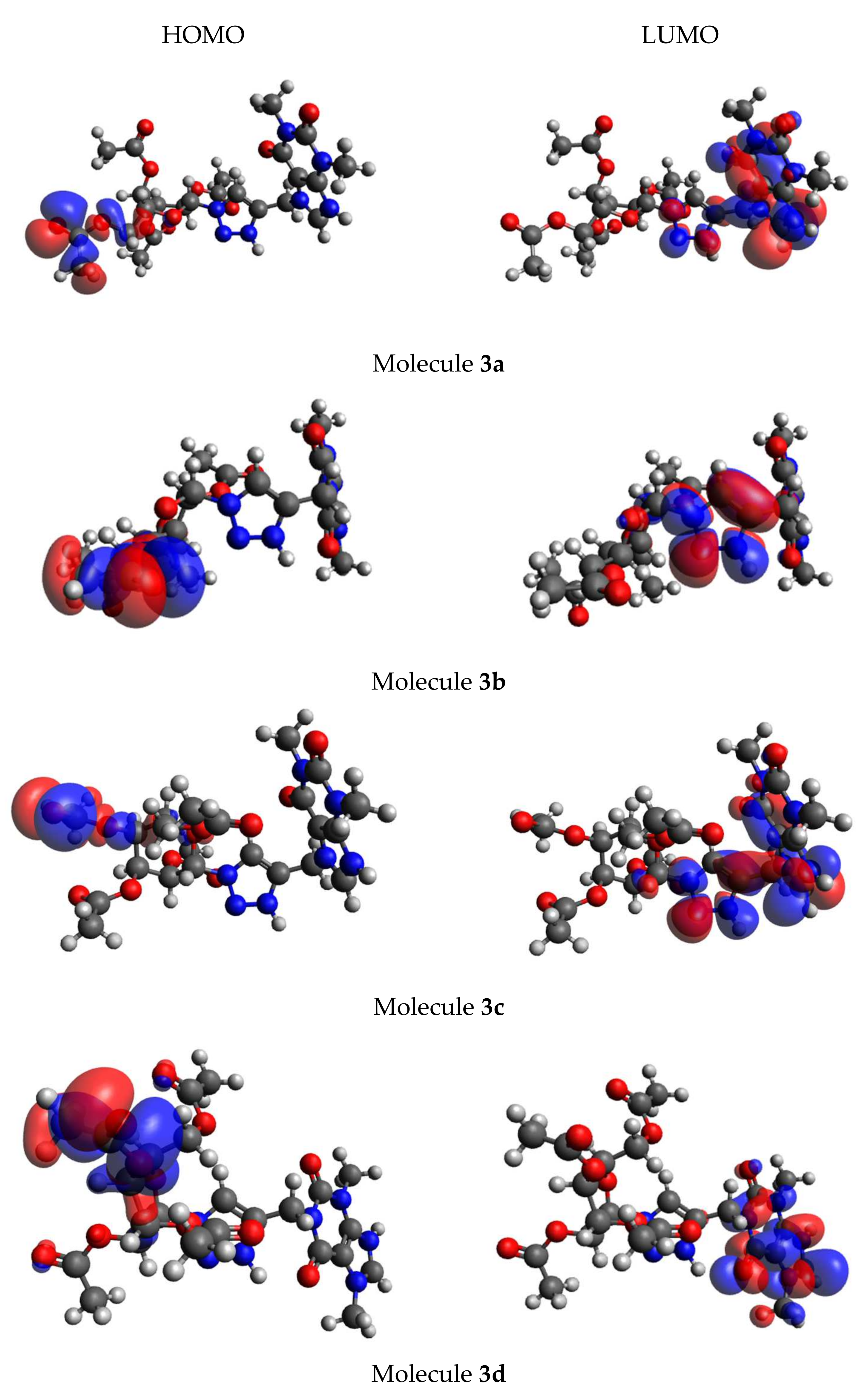
2.2.8. Theoretical Assessment
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of Carbohydrate-Xanthine Conjugates
3.1.2. Synthesis of Glucose-Triazole-Theophylline Conjugate (3a)
3.1.3. Synthesis of Glucose-Triazole-Theobromine Conjugate (3b)
3.1.4. Synthesis of Galactose-Triazole-Theophylline Conjugate (3c)
3.1.5. Synthesis of Galactose-Triazole-Theobromine Conjugate (3d)
3.1.6. Synthesis of Lactose-Triazole-Theophylline Conjugate (3e)
3.1.7. Synthesis of Lactose-Triazole-Theobromine Conjugate (3f)
3.2. Electrochemical Evaluation
3.3. Characterization by SEM-EDS and AFM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, V.S. Corrosion Inhibitors in the Oil and Gas Industry; Wiley: Hoboken, NJ, USA, 2020; p. 229. [Google Scholar]
- Tamalmani, K.; Husin, H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Appl. Sci. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2011, 2012, 380217. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic biomolecules as green corrosion inhibitors. J. Mol. Liq. 2021, 341, 117265. [Google Scholar] [CrossRef]
- Yang, H.M. Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments—A State-of-Art Review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Fouda, A.S.; Ahmed, R.E.; EI-Hossiany, A. Chemical, Electrochemical and Quantum Chemical Studies for Famotidine Drug as a Safe Corrosion Inhibitor for α-Brass in HCl Solution. Prot. Met. Phys. Chem. Surf. 2021, 57, 398–411. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardas, G.; Yazici, B.; Erbil, M. Adsorption and corrosion inhibitive properties of 2-amino-5-mercapto-1,3,4-thiadiazole on mild steel in hydrochloric acid media. Colloids Surf. A 2008, 312, 7–17. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Verma, C.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Experimental and computational insights. J. Mol. Struct. 2021, 1227, 129374. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Bhaumick, P.; Murmu, N.C.; Hirani, H.; Banerjee, P. Corrosion inhibition property of azomethine functionalized triazole derivatives in 1 mol L−1 HCl medium for mild steel: Experimental and theoretical exploration. J. Mol. Liq. 2020, 313, 113508. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical insight into an empirical rule about organic corrosión inhibitors containing nitrogen, oxygen, and sulfur atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Trindade, L.G.; Goncalves, R.S. Evidence of caffeine adsorption on a low-carbon steel surface in ethanol. Corros. Sci. 2009, 51, 1578–1583. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Reza, N.A.; Akhmal, N.H.; Fadil, N.A.; Taib, M.F.M. A Review on Plants and Biomass Wastes as Organic Green Corrosion Inhibitors for Mild Steel in Acidic. Environ. Met. 2021, 11, 1062. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green Corrosion Inhibitors from Natural Sources and Biomass Wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Vázquez, A.; Cervantes-Robles, M.A.; Negrón-Silva, G.E.; Rodríguez-Gómez, F.J.; Palomar-Pardavé, M.; Lomas-Romero, L.; Ángeles-Beltrán, D.; Pérez- Martínez, D. Carbohydrates as Corrosion Inhibitors of API 5L X70 Steel Immersed in Acid Medium. Electrochem. Sci. 2019, 14, 9206–9220. [Google Scholar] [CrossRef]
- Yadav, M.; Sarkara, T.K.; Obot, I.B. Carbohydrate compounds as green corrosion inhibitors: Electrochemical, XPS, DFT and molecular dynamics simulation studies. RSC Adv. 2016, 6, 110053–110069. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Madhankumar, A.; Gasem, Z. Performance evaluation of pectin as ecofriendly corrosion inhibitor for X60 pipeline steel in acid medium: Experimental and theoretical approaches. Carbohydr. Polym. 2015, 124, 280–291. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Madufor, I.C.; Ogbobe, O.; Oguzie, E.E. Inhibition of Mild Steel Corrosion in Sulfuric Acid Medium by Hydroxyethyl Cellulose. Chem. Eng. Commun. 2014, 202, 112–122. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose--inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.M.; Umoren, S.A.; Udosoro, I.I.; Udoh, A.P. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Su, P.; Li, L.; Li, W.; Huang, C.; Wang, X.; Liu, H.; Singh, A. Expired Drug Theophylline as Potential Corrosion Inhibitor for 7075 Aluminium Alloy in 1M NaOH Solution. Int. J. Electrochem. Sci. 2020, 15, 1412–1425. [Google Scholar] [CrossRef]
- Espinoza-Vazquez, A.; Rodríguez-Gomez, F.J.; Martínez-Cruz, I.K.; Negrón-Silva, G.E.; Palomar-Pardave, M. Adsorption and corrosion inhibition behaviour of new theophylline–triazole-based derivatives for steel in acidic medium. ECS Trans. 2018, 84, 165–171. [Google Scholar] [CrossRef]
- Elmsellem, H.; Elyoussfi, A.; Steli, H.; Sebbar, N.K.; Essassi, E.M.; Dahmani, M.; Ouadi, Y.E.; Aouniti, A.; Mahi, B.E.; Hammouti, B. The theobromine (chocolate) as green inhibitor of mild steel corrosion in hydrochloric acid: Electrochemical and theoretical Quantum studies. Pharma Chem. 2016, 8, 248–256. [Google Scholar]
- Espinoza-Vázquez, A.; Rodríguez-Gómez, F.J. Caffeine and nicotine in 3% NaCl solution with CO2 as corrosion inhibitors for low carbon steel. RSC Adv. 2016, 6, 70226–70236. [Google Scholar] [CrossRef]
- De Souza, F.S.; Gonçalves, R.S.; Spinelli, A. Assessment of Caffeine Adsorption onto Mild Steel Surface as an Eco-Friendly Corrosion Inhibitor. J. Braz. Chem. Soc. 2014, 25, 81–90. [Google Scholar] [CrossRef]
- De Souza, F.S.; Giacomelli, B.; Gonçalves, R.S.; Spinelli, A. Adsorption behavior of caffeine as a green corrosion inhibitor for copper. Mater. Sci. Eng. C 2012, 32, 2436–2444. [Google Scholar] [CrossRef]
- Ebadi, M.; Basirun, W.J.; Leng, S.Y.; Mahmoudian, M.R. Investigation of Corrosion Inhibition Properties of Caffeine on Nickel by Electrochemical Techniques. Int. J. Electrochem. Sci. 2012, 7, 8052–8063. [Google Scholar]
- Elmsellem, H.; Aouniti, A.; Youssoufi, M.; Bendaha, H.; Ben hadda, T.; Chetouani, A.; Warad, I.; Hammouti, B. Caffeine as a corrosion inhibitor of mild steel in hydrochloric acid. Phys. Chem. News 2013, 70, 84–90. [Google Scholar]
- Fallavena, T.; Antonow, M.; Goncalves, R.S. Caffeine as non-toxic corrosion inhibitor for copper in aqueous solutions of potassium nitrate. Appl. Surf. Sci. 2006, 253, 566–571. [Google Scholar] [CrossRef]
- Messaoudi, H.; Djazi, F.; Litim, M.; Keskin, B.; Slimane, M.; Bekhiti, D. Surface analysis and adsorption behavior of caffeine as an environmentally friendly corrosion inhibitor at the copper/aqueous chloride solution interface. J. Adhes. Sci. Technol. 2020, 34, 2216–2244. [Google Scholar] [CrossRef]
- Diki, N.Y.S.; Diomandé, G.G.D.; Akpa, S.J.; Ouédraogo, A.; Pohan, L.A.G.; Niamien, P.M.; Trokourey, A. Aluminum Corrosion Inhibition by 7-(Ethylthiobenzimidazolyl) Theophylline in 1M Hydrochloric Acid: Experimental and DFT Studies. Int. J. Appl. Pharm. Sci. Res. 2018, 3, 41–53. [Google Scholar] [CrossRef]
- Ouédraogo, A.; Akpa-Sagne, J.; Yao, S.D.N.; Gbe-Gondo, D.; Coulibaly-Nagnonta, H.; Trokourey, A. 7-(2-Ethyltiophenyl) Theophylline as Copper Corrosion Inhibitor in 1M HNO3. J. Mater. Sci. Chem. Eng. 2018, 6, 31–49. [Google Scholar]
- Hajjaji, F.E.; Salim, R.; Taleb, M.; Benhiba, F.; Rezki, N.; Chauhan, D.S.; Quraishi, M.A. Pyridinium-based ionic liquids as novel eco-friendly corrosion inhibitors for mild steel in molar hydrochloric acid: Experimental & computational approach. Surf. Interfaces 2021, 22, 100881. [Google Scholar]
- Rbaa, M.; Ouakki, M.; Galai, M.; Berisha, A.; Lakhrissi, B.; Jama, C.; Zarrouk, A. Simple preparation and characterization of novel 8-Hydroxyquinoline derivatives as effective acid corrosion inhibitor for mild steel: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125094. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, F.; Hu, S.; Cao, Z.; Wu, Z.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 2013, 74, 271–282. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Obot, I.B.; Sorour, A.A.; Abdul-Rashid, M.I. Green corrosion inhibitor for oilfield application I: Electrochemical assessment of 2-(2-pyridyl) benzimidazole for api ×60 steel under sweet environment in nace brine. Corros. Sci. 2019, 150, 183–193. [Google Scholar] [CrossRef]
- Wan, S.; Wei, H.; Quan, R.; Luo, Z.; Wang, H.; Liao, B.; Guo, X. Soybean extract firstly used as a green corrosion inhibitor with high efficacy and yield for carbon steel in acidic medium. Ind. Crops Prod. 2022, 187, 115354. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Q.; Li, H.; Wang, J.; Wang, X.; Wu, Y. Investigation of adsorption and corrosion inhibition property of Hyperoside as a novel corrosion inhibitor for Q235 steel in HCl medium. J. Mol. Liq. 2022, 364, 120009. [Google Scholar] [CrossRef]
- Fischer, B.; Yefidoff, R.; Major, D.; Rutman-Halili, I.; Shneyvays, V.; Zinman, T.; Jacobson, K.; Shainberg, A. Characterization of “Mini-Nucleotides” as P2X Receptor Agonists in Rat Cardiomyocyte Cultures. An Integrated Synthetic, Biochemical, and Theoretical Study. J. Med. Chem. 1999, 42, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Wang, B.; Gong, P.; Liu, Y.; Li, H.; Wu, Y. Lentinan as an eco-friendly corrosion inhibitor for Q235 steel in acid medium: Experimental and theoretical studies. J. Mol. Liq. 2022, 360, 119513. [Google Scholar] [CrossRef]
- Sun, X.; Qiang, Y.; Hou, B.; Zhu, H.; Tian, H. Cabbage extract as an eco-friendly corrosion inhibitor for X70 steel in hydrochloric acid medium. J. Mol. Liq. 2022, 362, 119733. [Google Scholar] [CrossRef]
- Kalkhambkar, A.G.; Rajappa, S.K.; Manjanna, J.; Malimath, G.H. Saussurea obvallatta leaves extract as a potential eco-friendly corrosion inhibitor for mild steel in 1 M HCl. Inorg. Chem. Commun. 2022, 143, 109799. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian, Version 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Khalil, N. Quantum chemical approach of corrosion inhibition. Electrochim. Acta 2003, 48, 2635–2640. [Google Scholar] [CrossRef]
- Lukovits, I.; Palfi, K.; Bako, I.; Kalman, E. LKP model of the inhibition mechanism of thiourea compounds. Corrosion 1997, 53, 12. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Vezin, H.; Hildebrand, H.F.; Lagrenee, M. 2,5-Bis (4-dimethylaminophenyl)-1,3,4-oxadiazole and 2,5-bis (4-dimethylaminophenyl)-1,3,4-thiadiazole as corrosion inhibitors for mild steel in acidic media. Corros. Sci. 2004, 46, 2781–2792. [Google Scholar] [CrossRef]
- Gómez, B.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Martínez-Palou, R.; Vela, A.; Gázquez, J. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J. Phys. Chem. B 2006, 110, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chem. Acc. 1977, 44, 129–138. [Google Scholar] [CrossRef]
























| C (ppm) | Rs (Ω cm2) | n | Cdl (µF/cm2) | Rct (Ω cm2) | Rmol (Ω cm2) | Rp (Ω cm2) | IE (%) | χ2 | |
|---|---|---|---|---|---|---|---|---|---|
| Blank | 0 | 5.0 | 0.8 | 7188.9 | 50.0 | - | 50.0 | - | 0.000452 |
| 3a | 5 | 1.3 | 0.8 | 424.3 | 78.5 | 12.4 | 90.9 | 36.3 | 0.003262 |
| 10 | 1.3 | 0.8 | 378.1 | 82.4 | 17.5 | 100.0 | 39.3 | 0.002553 | |
| 20 | 1.2 | 0.8 | 355.4 | 103.2 | 22.5 | 125.8 | 51.6 | 0.002984 | |
| 50 | 1.2 | 0.8 | 297.1 | 119.8 | 30.3 | 150.1 | 58.3 | 0.002348 | |
| 3b | 5 | 29.9 | 0.8 | 33.0 | 2168.4 | 19.3 | 2187.7 | 97.7 | 0.000488 |
| 10 | 30.0 | 0.8 | 45.1 | 3617.6 | 13.1 | 3630.7 | 98.6 | 0.000217 | |
| 20 | 30.1 | 0.8 | 63.7 | 6962.0 | 451.1 | 7413.1 | 99.3 | 0.000259 | |
| 50 | 29.8 | 0.8 | 60.7 | 7200.0 | 425.6 | 7625.6 | 99.2 | 0.000238 | |
| 3c | 5 | 1.3 | 0.8 | 140.5 | 588.8 | - | 588.8 | 91.5 | 0.004295 |
| 10 | 1.9 | 0.8 | 23.5 | 912.0 | - | 912.0 | 94.5 | 0.010460 | |
| 20 | 1.8 | 0.7 | 20.6 | 851.1 | - | 851.1 | 94.1 | 0.007602 | |
| 50 | 1.9 | 0.7 | 46.3 | 870.9 | - | 870.9 | 94.3 | 0.008256 | |
| 3d | 5 | 12.8 | 0.7 | 73.4 | 1077.4 | 401.7 | 1479.1 | 95.4 | 0.002924 |
| 10 | 15.6 | 0.7 | 84.0 | 887.4 | 342.8 | 1230.2 | 94.4 | 0.003157 | |
| 20 | 24.2 | 0.8 | 15.9 | 3914.1 | 872.2 | 4786.3 | 94.3 | 0.001092 | |
| 50 | 11.2 | 0.8 | 13.0 | 3634.6 | 936.2 | 4570.8 | 94.7 | 0.000824 | |
| 3e | 5 | 6.6 | 0.8 | 250.5 | 259.6 | 22.1 | 281.8 | 80.7 | 0.000697 |
| 10 | 6.7 | 0.8 | 398.1 | 332.6 | 30.3 | 363.0 | 85.0 | 0.000926 | |
| 20 | 11.9 | 0.7 | 101.8 | 1434.5 | 44.5 | 1479.1 | 96.5 | 0.001915 | |
| 50 | 10.5 | 0.8 | 117.1 | 465.2 | 366.5 | 831.7 | 89.3 | 0.001720 | |
| 3f | 5 | 8.5 | 0.4 | 86.2 | 1202.2 | - | 1202.2 | 95.8 | 0.001325 |
| 10 | 14.3 | 0.6 | 94.9 | 1679.0 | 941.4 | 2620.4 | 97.0 | 0.001191 | |
| 20 | 16.8 | 0.6 | 102.1 | 1205.0 | 922.1 | 2127.1 | 95.9 | 0.001179 | |
| 50 | 12.5 | 0.5 | 171.3 | 1078.0 | 330.7 | 1408.7 | 95.4 | 0.001166 |
| Inhibitor (C (ppm)) | Ecorr (mV) vs. Ag/AgCl sat | icorr (mA/cm2) | ba (mV/dec) | bc (mV/dec) | η (%) |
|---|---|---|---|---|---|
| 0 | −447.8 | 339.5 | 133.4 | −107.2 | - |
| 3b (5 ppm) | −408.5 | 25.9 | 82.5 | −115.9 | 92.4 |
| 3b (10 ppm) | −535.2 | 63.0 | 70.4 | −144.0 | 81.4 |
| 3d (50 ppm) | −453.5 | 7.9 | 371.1 | −237.7 | 97.7 |
| 3e (5 ppm) | −525.6 | 81.5 | 75.0 | −144.8 | 76.0 |
| 3f (5 ppm) | −493.0 | 43.5 | 80.2 | −153.7 | 87.2 |
| Inhibitor | ln Kads | ΔG°ads (kJ mol−1) | Linear Regression Equation | R2 |
|---|---|---|---|---|
| 3a | 8.67 | −19.6 | C/Ɵ = 1.6051 C + 0.0095 | 0.9999 |
| 3b | 12.5 | −28.4 | C/Ɵ = 1.0021 C + 0.0002 | 0.9995 |
| 3c | 13.2 | −30.0 | C/Ɵ = 1.0542 C + 0.0001 | 0.9995 |
| 3d | 16.4 | −37.3 | C/Ɵ = 1.0569 C + 4 × 10−6 | 0.9995 |
| 3e | 11.4 | −25.9 | C/Ɵ = 1.0467 C + 0.0006 | 0.9991 |
| 3f | 14.1 | −32.1 | C/Ɵ = 1.0262 C + 4 × 10−5 | 0.9999 |
| Conditions | C | O | Al | Si | S | Cl | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| Without inhibitor | - | 44.7 | - | 0.0901 | - | 0.6275 | 0.8837 | balance |
| 3b | 34.98 | 39.99 | 4.44 | - | - | - | - | balance |
| 3f | 26.15 | 1.70 | 0.67 | - | 1.08 | 0.63 | - | balance |
| AFM Image | Ra (nm) | Rq (nm) |
|---|---|---|
| Polish | 3.4 | 4.3 |
| Without inhibitor | 118.0 | 149 |
| Compound 3b | 97.9 | 124 |
| Compound 3f | 54.6 | 86 |
| Molecule | N (eV) |
|---|---|
| 3a | 2.78 |
| 3b | 2.87 |
| 3c | 2.86 |
| 3d | 2.77 |
| 3e | 2.91 |
| 3f | 3.12 |
| Molecule | Unprotonated | Protonated | Protonated in Solvent |
|---|---|---|---|
| 3a | −6.72 | −11.51 | −7.31 |
| 3b | −6.63 | −11.43 | −7.35 |
| 3c | −6.64 | −11.98 | −7.31 |
| 3d | −6.73 | −11.89 | −7.34 |
| 3e | −6.59 | −10.30 | −7.19 |
| 3f | −6.38 | −11.55 | −7.19 |
| Molecule | Unprotonated | Protonated | Protonated in Solvent |
|---|---|---|---|
| 3a | 4.90 | 2.76 | 4.51 |
| 3b | 4.99 | 3.01 | 4.67 |
| 3c | 4.91 | 3.44 | 4.57 |
| 3d | 5.01 | 3.38 | 4.80 |
| 3e | 4.93 | 1.85 | 4.69 |
| 3f | 4.93 | 3.63 | 4.80 |
| Molecule | Unprotonated | Protonated | Protonated in Solvent |
|---|---|---|---|
| 3a | 2.45 | 1.38 | 2.26 |
| 3b | 2.49 | 1.50 | 2.33 |
| 3c | 2.46 | 1.72 | 2.29 |
| 3d | 2.51 | 1.69 | 2.40 |
| 3e | 2.47 | 0.93 | 2.34 |
| 3f | 2.47 | 1.82 | 2.40 |
| Inhibitor Molecules | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 3d | 3e | 3f | ||||||
| Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge | Atom | Charge |
| C5 | 0.153 | C5 | 0.161 | C5 | 0.147 | C5 | 0.122 | C5 | 0.134 | C5 | 0.123 |
| C4 | 0.054 | C4 | 0.067 | C4 | 0.057 | C4 | 0.056 | C4 | 0.058 | C4 | 0.056 |
| N3 | 0.260 | N3 | 0.257 | N3 | 0.252 | N3 | 0.218 | N3 | 0.237 | N3 | 0.209 |
| N2 | 0.047 | N2 | 0.034 | N2 | 0.026 | N2 | 0.004 | N2 | 0.018 | N2 | 0.006 |
| N1 | 0.086 | N1 | 0.071 | N1 | 0.090 | N1 | 0.067 | N1 | 0.085 | N1 | 0.066 |
| O10 | −0.227 | O8 | −0.256 | O10 | −0.241 | O8 | −0.242 | O10 | −0.231 | O8 | −0.238 |
| N9 | −0.039 | N6 | −0.049 | N9 | −0.034 | N6 | −0.051 | N9 | −0.041 | N6 | −0.045 |
| O12 | −0.263 | O7 | −0.274 | O12 | −0.265 | O7 | −0.259 | O12 | −0.266 | O7 | −0.266 |
| N11 | −0.037 | N9 | −0.028 | N11 | −0.034 | N9 | −0.037 | N11 | −0.041 | N9 | −0.028 |
| Oxygen atoms present in carbohydrate moieties | |||||||||||
| O | −0.143 | O | −0.178 | O | −0.150 | O | −0.162 | O | −0.150 | O | −0.143 |
| O | −0.305 | O | −0.287 | O | −0.287 | O | −0.219 | O | −0.291 | O | −0.251 |
| O | −0.154 | O | −0.170 | O | −0.163 | O | −0.171 | O | −0.166 | O | −0.152 |
| O | −0.253 | O | −0.236 | O | −0.233 | O | −0.279 | O | −0.242 | O | −0.254 |
| O | −0.168 | O | −0.163 | O | −0.169 | O | −0.160 | O | −0.165 | O | −0.193 |
| O | −0.252 | O | −0.225 | O | −0.234 | O | −0.233 | O | −0.154 | O | −0.137 |
| O | −0.167 | O | −0.174 | O | −0.167 | O | −0.158 | O | −0.257 | O | 0.307 |
| O | −0.247 | O | −0.253 | O | −0.304 | O | −0.246 | O | −0.164 | O | −0.156 |
| O | −0.194 | O | −0.164 | O | −0.170 | O | −0.172 | O | −0.253 | O | −0.240 |
| O | −0.153 | O | −0.144 | ||||||||
| O | −0.254 | O | −0.277 | ||||||||
| O | −0.163 | O | −0.136 | ||||||||
| O | −0.264 | O | −0.274 | ||||||||
| O | −0.181 | O | −0.185 | ||||||||
| O | −0.136 | O | −0.152 | ||||||||
| O | −0.279 | O | −0.252 | ||||||||
| O | −0.179 | O | −0.171 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Eleuterio, A.; Mendoza-Merlos, C.; Corona Sánchez, R.; Navarrete-López, A.M.; Martínez Jiménez, A.; Ramírez-Domínguez, E.; Lomas Romero, L.; Orozco Cruz, R.; Espinoza Vázquez, A.; Negrón-Silva, G.E. Experimental and Theoretical Studies on Acid Corrosion Inhibition of API 5L X70 Steel with Novel 1-N-α-d-Glucopyranosyl-1H-1,2,3-Triazole Xanthines. Molecules 2023, 28, 460. https://doi.org/10.3390/molecules28010460
Sánchez-Eleuterio A, Mendoza-Merlos C, Corona Sánchez R, Navarrete-López AM, Martínez Jiménez A, Ramírez-Domínguez E, Lomas Romero L, Orozco Cruz R, Espinoza Vázquez A, Negrón-Silva GE. Experimental and Theoretical Studies on Acid Corrosion Inhibition of API 5L X70 Steel with Novel 1-N-α-d-Glucopyranosyl-1H-1,2,3-Triazole Xanthines. Molecules. 2023; 28(1):460. https://doi.org/10.3390/molecules28010460
Chicago/Turabian StyleSánchez-Eleuterio, Alma, Carlos Mendoza-Merlos, Ricardo Corona Sánchez, Alejandra M. Navarrete-López, Anatolio Martínez Jiménez, Elsie Ramírez-Domínguez, Leticia Lomas Romero, Ricardo Orozco Cruz, Araceli Espinoza Vázquez, and Guillermo E. Negrón-Silva. 2023. "Experimental and Theoretical Studies on Acid Corrosion Inhibition of API 5L X70 Steel with Novel 1-N-α-d-Glucopyranosyl-1H-1,2,3-Triazole Xanthines" Molecules 28, no. 1: 460. https://doi.org/10.3390/molecules28010460
APA StyleSánchez-Eleuterio, A., Mendoza-Merlos, C., Corona Sánchez, R., Navarrete-López, A. M., Martínez Jiménez, A., Ramírez-Domínguez, E., Lomas Romero, L., Orozco Cruz, R., Espinoza Vázquez, A., & Negrón-Silva, G. E. (2023). Experimental and Theoretical Studies on Acid Corrosion Inhibition of API 5L X70 Steel with Novel 1-N-α-d-Glucopyranosyl-1H-1,2,3-Triazole Xanthines. Molecules, 28(1), 460. https://doi.org/10.3390/molecules28010460







